| Research Article | ||
Open Vet. J.. 2025; 15(8): 3645-3654 Open Veterinary Journal, (2025), Vol. 15(8): 3645-3654 Research Article Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamateAstika Gita Ningrum1*, Endyka Erye Frety1, Ivon Diah Wittiarika1, Khadizah H. Abdul-Mumin2 and Renata Alya Ulhaq11Midwifery Study Program, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Nursing and Midwifery Program, PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam, Gadong, Brunei Darussalam *Corresponding Author: Astika Gita Ningrum. Midwifery Study Program, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: astika.gita.n [at] fk.unair.ac.id Submitted: 15/01/2025 Revised: 20/06/2025 Accepted: 10/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Excessive monosodium glutamate (MSG) consumption increases blood plasma glutamate levels, forming hydroxyl radicals (O.H.) that cause oxidative stress. Antioxidants stabilize free radicals, mitigating this oxidative damage in the body. Purslane (Portulaca oleracea L.), a native plant commonly found in Indonesia and often regarded as a weed, offers significant benefits as it can be consumed as a vegetable and used for its medicinal properties. Purslane plants have the highest levels of antioxidant activity in their leaves and stems. Aim: This study investigated the potential of Purslane (Portulaca oleracea L.) in mitigating oxidative stress induced by MSG and its effects on reproductive health indicators in female Wistar rats. Methods: Over 28 days, 30 rats were divided into two groups: one receiving MSG alone and the other receiving MSG with varying doses of Purslane. Two groups: one receiving MSG alone and the other receiving varying doses of Purslane over 28 days Results: This study demonstrated that Purslane significantly reduced malondialdehyde (MDA) levels, with optimal effects observed at doses of 250–500 mg. Follicle-stimulating hormone levels notably increased at 250 mg, and endometrial thickness showed a dose-dependent increase. Conclusion: Our study reveals that purslane supplementation may help counteract MSG-induced oxidative damage and enhance reproductive health markers. Further research is needed to elucidate its underlying mechanisms and therapeutic potential in conditions related to oxidative stress. Keywords: Endometrial thickness, Follicle-stimulating hormone, Malondialdehyde, Monosodium glutamate, Purslane, Reproductive health. IntroductionMonosodium glutamate (MSG) is a widely recognized food additive that enhances flavor in various dishes. According to the U.S. Food and Drug Administration and the Joint FAO/WHO Expert Committee on Food Additives, MSG is generally recognized as safe (GRAS) for consumption at levels typically found in foods, with no specified upper limit for daily intake. However, studies suggest that excessive consumption, particularly above 3 g per day, may lead to adverse health effects, including oxidative stress and neurotoxicity (He et al., 2011; FDA, 2021; WHO, 2022). MSG is the sodium salt of glutamic acid found in hydrolyzed protein form, which is purified and commonly found in processed foods (Abdel Moneim et al., 2018; Albrahim and Binobead, 2018). Indonesian Basic Health Research (2018) indicates that flavor enhancers are among the most consumed risky foods by the Indonesian population (77.6%), surpassing other risky foods such as sweet foods (61.27%) and fatty foods (41.7%) (Badan Penelitian dan Pengembangan Kesehatan, 2020). Excessive MSG consumption increases the concentration of glutamate in the blood plasma, which can transform into hydroxyl radicals (O.H.), triggering oxidative stress. Oxidative stress arises from increased levels, causing cellular damage in the arcuate nucleus of the hypothalamus. This disruption subsequently affects the pituitary gland’s secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH secretion influences follicle development, which releases estrogen (Hermanussen et al., 2006; Eweka et al., 2007; Fatmaningrum and Ningtyas, 2019). Decreased estrogen hormone levels play a crucial role in the menstrual cycle and endometrial proliferation, thereby affecting endometrial thickness via antioxidant chemical function, which neutralizes free radicals and prevents oxidative stress within the body. Purslane (Portulaca oleracea L.), a plant commonly found in Indonesia, is an example of a species rich in antioxidants (Nurcholis et al., 2023). Purslane plants contain various antioxidant chemicals, including gallotannins, omega-3 fatty acids, ascorbic acid, α-tocopherols, kaempferol, quercetin, and apigenin (Zhou et al., 2015). Research on the antioxidant properties of Purslane indicates that its extract lowers malondialdehyde (MDA) levels and elevates superoxide dismutase (SOD) levels in the ovaries and uterus, thereby influencing oxidative stress levels in menopausal female mice (Ahangarpour et al., 2016). To the best of our knowledge, this is the first in vivo experimental study evaluating the protective effect of the ethanol extract of purslane leaves on oxidative stress and reproductive indicators (MDA, FSH, and endometrial thickness) in female Wistar rats exposed to MSG. Addressing this gap is crucial, given MSG’s widespread consumption and the increasing prevalence of reproductive health disturbances that may be linked to environmental and dietary factors. Based on the information outlined above, this study aims to evaluate MDA levels, blood follicle-stimulating hormone (FSH) levels, and endometrium thickness in female Rattus norvegicus rats that were given an ethanol extract of purslane leaves and were also administered MSG orally. Materials and MethodsChemicalsThe MSG exposure provided was a single dose of 0.7 mg/g body weight (BW) orally for 4 weeks (once daily in the morning) after the rats were in the proestrus phase (Fatmaningrum and Ningtyas, 2019). Purslane leaf extractPurslane leaf extract was obtained through the maceration method at the Pharmacology Laboratory of Universitas Airlangga. Fresh Portulaca oleracea L. leaves were collected, cleaned, and shade-dried at room temperature for 5–7 days to preserve phytochemicals. The dried material was ground into a fine powder using an electric blender. Approximately 500 g of leaf powder was macerated in 96% ethanol (1:5 w/v) for 72 hours with occasional stirring. The solution was filtered using Whatman No. 1 filter paper and re-extracted twice to maximize the yield. The combined filtrates were concentrated using a rotary evaporator at 40°C under reduced pressure to obtain a thick ethanol extract. The extract was stored in a sterile amber glass container at 4°C until use. According to previous phytochemical profiling studies, the ethanol extract contains a mixture of antioxidant bioactives, including flavonoids (kaempferol, apigenin, and quercetin), phenolic acids, omega-3 fatty acids, ascorbic acid, and α-tocopherol (Zhou et al., 2015; Desta et al., 2020). Each treatment dose (125, 250, and 500 mg/kgBW) was prepared by dissolving the extract in a 0.9% NaCl solution. Oral gavage was used for administration once daily for 28 consecutive days in the evening, following the estrous cycle synchronization. The doses of Purslane extract (125, 250, and 500 mg/kg BW/day) were chosen based on previous studies that demonstrated its antioxidant and reproductive protective effects at these concentrations (Zhou et al., 2015; Ahangarpour et al., 2016; Ningrum et al., 2021a). AnimalsThirty healthy female Wistar rats (Rattus norvegicus) aged 10–12 weeks and weighing 150–200 g were used in this study. Rats were procured from a certified breeding facility and acclimatized for 7 days under standard laboratory conditions (22°C–25°C, 12-hour light/dark cycle, and ad libitum access to food and water). Synchronization of the estrous cycle was synchronized before treatment initiation to ensure uniform reproductive status. Every effort was made to minimize animal suffering throughout the study. All handling and administration procedures were performed by trained personnel, and the rats were monitored daily for signs of pain or distress. At the end of the treatment period, euthanasia was conducted humanely under deep anesthesia prior to sample collection to ensure minimal discomfort. Animal groups
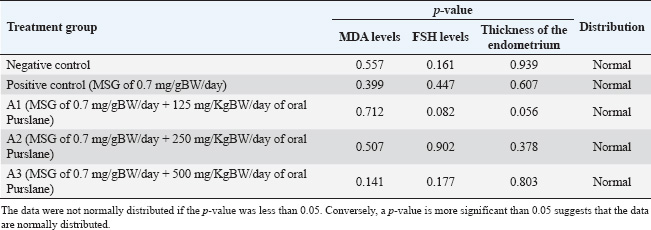
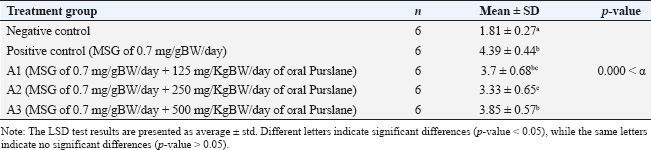
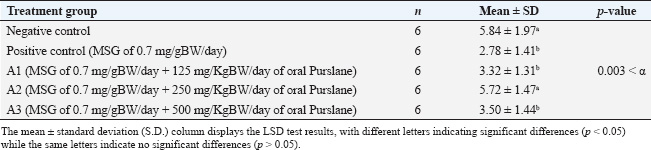
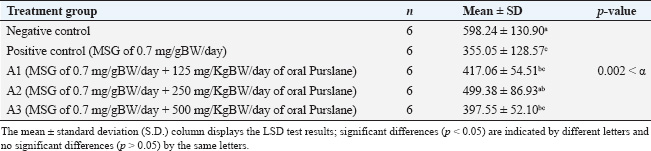
Sample collectionThe rats’ estrous cycles were checked on the day preceding and following the last day of MSG and Purslane treatment (28 days). The rats’ proestrus phase blood samples were taken intracardially via the right ventricle of the heart. Using an injection syringe, approximately 3 ml of blood was drawn into a test tube devoid of anticoagulant and sealed with a rubber stopper. Once retrieved, the uterine tissue was kept in a 10% formalin buffer container. Spectrophotometric measurement of serum MDA levelsSpectrophotometric measurements of MDA serum levels were performed using the BIOXYTECH MDA-586TM kit (Catalogue Number 21044). We performed the assay according to the manufacturer’s instructions for the kit. The reagent and standard preparations were performed according to the kit instructions. A reaction mixture, comprising the serum samples and pertinent reagents, was mixed and incubated at a specific temperature for a predetermined period. After incubation, the absorbance of the produced chromogen was measured using a spectrophotometer at a specific wavelength. To determine the MDA concentration, we compared the absorbance readings of the serum to a standard curve created from known MDA concentrations. Three assays were performed to verify the precision and repeatability of the findings. Quantification of FSH levels via enzyme-linked immunosorbent assay (ELISA) techniqueFSH levels were quantified via the ELISA technique. The process was modified according to the instructions outlined in the Rat FSH ELISA antibody kit (Catalog Number: E1393Ra), produced by B.T. Laboratory, which is manufactured in China. The ELISA assay comprised multiple sequential steps. Initially, the standards and samples were created according to the instructions provided in the kit. Subsequently, the microplate wells were coated with an FSH-targeting monoclonal antibody. The standards, samples, and controls were introduced into their corresponding wells. After a suitable incubation period, the wells were rinsed to remove any unbound compounds. Subsequently, a biotinylated and specific FSH detection antibody was introduced into each well, followed by an additional incubation period and subsequent washing. Subsequently, a streptavidin-HRP conjugate was introduced to the wells and bound to the biotinylated antibody. Following incubation and washing, a substrate solution was introduced to initiate a chromogenic reaction. The strength of the resulting color was directly correlated with the FSH concentration in the samples. The absorbance of the resulting color at a specified wavelength was determined by spectrophotometric measurement. The absorbance readings in the samples were compared to a standard curve built from known FSH concentrations to ascertain the FSH levels in those samples. The assay was performed in triplicate to ensure the accuracy and reliability of the results. Histological staining and analysis of uterus samplesThe staining of the uterus sample was conducted in the Laboratory of Anatomical Pathology, Faculty of Medicine, Universitas Airlangga. Previously stored in formalin buffers, the uterus was paraffin-embedded, stained with Hematoxylin, and then counterstained with Eosin 1% for 10–15 minutes. It was subsequently dehydrated using 70%, 80%, and 96% alcohol for 3 minutes each. The preparations were then drenched in Xylol for 60 minutes, mounted using Entellan, covered using cover glasses, and left to dry for observation. Each preparation slide was observed under an Olympus XC microscope using 100× magnification. To identify the thickness of the endometrium on the HE-stained uterus tissue when scanned using an Olympus microscope supported by the Dot Slide software application. The scan was then registered in the ImageJ software application to measure the endometrial thickness. The thickness of each endometrium was summed, and the mean was calculated. Statistical analysesAll quantitative data (MDA levels, FSH levels, and endometrial thickness) were first assessed for normality using the Shapiro–Wilk test. The results confirmed a normal distribution in all groups (p > 0.05), allowing the use of parametric statistical tests. Comparisons between the negative and positive control groups were performed using independent sample t-tests. To analyze differences among the four MSG-exposed groups (positive control and three Purslane treatment groups), a one-way analysis of variance (ANOVA) was used. When the ANOVA yielded a statistically significant result (p < 0.05), a post hoc least significant difference (LSD) test was applied to determine the pairwise differences between the groups. The LSD test calculates a critical difference value based on the standard error of the mean and the t-distribution. Differences in group means greater than this critical value were considered statistically significant. All p-values were two-tailed and considered significant at α=0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. Data are presented as mean ± standard deviation (SD), and statistically significant differences between groups were indicated using superscript letters. Ethical approvalAll procedures involving animals were reviewed and approved by the Universitas Airlangga Faculty of Dental Medicine Health Research Ethical Clearance Commission, under approval number 522/HRECC.FODM/IX/2021. ResultsThe Shapiro–Wilk test results showed that the MDA, FSH levels, and thickness of the endometrium data for each group of observations had a p-value more significant than the significance level of 0.05. All the data satisfied the parametric test conditions, confirming that the data follow a normal distribution (Table 1). Serum MDA level measurementTable 2 shows the effect of the ethanol extract of P. oleracea on serum MDA levels. A one-way ANOVA revealed significant differences across groups: F (4, 25)=24.19, p < 0.0001, with a large effect size based on eta squared (η²=0.79), indicating that 79% of the variation in MDA levels can be attributed to treatment differences. The treatment groups (125, 250, and 500 mg/kg BW) of Purslane had significantly lower MDA levels than the MSG-only group, with the 250 mg/kg group showing the most substantial reduction. A non-linear dose-response model is suggested. More precisely, when administered orally, Purslane at dosages of 125, 250, and 500 mg resulted in MDA levels of 3.7 ± 0.68, 3.33 ± 0.65, and 3.85 ± 0.57 μM, respectively. The use of letters to identify significant differences emphasizes the distinctness of each treatment group. For example, using various letters (a, b, c) indicates significant differences (p-value < 0.05), whereas using the same letter indicates no significant changes. This implies that the Ethanol Extract of Purslane Leaves has a dosage-dependent protective effect against MSG-induced oxidative stress, as indicated by the reduction in MDA levels. The optimal dose is probably between 250 and 500 mg of oral Purslane. The histogram below displays, in total, the average MDA levels of the five sample groups (Fig. 1). Measurement of the FSH levelsTable 3 presents the FSH levels across the groups. One-way ANOVA showed: F (4, 25)=4.87, p=0.005, with η²=0.44, indicating a moderate effect size, suggesting that the treatment accounted for 44% of the variability in FSH levels. Only the 250 mg/kg dose significantly restored FSH levels, nearly matching the negative control. The other doses showed non-significant increases. The treatment groups that administered oral Purslane supplementation (A1, A2, and A3) had different impacts on FSH levels compared to the positive control group. The p-values obtained from the LSD test confirm the statistically significant differences between the treatment and positive control groups. More precisely, when given oral Purslane at doses of 125 and 500 mg, the FSH levels were measured to be 3.32 ± 1.31 and 3.50 ± 1.44 μM, respectively. No notable variations were observed in both cases compared to the positive control group. Notably, the group receiving 250 mg of oral Purslane (A2) demonstrated a significant increase in FSH levels (5.72 ± 1.47 μM), denoted by the different letter “a” in comparison to the positive control. This suggests a dose-dependent response, with the optimal effect on FSH levels observed at 250 mg of oral Purslane. The histogram below shows the average FSH levels of all five sample groups (Fig. 2). Measurement of the endometrium thicknessTable 4 shows the endometrial thickness data. One-way ANOVA yielded the following results: F (4, 25)=6.51, p=0.001, with η²=0.51, denoting a moderate to large effect size, with over half of the variance explained by treatment. The 250 mg/kg group showed the most consistent improvement, whereas the 125 mg/kg group also showed improvement. The 500 mg/kg group exhibited weaker effects, suggesting a non-linear relationship. Significant differences in endometrium thickness were observed among the treatment groups receiving oral Purslane supplementation (A1, A2, and A3) compared with the positive control group. The p-values obtained from the LSD test confirm these differences. Specifically, at doses of 125, 250, and 500 mg of oral Purslane, the endometrium thicknesses were 417.06 ± 54.51, 499.38 ± 86.93, and 397.55 ± 52.10 μm, respectively. Table 1. Statistical analysis was performed to assess the normality of serum MDA and FSH levels and the thickness of the endometrium.
Table 2. Ethanol extract of purslane leaves (Portulaca oleracea L.) affects serum MDA levels (μM).
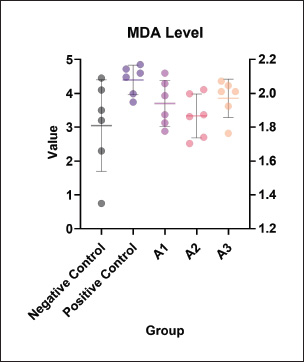
Fig. 1. MDA levels (μM) in different experimental groups. The positive control group (MSG 0.7 mg/g BW/day) showed a significant increase in MDA levels compared to the negative control, indicating elevated oxidative stress. Co-administration of oral P. oleracea (purslane) at various doses (125, 250, and 500 mg/g BW/day) resulted in a reduction of MDA levels, with the 250 mg/g BW/day group (A2) showing the most pronounced decrease. These findings suggest that purslane has antioxidant potential in mitigating MSG-induced oxidative damage. Letters are used to highlight the essential distinctions between each treatment group. While using the same letter shows no significant differences, using different letters (a, b, c), for instance, reveals substantial differences (p-value < 0.05). The ethanol extract of purslane leaves dose-dependently enhanced endometrial thickness in rats exposed to MSG. Among the tested doses, 250 mg/kg BW demonstrated the most consistent improvements across parameters; however, the relative performance of 125 mg/kg BW in reducing MDA levels and increasing endometrial thickness suggests that the dose-response relationship may be nonlinear and requires further investigation.
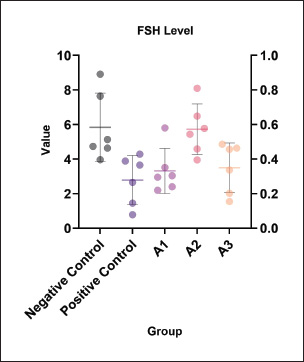
Fig. 2. FSH levels (μM) across the experimental groups. The positive control group (MSG 0.7 mg/g BW/day) exhibited a significant decrease in FSH levels compared with the negative control, indicating suppression of reproductive hormonal function. Co-treatment with P. oleracea (purslane) at doses of 125, 250, and 500 mg/g BW/day showed dose-dependent improvement, with the 250 mg/g BW/day group (A2) restoring FSH levels nearly to baseline. These results suggest that moderate-dose purslane may effectively suppress MSG-induced FSH suppression. Table 3. Effect of the ethanol extract of purslane leaves (Portulaca oleracea L.) on FSH levels (μM).
Table 4. Effect of ethanol extract of purslane leaves (Portulaca oleracea L.) on endometrium thickness.
DiscussionMSG is a widely used additive in processed foods, valued for its flavor-enhancing properties and its role as a preservative. It is found in many food products, making it accessible to people of all ages. Glutamate, the main ingredient in MSG, is such an essential molecule in biological systems that it also regulates gene expression, acts as an antioxidant, and is involved in immune responses. Despite its biological significance, MSG consumption has raised health issues mainly due to its ability to cause oxidative stress (Hoesada et al., 2016). Experimental animal studies indicate that MSG is associated with oxidative damage. This included high blood levels of MDA, a marker of lipid peroxidation, in rats exposed to MSG (Fig. 1). High MDA levels reflect a high degree of lipid peroxidation, which can also compromise cellular integrity. Decreased levels of FSH synthesis and secretion due to MSG have been reported; FSH plays crucial roles in gametogenesis, sex steroid activation, and mature reproductive organ development in both sexes (Fig. 2). In animal model studies, exposure to MSG has been associated with decreased circulating concentrations of LH and FSH (França et al., 2006; Fernandes et al., 2012; Ningrum et al., 2021b). MSG triggers heightened glutamate receptor activity, leading to elevated free radical production and Ca²+ influx into neurons. Additionally, this cascade promotes excitotoxicity and neuronal damage, particularly in hypothalamic nuclei that control the pituitary-adrenal axis and regulate reproductive hormones. Consequently, hypothalamic glutamate receptor impairment inhibits FSH and LH release and further disturbs normal hormonal regulation (Khaled et al., 2016; Rueda et al., 2016). Ovarian function primarily depends on FSH, which affects the rapid development of follicles and impacts endometrial thickness and receptivity. Previous experimental findings supporting this association also showed that MSG reduces serum FSH levels in female rats, compromising ovarian and endometrial functions. A fall in FSH levels not only inhibits follicle progression but also decreases endometrial receptivity, potentially threatening fertility (Abdulghani et al., 2022). This body of work highlights the significant influence MSG has on the hypothalamic-pituitary-gonadal axis, which may lead to reproductive health consequences. The body’s endogenous antioxidants typically protect against oxidative stress caused by reactive oxygen species (ROS). However, excessive ROS production overwhelms these defenses, necessitating the use of external antioxidants to restore the balance between prooxidants and antioxidants. Purslane (Portulaca oleracea) offers a potential therapeutic solution due to its rich content of bioactive compounds, including flavonoids, phenolic compounds, and vitamins, with potent antioxidant properties. The antioxidants in Purslane play a crucial role in scavenging free radicals, reducing oxidative stress, and mitigating cellular damage. This is particularly relevant in counteracting oxidative stress, which contributes to various chronic diseases and the aging process (Desta et al., 2020). The effect of both MSG and P. oleracea (purslane) on FSH regulation remains a subject of ongoing debate in the literature. Some studies have reported a suppressive effect of MSG on gonadotropins, including FSH and LH, due to excitotoxicity and oxidative stress-induced hypothalamic-pituitary disruption (Mondal et al., 2018). In contrast, other reports suggest potential compensatory endocrine responses that can vary based on dosage, exposure duration, and physiological status. Similarly, the influence of P. oleracea on FSH appears to be dose- and context-dependent. For instance, Ahangarpour et al. (2016) demonstrated that the ethanolic extract of purslane improved ovarian function and significantly increased FSH levels in aging female mice, supporting its estrogen-modulatory and pro-fertility roles. Conversely, Obinna et al. (2019) found that both lipophilic and hydrophilic P. oleracea extracts disrupted serum levels of FSH, LH, and estradiol in reproductive-aged female rats, suggesting that extract polarity and concentration may critically influence endocrine outcomes (Ahangarpour et al., 2016). The present study provides new insights by demonstrating that the administration of 250 mg/kg BW of purslane extract significantly increases FSH levels in MSG-exposed female rats compared with the MSG-only and low-dose groups. This supports the hypothesis that P. oleracea exerts protective effects against MSG-induced hormonal suppression, possibly via antioxidant and phytoestrogenic mechanisms. However, the observation that higher doses (500 mg/kg BW) did not produce a greater FSH elevation also suggests a non-linear or saturable dose-response relationship, highlighting the need for further mechanistic studies to clarify these dynamics. Mechanistic insightsThe protective effects of P. oleracea are primarily attributed to its content of antioxidant and anti-inflammatory compounds, such as flavonoids, omega-3 fatty acids, and vitamins C and E (Zhou et al., 2015; Skibska et al., 2023). These molecules act not only as free radical scavengers but also as modulators of key intracellular signaling pathways, such as NF-κB, Nrf2, and MAPK, which regulate oxidative stress response and inflammation (Zhou et al., 2015; Skibska et al., 2023). Additionally, flavonoids, such as kaempferol and quercetin, have been demonstrated to modulate estrogenic activity by binding to estrogen receptors, which may influence endometrial receptivity and hormonal balance (Park et al., 2022). These mechanistic pathways support the observed improvement in oxidative stress markers and reproductive parameters in MSG-exposed rats treated with Purslane. Purslane’s antioxidants, including flavonoids and phenolic substances, effectively neutralize ROS and decrease oxidative stress in endometrial tissues (Adjuvant et al., 2018; Skibska et al., 2023). By donating electrons to lipid radicals, these antioxidants interrupt lipid peroxidation—a chain reaction caused by ROS that leads to MDA formation—and subsequently lower MDA levels. The ethanol extract of Purslane leaves demonstrated a dose-dependent protective effect in rats treated with MSG, particularly within the 250–500 mg range, as evidenced by reduced MDA levels (Table 2). Purslane also enhances the body’s natural antioxidant defense system by boosting the activity of enzymes such as SOD, CAT, and glutathione peroxidase. These enzymes work synergistically to neutralize ROS, thereby protecting cells from oxidative damage. Enhanced enzymatic activity through Purslane supplementation leads to more effective ROS removal and reduced oxidative stress markers, including MDA (Skibska et al., 2023). In addition to its antioxidant effects, Purslane influences hormonal balance, notably FSH, which is critical for follicular growth and endometrial health. Administration of Purslane (250 mg) significantly increased FSH levels (Table 3), stimulating ovarian follicle development and estrogen synthesis (Abdulghani et al., 2022). These hormonal effects improve endometrial thickness and receptivity, which are essential for successful implantation. Safety and toxicity considerationsPortulaca oleracea (purslane) is widely consumed as a food and traditional medicine in many cultures and is generally regarded as safe. However, several experimental and preclinical studies have raised concerns about dose-related toxicity. Although moderate doses (≤ 500 mg/kg BW) are typically considered non-toxic, higher concentrations have been associated with hepatic stress and biochemical alterations. Musa et al. (2007) reported mild histological changes in the liver tissues of rats treated with high-dose methanolic extracts of purslane, despite the absence of mortality at or below 500 mg/kg BW. These findings underscore the need for comprehensive toxicological and histopathological evaluations when assessing the safety of botanical extracts for pharmacological use (Musa et al., 2007). Supporting this caution, Darvish Damavandi et al. (2021) conducted a randomized, double-blind, placebo-controlled clinical trial in patients with non-alcoholic fatty liver disease. They found that administering 300 mg/day of purslane extract for 12 weeks did not significantly alter liver enzyme levels (ALT, AST, ALP, GGT). This suggests that purslane extract is well tolerated in humans at moderate doses, although minor gastrointestinal complaints were reported. Nevertheless, the evidence from human studies remains limited, especially concerning long-term and high-dose applications (Darvish Damavandi et al., 2021). In this study, no signs of toxicity or distress were observed in rats treated with Purslane extract at up to 500 mg/kgBW. However, long-term administration and histopathological assessments are necessary to fully confirm the absence of systemic toxicity, especially in therapeutic applications. Purslane supplementation demonstrated a gradual, dose-dependent increase in endometrial thickness, with significant improvements observed at dosages of 250–500 mg (Table 4). This effect can be attributed to Purslane’s ability to enhance tissue integrity, mitigate oxidative damage, and reduce inflammation. Moreover, Purslane antioxidants protect endometrial epithelial and stromal structures, improving overall endometrial health and implantation receptivity. Although this study did not include a group treated with Purslane extract alone, previous research suggests that P. oleracea may exert reproductive and systemic benefits beyond its antioxidant properties. Ahangarpour et al. (2016) reported that the ethanolic extract of Purslane enhanced ovarian function and increased the levels of FSH in aging female mice, even without prior exposure to toxicants. Similarly, Ningrum et al. (2021a) observed improvements in oxidative stress markers and reproductive hormone balance in rats treated with Purslane alone. These findings raise the possibility that Purslane’s protective actions may also involve hormonal modulation, phytoestrogenic effects, or anti-inflammatory pathways (Ahangarpour et al., 2016; Ningrum et al., 2021a). Portulaca oleracea (purslane) is widely recognized for its rich antioxidant and flavonoid content, such as quercetin, kaempferol, and apigenin. These compounds also belong to the class of phytoestrogens—plant-derived molecules capable of modulating estrogenic activity by binding to estrogen receptors ERα and Erβ (Patisaul and Jefferson, 2010). Although this may offer therapeutic benefits, particularly in menopausal and aging models, the long-term safety of phytoestrogens remains controversial due to potential endocrine-disrupting effects, carcinogenicity, reproductive toxicity, and thyroid interference. In sensitive populations such as pregnant women, individuals with estrogen-sensitive malignancies, or those undergoing hormonal therapy, phytoestrogens may act as agonists or antagonists, leading to alterations in reproductive hormone regulation. Excessive intake of soy isoflavones—another well-known phytoestrogen—can stimulate estrogen-dependent tumor growth in animal models (Allred et al., 2001) and disrupt reproductive development in infants (Jefferson et al., 2012; Adgent et al., 2018). Although similar studies on purslane are limited, Obinna et al. (2019) found that both hydrophilic and lipophilic leaf extracts of purslane altered serum levels of FSH, LH, and estradiol in rats, suggesting its potential hormonal activity. The possibility of thyroid disruption has also been raised. Isoflavones in soy are known to inhibit thyroid peroxidase, particularly in iodine-deficient individuals (Doerge and Sheehan, 2002; Messina and Redmond, 2006). Although purslane-specific data on thyroid function are lacking, its polyphenolic profile suggests a need for caution among individuals with thyroid disorders. Therefore, the current study did not observe any adverse estrogenic effects in rats, and prior studies suggest reproductive benefits at moderate doses (Ahangarpour et al., 2016). Further investigation is warranted. This includes hormonal profiling, chronic toxicity studies, uterotrophic assays, and evaluation of reproductive and thyroid outcomes, especially if purslane is recommended for regular human consumption or as a nutraceutical product. Generalizability and translational potentialAlthough this study used a rodent model, the fundamental biochemical and hormonal pathways influenced by MSG and antioxidant agents are conserved across mammalian species. Therefore, the observed effects, particularly those related to oxidative stress, hormonal regulation, and endometrial structure, can be cautiously extrapolated to human physiology. These findings underscore the importance of evaluating Purslane-based interventions in clinical or translational research, given the growing global concern about endocrine disruptors and fertility decline. Nevertheless, interspecies variations in pharmacokinetics and hormonal feedback mechanisms warrant careful investigation before human application. The selection of doses of the purslane extract (125, 250, and 500 mg/kgBW) in this study was based on previous experimental literature that demonstrated pharmacological activity and safety within this range (Ahangarpour et al., 2016; Ningrum et al., 2021a). These doses were chosen to observe both sub-therapeutic and potentially optimal responses. Therefore, 500 mg/kgBW was designated as the upper limit in this study to minimize the potential risk of toxicity. Further investigations are necessary to define the long-term safety profile of higher doses of P. oleracea and to establish its no-observed-adverse-effect level. One limitation of this study is the absence of a treatment group receiving Purslane extract alone without MSG exposure, which would have allowed a more precise evaluation of the independent physiological effects of Purslane on reproductive parameters. Future studies should include a Purslane-only group to clarify whether the observed benefits are purely protective against oxidative damage or involve additional mechanisms such as endocrine modulation in healthy conditions. Further research is warranted to elucidate the precise mechanisms underlying Purslane’s protective effects and to explore its therapeutic applications in clinical settings. Harnessing the medicinal properties of P. oleracea could pave the way for novel treatments and preventative strategies for reproductive disorders associated with MSG consumption. ConclusionThis study demonstrates that the ethanolic extract of P. oleracea (Purslane) effectively mitigates MSG-induced oxidative stress, as evidenced by reduced MDA levels and improved FSH concentrations and endometrial thickness in female Wistar rats. Among the tested doses, 250 mg/kgBW showed the most consistent effect on hormonal regulation, whereas 125 mg/kgBW produced superior outcomes in oxidative markers and endometrial recovery, indicating a variable dose-response relationship. These findings highlight Purslane’s potential as a natural antioxidant therapy for reproductive toxicity, while also underscoring the need for further studies with refined dose intervals and mechanistic validation to support its translational applicability. AcknowledgmentThe authors declare no acknowledgment. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThis study was funded by Universitas Airlangga (Grant ID: 9520/UN3.FK/PT.01.03/2023). Authors’ contributionsAstika Gita Ningrum: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review and editing; Endyka Erye Frety: Conceptualization, Methodology, Formal analysis; Ivon Diah Wittiarika: Conceptualization, Methodology, Formal analysis; Khadizah H. Abdul-Mumin: Formal analysis, Writing-original draft, Writing-review and editing; Renata Alya Ulhaq: Writing-review and editing, Data visualization. Data availabilityData are available from the corresponding author upon reasonable request. ReferencesAbdel Moneim, W.M., Yassa, H.A., Makboul, R.A. and Mohamed, N.A. 2018. Monosodium glutamate affects cognitive function in male albino rats. Egypt. J. Forensic Sci. 8, 9; doi:10.1186/s41935-018-0038-x Abdulghani, M.A.M., Alshehade, S.A., Kamran, S. and Alshawsh, M.A. 2022. Effect of monosodium glutamate on serum sex hormones and uterine histology in female rats: molecular docking and in silico toxicity. Heliyon 8, e10967; doi: 10.1016/j.heliyon.2022.e10967 Adgent, M.A., Umbach, D.M., Zemel, B.S., Kelly, A., Schall, J.I., Ford, E.G., James, K., Darge, K., Botelho, J.C., Vesper, H.W., Chandler, D.W., Nakamoto, J.M., Rogan, W.J. and Stallings, V.A. 2018. A longitudinal study of estrogen-responsive tissues and hormone concentrations in infants fed soy formula. J. Clin. Endocrinol. Metab. 103, 1899–1909; doi:10.1210/jc.2017-02249 Adjuvant, C.F., Azizah, R.N., Asmaliani, I. and Putra, B. 2018. Antirheumatoid artritis effect of Purslane herb extract (Portulaca oleracea L.) to rat (Rattus norvegicus) induced by complete freuds adjuvant. J. Glob. Pharma. Technol. 11(04), 314–319. Ahangarpour, A., Lamoochi, Z., Fathi Moghaddam, H. and Mansouri, S.M.T. 2016. Effects of Portulaca oleracea ethanolic extract on reproductive system of aging female mice. Int. J. Reprod. Biomed. 14, 205–212. Albrahim, T. and Binobead, M.A. 2018. Roles of Moringa oleifera leaf extract in improving the impact of high dietary intake of monosodium glutamate-induced liver toxicity, oxidative stress, genotoxicity, DNA damage, and PCNA alterations in male rats. Oxid. Med. Cell. Longev. 2018, 4501097; doi:10.1155/2018/4501097 Allred, C.D., Allred, K.F., Ju, Y.H., Virant, S.M. and Helferich, W.G. 2001. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 61, 5045–5050. Badan Penelitian dan Pengembangan Kesehatan. 2020. Laporan Nasional Riskesdas, 2018. Jakarta, Indonesia: Kementerian Kesehatan RI. Darvish Damavandi, R., Shidfar, F., Najafi, M., Janani, L., Masoodi, M., Akbari-Fakhrabadi, M. and Dehnad, A. 2021. Effect of Portulaca oleracea (purslane) extract on liver enzymes, lipid profile, and glycemic status in nonalcoholic fatty liver disease: a randomized, double-blind clinical trial. Phytother. Res. 35, 3145–3156; doi:10.1002/ptr.6972 Desta, M., Molla, A. and Yusuf Z. 2020. Characterization of the physicochemical properties and antioxidant activity of oil obtained from the seed, leaf, and stem of purslane (Portulaca oleracea L.). Biotechnol. Rep. 27, e00512; doi:10.1016/j.btre.2020.e00512 Doerge, D.R. and Sheehan, D.M. 2002. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 110(Suppl 3), 349–353; doi: 10.1289/ehp.02110s3349 Eweka, A. and Om’iniabohs, F. 2011. Histological studies of the effects of monosodium glutamate on the ovaries of adult wistar rats. Ann. Med. Health Sci. Res. 1(1), 37–43. Fatmaningrum, W. and Ningtyas, W.S. 2019. Mung bean sprout extract suppresses monosodium glutamate (MSG) effect on the reproductive hormones (FSH and Estrogen) in female wistar rats. Maj. Obstet. Ginekol. 27, 24–27; doi:10.20473/mog. V27I12019.24-27 Fernandes, G.S.A., Arena, A.C., Campos, K.E., Volpato, G.T., Anselmo-Franci, J.A., Damasceno, D.C. and Kempinas, W.G. 2012. Glutamate-induced obesity leads to decreased sperm reserves and acceleration of transit time in the epididymis of adult male rats. Reprod. Biol. Endocrinol. 10, 105; doi:10.1186/1477-7827-10-105 França, L.R., Suescun, M.O., Miranda, J.R., Giovambattista, A., Perello, M., Spinedi, E. and Calandra, R.S. 2006. Structure and function of testis in a nongenetic hyperadipose rat model at prepubertal and adult ages. Endocrinology 147, 1556–1563; doi: 10.1210/en.2005-0640 He, K., Du, S., Xun, P., Sharma, S., Wang, H., Zhai, F. and Popkin, B. 2011. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 93, 1328–1336; doi:10.3945/ajcn.110.008870 Hermanussen, M., García, A.P., Sunder, M., Voigt, M., Salazar, V. and Tresguerres, J.A.F. 2006. Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. Eur. J. Clin. Nutr. 60, 25–31; doi:10.1038/sj.ejcn.1602263 Hoesada, I., Nasihun, T. and Isradji, I. 2016. Effect of propolis extract on MDA levels (Malondialdehyde) and sperm quality on epididimis (Experimental Study on Wistar Strain Male Rats Exposed to Kretek Cigarettes). Sains Med. 7, 09–14. Jefferson, W.N., Padilla-Banks, E., Phelps, J.Y., Cantor, A.M. and Williams, C.J. 2012. Neonatal phytoestrogen exposure alters oviduct mucosal immune response to pregnancy and affects preimplantation embryo development in the mouse. Biol. Reprod. 87, 1–10,10; doi:10.1095/biolreprod.112.099846 Khaled, F.A., Al, O., Yousef, M.I., Kamel, K.I., Fayrouz, C. and Khaled, A. 2016. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Int. J. Chem. Stud. 4, 04–09. Messina, M. and Redmond, G. 2006. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid® 16, 249–258; doi: 10.1089/thy.2006.16.249 Mondal, M., Sarkar, K., Nath, P.P. and Paul, G. 2018. Monosodium glutamate suppresses the female reproductive function by impairing the functions of ovary and uterus in rat. Environ. Toxicol. 33, 198–208; doi:10.1002/tox.22508 Musa, K.Y., Ahmed, A., Ibrahim, G., Ojonugwa, O.E., Bisalla, M., Musa, H. and Danmalam, U.H. 2007. Toxicity studies on the methanolic extract of Portulaca oleracea L (Fam. Portulacaceae). J. Biol. Sci. 7, 1293–1295. Ningrum, A.G., Frety, E.E., Diah, I., Shabran, Z.H., Setiani, R.E. and Dewi, E.R. 2021a. Antioxidant activity of purslane (Portulaca oleracea L.) leaf extract on the levels of ovarian oxidative stress and reproductive hormone in Rattus norvegicus exposed to cigarette smoke. Open Access Maced. J. Med. Sci. 9, 1535–1540; doi:10.3889/oamjms.2021.7081 Ningrum, A.G., Ratnawati, R. and Andarini, S. 2021b. Effect of sweet potato anthocyanin (Ipomoea batatas L.) on levels of follicle stimulating hormone and folliculogenesis in Rattus norvegicus exposed to cigarette smoke. Indian J. Forensic Med. Toxicol. 15, 1820–1826; doi:10.37506/ijfmt.v15i1.13674 Nurcholis, W., Putera Irsal, R.A., Rosyidah, R.A., Agung Kurnia, M.R. and Aisyah, S.I. 2023. Potensi Senyawa Antioksidan Dari Tanaman Krokot (Portulaca grandiflora): narrative review. J. Farmamedika (Pharm J) 8, 25–35; doi:10.47219/ath.v8i1.192 Park, S. R., Kim, S.-R., Kim, S.-K., Park, J.-R. and Hong, I.-S. 2022. A novel role of follicle-stimulating hormone (FSH) in various regeneration-related functions of endometrial stem cells. Exp. Mol. Med. 54, 1524–1535; doi:10.1038/s12276-022-00858-1 Patisaul, H.B. and Jefferson, W. 2010. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 31, 400–419; doi:10.1016/j.yfrne.2010.03.003 Rueda, C.B., Llorente-Folch, I., Traba, J., Amigo, I., Gonzalez-Sanchez, P., Contreras, L., Juaristi, I., Martinez-Valero, P., Pardo, B., Del Arco, A. and Satrustegui, J. 2016. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim. Biophys. Acta 1857, 1158–1166; doi:10.1016/j.bbabio.2016.04.003 Skibska, B., Kochan, E., Stanczak, A., Lipert, A. and Skibska, A. 2023. Antioxidant and anti-inflammatory effects of α-lipoic acid on lipopolysaccharide-induced oxidative stress in rat kidney. Arch. Immunol. Ther. Exp. (Warsz) 71, 16; doi:10.1007/s00005-023-00682-z U.S. Food and Drug Administration. 2021. Questions and answers on monosodium glutamate (MSG). Silver Spring, MD: U.S. Food Drug Administration. Available via https://www.fda.gov WHO. 2022. Evaluation of certain food additives and contaminants: 88th Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 1036th ed. Geneva, Switzerland: World Health Organization. Zhou, Y.X., Xin, H.L., Rahman, K., Wang, S.J., Peng, C. and Zhang, H. 2015. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. Biomed. Res. Int. 2015, 925631; doi:10.1155/2015/925631 | ||
| How to Cite this Article |
| Pubmed Style Ningrum AG, Frety EE, Wittiarika ID, Abdul-mumin KH, Ulhaq RA. Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Vet. J.. 2025; 15(8): 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 Web Style Ningrum AG, Frety EE, Wittiarika ID, Abdul-mumin KH, Ulhaq RA. Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. https://www.openveterinaryjournal.com/?mno=237865 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.28 AMA (American Medical Association) Style Ningrum AG, Frety EE, Wittiarika ID, Abdul-mumin KH, Ulhaq RA. Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Vet. J.. 2025; 15(8): 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 Vancouver/ICMJE Style Ningrum AG, Frety EE, Wittiarika ID, Abdul-mumin KH, Ulhaq RA. Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 Harvard Style Ningrum, A. G., Frety, . E. E., Wittiarika, . I. D., Abdul-mumin, . K. H. & Ulhaq, . R. A. (2025) Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Vet. J., 15 (8), 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 Turabian Style Ningrum, Astika Gita, Endyka Erye Frety, Ivon Diah Wittiarika, Khadizah H. Abdul-mumin, and Renata Alya Ulhaq. 2025. Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Veterinary Journal, 15 (8), 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 Chicago Style Ningrum, Astika Gita, Endyka Erye Frety, Ivon Diah Wittiarika, Khadizah H. Abdul-mumin, and Renata Alya Ulhaq. "Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate." Open Veterinary Journal 15 (2025), 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 MLA (The Modern Language Association) Style Ningrum, Astika Gita, Endyka Erye Frety, Ivon Diah Wittiarika, Khadizah H. Abdul-mumin, and Renata Alya Ulhaq. "Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate." Open Veterinary Journal 15.8 (2025), 3645-3654. Print. doi:10.5455/OVJ.2025.v15.i8.28 APA (American Psychological Association) Style Ningrum, A. G., Frety, . E. E., Wittiarika, . I. D., Abdul-mumin, . K. H. & Ulhaq, . R. A. (2025) Protective role of ethanol extract of purslane leaves (Portulaca oleracea L.) on serum malondialdehyde levels, follicle-stimulating hormone levels, and endometrial thickness in female rats exposed to monosodium glutamate. Open Veterinary Journal, 15 (8), 3645-3654. doi:10.5455/OVJ.2025.v15.i8.28 |