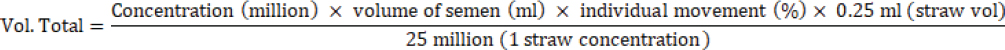| Research Article | ||
Open Vet. J.. 2025; 15(4): 1747-1756 Open Veterinary Journal, (2025), Vol. 15(4): 1747-1756 Research Article Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 yearsAhmad Budi Purnawan1, Rimayanti Rimayanti2*, Suherni Susilowati2, Erma Safitri2, Tatik Hernawati2, Imam Mustofa2, Zulfi Nur Amrina Rosyada3, Adeyinka Oye Akintunde4 and Aswin Rafif Khairullah51Singosari National Artificial Insemination Center, Malang, Indonesia 2Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Division of Veterinary Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 5Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Rimayanti Rimayanti. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: rimayanti [at] fkh.unair.ac.id Submitted: 14/01/2025 Accepted: 04/03/2025 Published: XX/XX/2025 © 2025 Open Veterinary Journal
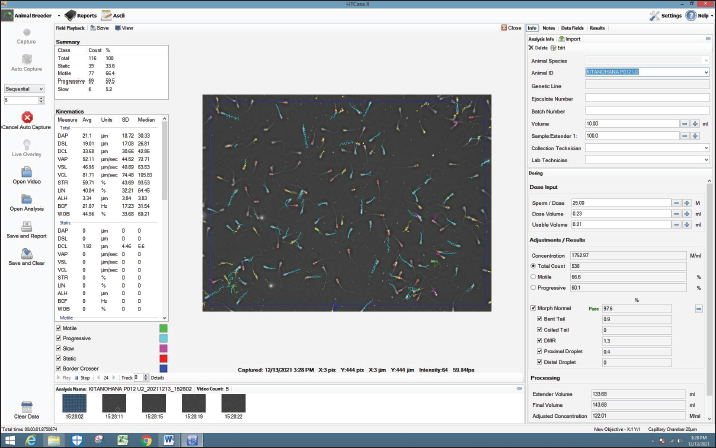
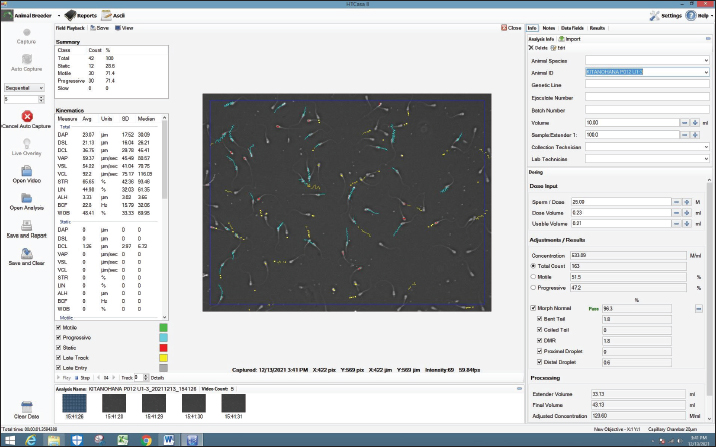
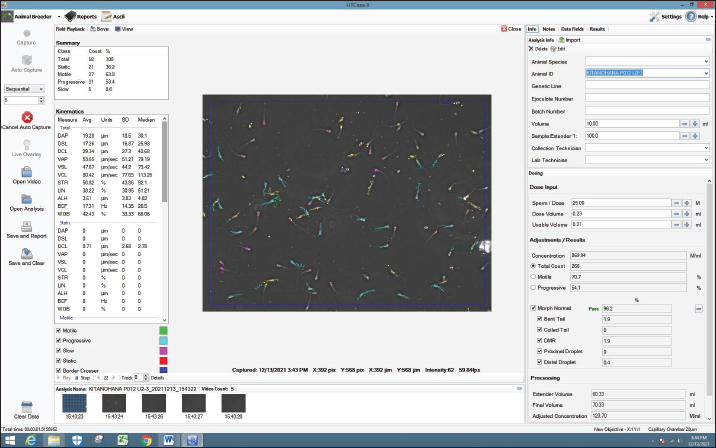
AbstractBackground: Fertility is determined by sperm motility, kinematics, and morphology. Motility is the most commonly used metric worldwide, aside from movement score and semen concentration, and is one of the semen quality indicators included in the Indonesian national standard (SNI 4869.1-2017) for frozen semen quality. Aim: This study aimed to determine the effect of frozen semen storage for 33, 30, 27, and 24 years on the sperm kinematics and morphology of frozen-thawed semen in FH bulls. Methods: Samples were collected from the frozen semen of FH bulls using five straws for each storage period. Evaluation of frozen-thawed sperm motility, kinematics, and morphology was performed using a computer-assisted sperm analysis IVOS® II sperm analyzer. Each sample was measured in five fields, observation included motility (%), progressivity (%), concentration (million spermatozoa/ml), kinematics parameters consisting of the amplitude of lateral head displacement (μm), curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), linearityLIN=VSL/VCL, %, wobbleWOB=VAP/VCL, %, and straightnessSTR=VSL/VAP, %. Results: Based on statistical observation, the results of the research were not significantly different (p > 0.05) among storage years for sperm concentration and motility, kinematics, static, and sperm morphological of FH bull frozen-thawed semen stored for 24, 27, 30, and 33 years. Moreover, sperm motility was >40%, meeting Indonesian frozen bovine semen standards. Conclusion: FH bull frozen-thawed semen of 33, 30, 27, and 24 years did not differ in sperm concentration, motility, kinematics, and sperm morphology and fulfilled the standards for artificial insemination. Keywords: FH bull, Food security, Frozen semen storage, Sperm kinematics, Sperm morphology. IntroductionTo ensure food and nutritional security, one project that needs to be implemented is extending the reach of artificial insemination (AI) programs to increase the number of livestock. AI is also a reproductive technology that has shown efficacy and broad-field application (Singh and Balhara, 2016). One of the success factors of the AI program is the quality of the cryopreserved semen. The quality of frozen semen can be affected by its shelf life, which is not determined by standard operational procedures (Purnawan et al., 2023). Moreover, according to Nagata et al. (2019), frozen high-quality semen suitable for AI is preserved in liquid nitrogen containers. The frozen semen should be preserved until use. Maintaining the quality of frozen semen requires proper storage and management. Improper handling might decrease spermatozoa quality (Nagata et al., 2019). Furthermore, when spermatozoa are stored for a long time and with poor management, oxidative stress can occur, leading to malondialdehyde production in seminal plasma (Prastika et al., 2018). Toxic lipid peroxide compounds can be generated more easily during oxidative stress. These compounds can lower fertility by damaging sperm membranes and DNA, leading to changes in mitochondrial structure and death (Longobardi et al., 2020). This risk is thought to be due to changes in temperature during storage up to –80°C, which cause crystal formation (recrystallization) in the cell (Bojic et al., 2021). Consequently, when introduced into a female bovine, this will lead to a decline in reproductive capacity. One artificial insemination center (AIC) in East Java, Indonesia. Singosari AIC has played a significant role in the production of cryopreserved semen for improving cattle in Indonesia since 1982. The straw utilized by Singosario to produce frozen semen is older than 20 years old. Studies on the longevity of cryopreserved semen yield different findings. A previous study by Leibo et al. (1994) discovered that frozen bovine semen maintained regular sperm motility even after storage for 37 years. Additionally, Malik et al. (2015) found that the viability and motility of thawed semen decreased significantly after 6 years of storage in liquid nitrogen compared to storage for 1 to 2 years. Furthermore, frozen semen maintained for an extended period decreases spermatozoa membrane integrity, mitochondrial function, and fertility while increasing the non-return rate in FH bull spermatozoa (Malik et al., 2015) and pigs (Fraser et al., 2014). In contrast, Ramírez et al. (2016) found no variations in the motility and viability of bovine spermatozoa preserved in liquid nitrogen at 196°C for varying lengths of time. Several studies have found no variations in the DNA integrity and fertility of human spermatozoa stored for various periods (Huang et al., 2019). Fertility is determined by sperm motility, kinematics, and morphology (Yãniz et al., 2018). Motility is the most commonly used metric worldwide, aside from movement score and semen concentration, and is one of the semen quality indicators included in the Indonesian national standard (SNI 4869.1-2017) for frozen semen quality (Rosyada et al., 2021; Bahmid et al., 2023). The study employed computer-assisted sperm analysis (CASA) to evaluate sperm motility in frozen semen after prolonged storage. Compared with conventional semen examination methods, CASA can predict fertility more accurately (Murphy et al., 2018; Tanga et al., 2021). Thus, using a CASA, this study aimed to determine the impact of frozen semen storage for 33, 30, 27, and 24 years on FH bull sperm characteristics, such as motility, kinematics, and morphology. Materials and MethodsResearch designThe research was conducted at the Animal Disease and Diagnostic Laboratory of the Faculty of Veterinary Medicine, Brawijaya University Malang, and the Laboratory of the Singosari AIC. The semen cryopreservation methods and extenders used are the same and are regulated by standard operating procedures that are strictly supervised by the Directorate General of Animal Husbandry and Animal Health, Ministry of Agriculture, Republic of Indonesia. This study used frozen semen samples from a Frisian Holstein bull (study code: 38619). This study used only one bull (Kitanohana) to avoid individual variability. Kitanohana bulls were imported from Japan on March 14, 1987, and frozen semen was produced from June 1987 until the end of 1996. Frozen semen was processed using skim milk extender compositions as follows: skim milk powder (10 g), fructose (1 ml), citric acid (1.70 g), penicillin (1,000 IU), streptomycin sulfate (0.1 g/ml), glycerol (88 ml), aquatides (100 ml), and mini straw (0.25 ml) (Hedah and Ma’sum, 1996). The samples were frozen semen with a shelf life of 33 years (1987 production), 30 years (1990 production), 27 years (1993 production), and 24 years (1996 production). Semen collection for frozen semen production is carried out twice a week (every Tuesday and Friday); therefore, in 1 year, there are 104 collections. The frozen semen was stored at the Sperm Bank of the Singosari AIC, Malang, East Java, Indonesia. The number of straw samples used in this study was five from each storage length. Motility was examined by thawing frozen semen in warm water at 37°C for 30 seconds. Semen that has been liquid is placed in a microtube and dripped on 20 μl slides. The examination of sperm motility, kinematics, and morphology were examined using a CASA IVOS® II sperm analyzer (IVOS II, Hamilton Thorne Inc., USA), as shown in Figures 1–4, for the CASA evaluation of sperm kinematics and morphology with each storage period of 24, 27, 30, and 33, respectively. Egg yolk skim diluterFirst, the final volume of extender A was 1,000 ml. The materials used according to the measurements were 100 g of skim milk, 50 ml of egg yolk, 2 g of antibiotics, and 7.5 g of fructose. Then 100 g of skim milk powder is put into a beaker boiled at 100°C for 10 minutes. The milk is cooled until it reaches 37°C. The skim milk solution was filtered using a layered gauze, 50 ml of egg yolk was added, and the mixture was stirred until homogeneous. The skim milk and egg yolk solutions were filtered using a layered gauze. Next, 2 g of antibiotics and 7.5 gg of fructose were added, and stirred until homogeneous. Skim milk, egg yolk, antibiotic, and fructose solutions were filtered using a layered gauze. Extender A is a mixture of skim milk, egg yolk, antibiotic, and fructose. The volume of extender B is half that of extender A (500 ml. Extender B consists of 435 ml of extender A, 65 ml of glycerol, and 10 g of glucose. Extender A was mixed with glycerol and stirred until homogeneous. Then, extender A and glycerol solutions are mixed with glucose and stirred until homogeneous. Extender B is a mixture of extender A, glycerol, and glucose. Fresh semen should be examined macroscopically and microscopically. After the examination, the cement is added with an extender A as much as the volume of fresh semen and this is called the addition of extender A1. The following formula is used to calculate the total extender requirement:
Note: Total diluter=Total volume volume of fresh semen Diluter B=Total Volume: 2 Diluter B=Diluter A1 + Diluter A2 + fresh semen.
Fig. 1. Screenshot on CASA for FH bull straw frozen semen 24 years storage.
Fig. 2. Screenshot on CASA of FH bull straw frozen semen 27 years storage.
Fig. 3. Screenshot on CASA of FH bull straw frozen semen 30 years storage.
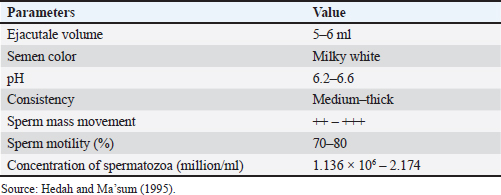
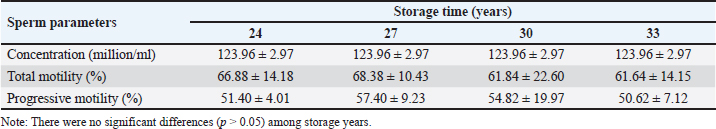
Fig. 4. Screenshot on CASA of FH bull straw frozen semen 33 years storage. Frozen semen manufacturingSemen storage using an artificial vagina. Macroscopic and microscopic examinations to assess sperm quality. Addition of diluters A1, A2, and B according to the microscopic examination results. The addition of diluters A1 and A2 was performed at room temperature (37°C). The addition of diluter B was performed in a cooltop at 4°C. The container is stored for 12 hours in a cooltop or chiller. The next day, the prefreezing examination process is carried out to ensure progressive spermatozoa motility is >70%. After the prefreezing examination is carried out and meets the requirements, the next step is the straw printing process to label the straw. The label on the straw contains information about the Indonesian National Standard for frozen bull semen, producer, batch code, stud name, stud code, breed, and type of diluter. The semen and diluter solutions are inserted into the straw using a filling sealer. The freezing process was performed using a freezer. The straw was inserted into liquid nitrogen to store frozen semen. Samples need to be taken for the postthaw motility examination process (PTM). A PTM that meets the requirements is at least 40% individual movement. Frozen semen that meets the requirements is stored in individual containers. Refilling should be performed periodically with liquid nitrogen to maintain the quality of frozen semen. Evaluation of sperm motility, kinematics, and morphologyThe semen post-thawed kinematics were evaluated using CASA (IVOS II, Hamilton Thorne Inc., USA) and equipped with a 10× negative phase contrast objective (Zeiss 10x NH IVOS-II 160 nm). The straw samples were thawed at 37°C for 30 seconds, emptied using a piston, diluted at a ratio of 1:4 (v/v) in pre-warmed Easy Buffer B (IMV Technologies, L’Aigle, France), and then incubated for 10 m at 37°C. Furthermore, a 3 μl of diluted semen was loaded into a prewarmed analysis chamber with a depth of 20 μm (Leja, Nieuw-Vennep, The Netherlands). Each sample was measured in five fields, observation included for motility (%), progressivity (%), concentration (million spermatozoa/ml), morphological abnormalities (coiled tail, bent tail, proximal and distal droplet, and distal mid-piece reflex (DMR) (%), kinematics parameters consisting of the amplitude of lateral head displacement (ALH, μm), curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), linearity (LIN=VSL/VCL, %), wobble (WOB=VAP/VCL, %), and straightness (STR=VSL/VAP, %) (O’Meara et al., 2022). Data analysisThe differences between each variable of the four storage duration treatments were tested using a one-way analysis of variance. The data were analyzed using Duncan’s test to identify significant differences, and the results are shown as means ± SEM. Ethical approvalNot needed for this study. ResultsSperm motility is an important determinant of semen quality and is essential for AI (Mustofa et al., 2021). For bovine ejaculates to be eligible for freezing, their sperm motility must be at least 70%. Frozen thawed semen must meet specific criteria for postthawing motility, with a minimum spermatozoa motility of 40% (INSA, 2024). Hedah and Ma’sum (1995) reported that the percentage of sperm motility of Kitanohana FH bull fresh semen was 70%–80% (Table 1); this value meets the freezing standard. As a result, the Kitanohana FH bulls used in this study are qualified for frozen semen production. Different storage times of frozen-thawed FH sperm motilityThe effects of liquid nitrogen storage for 24, 27, 30, and 33 years on the concentration and motility of frozen-thawed FH bull spermatozoa are presented in Table 2. The concentration among storage years remained the same, whereas the total motility of frozen sperm preserved for varying durations varied from 61.64% to 68.38%. Sperm preserved for 27 years had the highest progressive motility value at 57.40% ± 9.23%. Sperm preserved for 30 and 24 years were 54.02% and 51.40%, respectively. Table 2 also shows that sperm with a storage period of 33 years showed the lowest motility, observing just 61.64% ± 14.15%. The total and progressive motility did not differ significantly (p > 0.05) across all storage periods. Table 1. Fresh semen quality of Kitanohana FH bulls produced between 1987 and 1996.
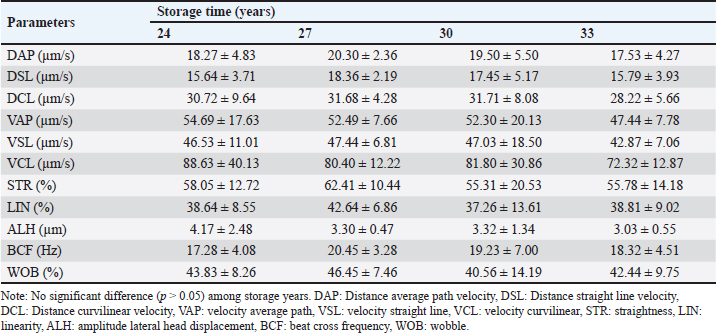
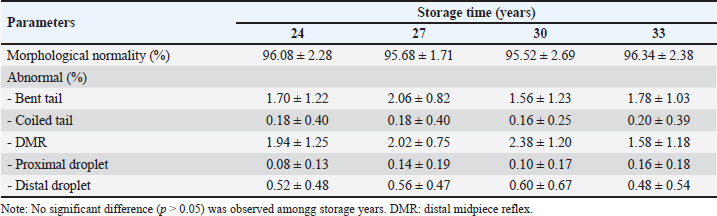
Kinematic characteristics of frozen-thawed FH bull sperm at different storage timesThe effects of storing frozen-thawed FH bull sperm for different lengths of time were examined on sperm kinematics, such as the distance traveled by spermatozoa at distance curvilinear velocity (DCL), distance average path velocity (DAP), and distance straight line velocity (DSL), and on the ability of sperm to fertilize eggs based on velocity characteristics (VAP, VCL, and VSL). Data in Table 3 show that the velocity characteristics and the variance among the different storage times of 24, 27, 30, and 33 years had velocity characteristics (VAP, VCL, and VSL) that were not statistically significant (p > 0.05). The results align with DAP, DCL, and DSL, and sperm movements in STR, LIN, ALH, BCF, and WOB did not show a significant difference (p > 0.05) among the different storage. Morphological characteristics of frozen-thawed FH bull sperm at different storage timesThis study investigated the impact of storage times for frozen-thawed semen in FH bulls at 24, 27, 30, and 33 years on spermatozoa morphology, including the overall proportion of normal spermatozoa and several types of defects (Table 4). The total normal morphological spermatozoa of frozen-thawed FH bull sperm at 24, 27, 30, and 33 years of storage in this study ranged from 95.52% to 96.34%. In this study, only several major tail abnormalities, such as twisted tails, coiled tails, DMR, and proximal and distal droplet-like defects, were detected using CASA IVOS® II (Perry, 2021). However, there were no significant differences among storage times for both normal and abnormal proportions of morphological sperm. DiscussionThe results of this research, which referenced Hedah and Ma’sum (1995) regarding the quality of fresh sperm, indicated that the average volume of fresh ejaculate varied between 5 and 6 ml. The results presented here align with the findings of Malik et al. (2015), which indicated that FH bull ejaculate volumes ranged from 5.8 to 6.2 ml. Furthermore, sperm motility in ejaculates met the minimum requirement of 70% (INSA, 2024). Additionally, the findings of this research, which cited Hedah and Ma’sum (1995), indicated that Kitanohana FH bulls produced from 1987 to 1996 contained higher concentrations of sperm than those discovered in prior investigations by Hayasi and Ishobe (2005) and Malik et al. (2015), which reported a sperm concentration of 455,106/ml in fresh ejaculate from Frisian Holstein bull spermatozoa. The fresh semen samples obtained from the Kitanohana FH bulls utilized in this study were normal and appropriate for cryopreservation, as determined by Hedah and Ma’sum’s (1995). Table 2. Sperm motility of frozen-thawed FH bull sperm stored for 24, 27, 30, and 33 years.
Table 3. Kinematics of frozen-thawed FH bull sperm stored for 24, 27, 30, and 33 years.
Table 4. Morphological analysis of frozen-thawed FH bull sperm stored for 24, 27, 30, and 33 years.
Different storage times of frozen thawed FH sperm motilityFrozen human and animal semen is commonly stored in liquid nitrogen. This study examined sperm kinematics and morphology 24, 27, 30, and 33 years after storage in liquid nitrogen. Sperm motility is crucial for assessing their ability to fertilize eggs (Nagy et al., 2015). The total motility of Kitanohana FH bull sperm, which had been cryopreserved for 24, 27, 30, and 33 years, was decreased compared with that of fresh sperm. However, the total sperm motility of the Kitanohana FH bull achieved the standards set for frozen semen (SNI 4869-1:2017), with a minimum post-thawed motility of 40% (INSA, 2024). In comparison with the findings of Rosyada et al. (2020) and Bahmid et al. (2023), the frozen-thawed Kitanohana FH bull showed higher percentages of total sperm motility. The aforementioned studies reported that the total motility percentage of FH bulls ranged from 43% to 45% and 47% (after being stored for 25 years), respectively. Regarding frozen-thawed FH bull sperm progressive motility (Table 2), this study of Kitanohana frozen-thawed semen stored from 24 to 33 years found a higher value for progressive motility than a recent study by Bahmid et al. (2023), which found that semen stored for 25 years only had progressive motility of 40.7%. Frozen-thawed sperm quality depends on the use of better equipment and skilled technicians; the freezing process is more efficient with the use of the same extender. Thus, frozen semen quality has likely improved in the past year. Nevertheless, the individual bull is unique and determines it as well (Indriastuti et al., 2020). Sperm are frozen at –196°C, a temperature much below zero. At this temperature, biological activity ceases (Getreu and Fuller, 2019). Sperm do not deplete energy reserves and remain in a state of inactivity with no metabolic activity. Biologists believe that properly frozen cells in long-term storage can remain indefinitely if the temperature stabilizes. As a result, it is possible to conclude that the AIC properly maintained frozen semen. Kinematic characteristics of frozen-thawed FH bull sperm at different storage timesIn this study, several kinematic parameters were determined, including sperm movement, DAP, DSL, and DCL, as well as velocity, namely VCL, VSL, and VAP (μm/s), and dimensionless motility indices, such as STR, LIN, WOB, ALH (μm), and beat cross frequency (BCF) (Hz). There were no significant differences between the storage times analyzed. This study’s VCL value is <100 μm/s, lower than the ideal value of >100 μm/s suggested by Oliveira et al. (2013). However, according to Agustinus and Pakpahan (2020), VCL is merely the velocity of sperm in its trajectories and indicates the strength of their movements. Additionally, VCL indicates the strength of sperm motility, not its direction and course. The most commonly observed sperm motions are VAP, VSL, and VCL. Velocity and LIN play crucial roles in sperm function. Fertilization potential is also linked to velocity. The VAP value can be used to predict the fertilization capability of frozen bull sperm (Nagy et al., 2015), whereas the VSL describes fundamental sperm function properties. According to the study findings, the kinematic properties of FH bull semen frozen for 24–33 years did not differ significantly. Each sample showed a similar freezing ability, and the quality of the semen remained steady until thawing. This also implies that long storage times did not impact sperm kinematics and should have good freezability. A previous study reported that the STR indicates swimming, whereas the LIN indicates progressive motility (Oliveira et al., 2013). According to Oliveira et al. (2013), sperm migrate linearly when LIN >35% and STR >50%. This study showed that preserved FH bull semen does not affect the percentage of STR or LIN. In frozen semen after 24, 27, 30, and 33 years, 55.78% to 62.41% and 37.26% to 42.64% of sperm STR and LIN met the requirements, respectively. A high percentage of LIN and STR assessments indicated progressive-swimming sperm, whereas a low LIN level indicated hyperactivity. The origin of sperm, individual differences, and temperature affect hyperactivity (Nagata et al., 2019). STR and LIN differ in frozen-thawed FH bull sperm stored for 24, 27, 30, and 33 years, presumably due to management influences. The maximum sperm count per second is WOB (Oliveira et al., 2013). ALH and BCF affect sperm wave patterns (Ratnawati et al., 2017). Similar to STR and LIN, our study found no significant differences in ALH or BCF samples preserved for 24, 27, 30, and 33 years and those that met the standard values. Research indicates that frozen semen with ALH concentrations <5 μm is not hyperactive during storage (Oliveira et al., 2013; Raafi et al., 2021). These results align with Rosyada et al. (2021), who found an ALH value of 2–5 μm in Madura bulls. According to Agustus and Pakpahan (2020), device setting, standard sperm trajectory, and CASA type alter ALH values. BCF predicts in vivo fertilization and sperm strength (Oliveira et al., 2013). The BCF ranged from 17.28 to 20.45 Hz. Our findings were consistent with Bahmid et al. (2023), who found a BCF range of 10.34–23.12 Hz in the FH breed. Morphological characteristics of frozen-thawed FH bull sperm at different storage timesRegarding sperm morphology, our results showed that frozen-thawed FH bull sperm morphology was unaffected by storage time (p > 0.05). In addition, normal spermatozoa tails are required for migration to reach the ovum. Morphological examination of the spermatozoa shows proximal and distal midpiece droplets. Barth and Oko (1989) and Chenoweth (2005) found a genetic association between proximal and distal droplet midpiece thickness. Bull spermiograms often show reflexes at the midpiece of the distal spermatozoa. Blom (1973) classified this anomaly as modest and has a low impact on fertility. In this study, bent-tail spermatozoa were detected by folding or bending the tail. A coiled tail was observed during the examination. Barth and Oko (1989) reported that a coiled tail does not indicate fertility. Chenoweth (2005) discovered a connection between the elevated prevalence of coiled tails and several nongenetic causes. Cytoplasmic droplets, a 2–3 μm spherical mass in the tail, are normally found in a small percentage of ejaculated spermatozoa. Sperms shed cytoplasmic droplets normally following ejaculation. Concerning tail abnormalities identified by CASA IVOS II, this research also revealed minor proportions of aberrant midpieces (including proximal and distal droplets) and bent, coiled, and contorted tails; no significant variation was observed across different storage durations. Officially, AI centers can only keep frozen semen with >40% motility. This study showed excellent sperm kinematics, morphology, and optimal motility throughout storage. Results indicate that the national AIC employs well-managed bulls, applicable freezing methods, and container system management. Frozen semen preserved for 33 years exhibits suitability for AI. To discover what changes might occur due to long-term storage, it also needs to look into other assessments like mitochondrial metabolism, reactive oxygen species levels, DNA fragmentation, and chromatin integrity. In vitro fecundation and field trials are needed to confirm the effects of long-term sperm storage at –196°C on fertility, embryo viability, and the calving rate. ConclusionOur study found no significant changes in crucial sperm kinematic and morphological parameters across storage durations of 24, 27, 30, and 33 years. Long-term storage at –196°C has little effect on the kinematics and morphology of bovine sperm and is still eligible for AI. AcknowledgmentsThe authors thank Dr. Kreso Suharto, DVM., MP for managerial support, Dikky Eka Mandala Putranto, DMV, M.Sc, and Yudit Oktanella, DVM., M.Sc. for technical support. Conflict of interestThe authors declare no conflict of interest. FundingThis study was funded by the authors. Author’s contributionsABP and RR compiled the ideas and designed the framework research. ZNAR and SS acquisition and analysis of data. ES, AOA, and TH conceived the manuscript. IM and ARK critically read and revised the manuscript for intellectual content. All authors have read and approved the final manuscript. Data availabilityAll data are available in the manuscript. ReferencesAgustinus, A. and Pakpahan, C. 2020. Computer assisted sperm analysis: a review. Indones. Androl. Biomed. J. 1(2), 60–66. Bahmid, N.A., Karja, N.W.K. and Arifiantini, R.I. 2023. The quality of frozen Friesian Holstein semen after long-term storage. Trop. Anim. Sci. J. 46(1), 13–19. Barth, A.D. and Oko, R. 1989. Abnormal morphology of bovine spermatozoa. Ames, IA: Iowa State University Press. Blom, E. 1973. Ultrastrukturen af nogle karakteristiske sperimedefekter og forslag til etnyt klassificerings-system for tyrens sperimiogram. Nord. Vet. Med. 25(7), 383–391. Bojic, S., Murray, A., Bentley, B.L., Spindler, R., Pawlik, P., Cordeiro, J.L., Bauer, R. and de Magalhães, J.P. 2021. Winter is coming: the future of cryopreservation. BMC Biol. 19(1), 56. Chenoweth, P.J. 2005. Genetic sperm defects. Theriogenology 64(3), 457–468. Fraser, L., Strzeek, J. and Kordan, W. 2014. Post-thaw sperm characteristics following long-term storage of boar semen in liquid nitrogen. Anim. Reprod. Sci. 147(3–4), 119–127. Getreu, N. and Fuller, B.J. 2019. Stopping the biological clock merging biology and cryogenics in applied cryobiology. IOP Conf. Ser. Mater. Sci. Eng. 502(1), 012003. Hayasi, I. and Isobe, N. 2005. Characteristics of cryopreserved spermatozoa from a holstein-friesian bull thawed at different temperatures. J. Int. Dev. Coop. 12(1), 107–110. Hedah, D. and Ma’sum, K. 1995. Prosiding Pertemuan Teknis Workshop Evaluasi Uji Zuriat Sapi Perah di Indonesia. Balai Inseminasi Buatan Singosari Malang. Jakarta, Indonesia: Direktorat Jenderal Peternakan Departemen Pertanian. Hedah, D. and Ma’sum, K. 1995. Prosiding Pertemuan Teknis Workshop Evaluasi Uji Zuriat Sapi Perah di Indonesia. Balai Inseminasi Buatan Singosari Malang. Direktorat Jenderal Peternakan Departemen Pertanian. Hedah, D. and Ma’sum, K. 1996. Prosiding Pertemuan Teknis Evaluasi Rekording Sapi Perah untuk Produksi Calon Pejantan Unggul Lokal. Jakarta, Indonesia: Balai Inseminasi Buatan Singosari Malang Direktorat Jenderal Peternakan Departemen Pertanian. Huang, C., Lei, L., Wu, H.L., Gan, R.X., Yuan, X.B., Fan, L.Q. and Zhu, W.B. 2019. Long-term cryostorage of semen in a human sperm bank does not affect clinical outcomes. Fertil. Steril. 112(4), 663–669.e1. Indriastuti, R., Ulum, M.F., Arifiantini, R.I. and Purwantara, B. 2020. Individual variation in fresh and frozen semen of Bali bulls (Bos sondaicus). Vet. World 13(5), 840–846. INSA. 2024. Frozen semen”part 1: bull. Jakarta, Indonesian: National Standard Agency. Available via https://www.bsn.go.id/uploads/attachment/rsni3_4869-1-2024.pdf (Accessed 10 February 2025) Leibo, S., Semple, M. and Kroetsch, T. 1994. In vitro fertilization of oocytes by 37-year-old cryopreserved bovine spermatozoa. Theriogenology 42(1), 1257–1262. Longobardi, V., Zullo, G., Cotticelli, A., Salzano, A., Albero, G., Navas, L., Rufrano, D., Claps, S. and Neglia, G. 2020. Crocin improves the quality of cryopreserved goat semen in different breeds. Animals 10(6), 1101. Malik, A., Laily, M. and Zakir, M.I. 2015. Effects of long-term storage of semen in liquid nitrogen on the viability, motility, and abnormality of frozen-thawed Frisian Holstein bull spermatozoa. Asian Pac. J. Reprod. 4(1), 22–25. Murphy, E.M., O’Meara, C., Eivers, B., Lonergan, P. and Fair, S. 2018. Comparison of plant- and egg yolk-based semen diluents on in vitro sperm kinematics and in vivo fertility of frozen-thawed bull semen. Anim. Reprod. Sci. 191(1), 70–75. Mustofa, I., Susilowati, S., Wurlina, W., Hernawati, T. and Oktanella, Y. 2021. Green tea extract increases the quality and reduced DNA mutation of post-thawed Kacang buck sperm. Heliyon 7(3), e06372 Nagata, M.B., Egashira, J., Katafuchi, N., Endo, K., Ogata, K., Yamanaka, K., Yamanouchi, T., Matsuda, H., Hashiyada, Y. and Yamashita, K. 2019. Bovine sperm selection procedure prior to cryopreservation for improvement of post-thawed semen quality and fertility. J. Anim. Sci. Biotechnol. 10(1), 91. Nagy, ã, Polichronopoulos, T., Gãspãrdy, A., Solti, L. and Cseh, S. 2015. Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Vet. Hung. 63(3), 370–381. Oliveira, L.Z., de Arruda, R.P., de Andrade, A.F., Celeghini, E.C., Reeb, P.D., Martins, J.P., dos Santos, R.M., Beletti, M.E., Peres, R.F., Monteiro, F.M. and de Lima, V.F.H. 2013. Assessment of in vitro sperm characteristics and their importance in the prediction of conception rate in a bovine timed-AI program. Anim. Reprod. Sci. 137(3–4), 145–155. O’Meara, C., Henrotte, E., Kupisiewicz, K., Latour, C., Broekhuijse, M., Camus, A., Gavin-Plagne, L. and Sellem, E. 2022. The effect of adjusting settings within a computer-assisted sperm analysis (CASA) system on bovine sperm motility and morphology results. Anim. Reprod. 19(1), e20210077. Perry, V.E.A. 2021. The role of sperm morphology standards in the laboratory assessment of bull fertility in Australia. Front. Vet. Sci. 8(1), 672058. Prastika, Z., Susilowati, S., Agustono, B., Safitri, E. and Prastiya, R.A. 2018. Motility and viability of Rambon cattle spermatozoa in Kemiren village Banyuwangi. J. Med. Vet. 1(2), 38–42. Purnawan, A.B., Rimayanti, R., Susilowati, S., Mustofa, I., Hernawati, T. and Safitri, E. 2023. Evaluation of motility, viability, and integrity plasma membranes of frozen semen in Friesian Holstein with storage periods of 33, 30, 27, and 24 years. J. Med. Vet. 6(2), 162–171. Raafi, M., Yusuf, M., Toleng, A.L., Diansyah, A.M., Surahman and Sahiruddin. 2021. Movement patterns of sperms at different bull breeds using computer assisted sperm analysis (CASA). IOP Conf. Ser. Earth Environ. Sci. 788(1), 012137. Ramírez, A.R., Hernãndez, J.L. and Aros, P. 2016. Long-term storing of frozen semen at 196°C does not affect the post-thaw sperm quality of bull semen. Cryopreservation in Eukaryotes. London, UK: IntechOpen Book Series, pp: 91-102. Ratnawati, D., Isnaini, N. and Susilawati, T. 2017. Pemanfaatan CASA dalam observasi motilitas spermatozoa semen cair sapi madura dalam pengencer berbeda. Indones. J. Ilmu-Ilmu Peternak. 27(1), 80–95. Rosyada, Z.N.A., Tumbelaka, L.I.T.A., Ulum, M.F., Solihin, D.D., Kaiin, E.M., Gunawan, M., Harsi, T., Suharto, K. and Purwantara, B. 2021. Meta data analysis of conception rate in relation to sperm motility in Madura superior bulls. IOP Conf. Ser. Earth Environ. Sci. 902(1), 012048. Rosyada, Z.N.A., Ulum, M.F., Tumbelaka, L.I.T.A. and Purwantara, B. 2020. Sperm protein markers for Holstein bull fertility at National Artificial Insemination Centers in Indonesia. Vet. World 13(5), 947–955. Singh, I. and Balhara, A.K. 2016. New approaches in buffalo artificial insemination programs with special reference to India. Theriogenology 86(1), 194–199. Tanga, B.M., Qamar, A.Y., Raza, S., Bang, S., Fang, X., Yoon, K. and Cho, J. 2021. Semen evaluation: methodological advancements in sperm quality-specific fertility assessment”a review. Anim. Biosci. 34(8), 1253–1270. Yãniz, J.L., Silvestre, M.A., Santolaria, P. and Soler, C. 2018. CASA-Mot in mammals: an update. Reprod. Fertil. Dev. 30(6), 799–809. | ||
| How to Cite this Article |
| Pubmed Style Purnawan AB, Rimayanti R, Susilowati S, Safitri E, Hernawati T, Mustofa I, Rosyada ZNA, Akintunde AO, Khairullah AR. Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Vet. J.. 2025; 15(4): 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 Web Style Purnawan AB, Rimayanti R, Susilowati S, Safitri E, Hernawati T, Mustofa I, Rosyada ZNA, Akintunde AO, Khairullah AR. Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. https://www.openveterinaryjournal.com/?mno=237765 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.26 AMA (American Medical Association) Style Purnawan AB, Rimayanti R, Susilowati S, Safitri E, Hernawati T, Mustofa I, Rosyada ZNA, Akintunde AO, Khairullah AR. Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Vet. J.. 2025; 15(4): 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 Vancouver/ICMJE Style Purnawan AB, Rimayanti R, Susilowati S, Safitri E, Hernawati T, Mustofa I, Rosyada ZNA, Akintunde AO, Khairullah AR. Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 Harvard Style Purnawan, A. B., Rimayanti, . R., Susilowati, . S., Safitri, . E., Hernawati, . T., Mustofa, . I., Rosyada, . Z. N. A., Akintunde, . A. O. & Khairullah, . A. R. (2025) Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Vet. J., 15 (4), 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 Turabian Style Purnawan, Ahmad Budi, Rimayanti Rimayanti, Suherni Susilowati, Erma Safitri, Tatik Hernawati, Imam Mustofa, Zulfi Nur Amrina Rosyada, Adeyinka Oye Akintunde, and Aswin Rafif Khairullah. 2025. Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Veterinary Journal, 15 (4), 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 Chicago Style Purnawan, Ahmad Budi, Rimayanti Rimayanti, Suherni Susilowati, Erma Safitri, Tatik Hernawati, Imam Mustofa, Zulfi Nur Amrina Rosyada, Adeyinka Oye Akintunde, and Aswin Rafif Khairullah. "Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years." Open Veterinary Journal 15 (2025), 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 MLA (The Modern Language Association) Style Purnawan, Ahmad Budi, Rimayanti Rimayanti, Suherni Susilowati, Erma Safitri, Tatik Hernawati, Imam Mustofa, Zulfi Nur Amrina Rosyada, Adeyinka Oye Akintunde, and Aswin Rafif Khairullah. "Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years." Open Veterinary Journal 15.4 (2025), 1747-1756. Print. doi:10.5455/OVJ.2025.v15.i4.26 APA (American Psychological Association) Style Purnawan, A. B., Rimayanti, . R., Susilowati, . S., Safitri, . E., Hernawati, . T., Mustofa, . I., Rosyada, . Z. N. A., Akintunde, . A. O. & Khairullah, . A. R. (2025) Kinematic and morphological evaluation of frozen Friesian Holstein semen stored for 33, 30, 27, and 24 years. Open Veterinary Journal, 15 (4), 1747-1756. doi:10.5455/OVJ.2025.v15.i4.26 |