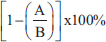| Research Article | ||
Open Vet. J.. 2025; 15(4): 1734-1746 Open Veterinary Journal, (2025), Vol. 15(4): 1734-1746 Research Article Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycolDewa Ketut Meles1, Imam Mustofa2*, Wurlina Wurlina1, Zulfi Nur Amrina Rosyada3, Aswin Rafif Khairullah4, Kadek Rachmawati1, Astri Nur Hidayah5, Niluh Suwasanti6, Rheza Imawan Mustofa7, Adeyinka Oye Akintunde8, Riza Zainuddin Ahmad4, Fitrine Ekawasti4, Katty Hendriana Priscilia Riwu9 and Audrey Gracelia Riwu101Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 5Profession Program of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Department of Clinical Pathology, Universitas Katolik Widya Mandala Surabaya, Surabaya, Indonesia 7Physician in Nayaka Hostpital, Surabaya, Indonesia 8Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 9Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 10Faculty of Medicine and Veterinary Medicine, Universitas Nusa Cendana, Kupang, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 12/01/2025 Accepted: 22/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
AbstractBackground: Nephrolithiasis is a kidney disorder caused by the formation of crystals through physiological and chemical processes in the body, with calcium oxalate being a major contributor. Moringa oleifera leaves are known for their antioxidant, diuretic, and anti-inflammatory properties and contain alkaloids, flavonoids, polyphenols, polysaccharides, saponins, phenolics, and tannins. Aim: This study aimed to investigate the potential of M. oleifera leaf extract (MLE) as a calcium oxalate crystal inhibitor, its effect on reducing malondialdehyde (MDA) levels, enhancing superoxide dismutase (SOD) activity, and histopathological changes in kidneys. Methods: Fifty male Rattus norvegicus aged 12 weeks and weighing 202.43 ± 30.39 g were divided into five groups of 10. The groups included: normal control (C) group without treatment, the untreated ethylene glycol (EG)-induced group (T0), and the EG-induced groups treated with MLE at doses of 200 mg/kgbw (T1), 316 mg/kgbw (T2), and 500 mg/kgbw (T3) at 4-hour intervals after EG induction. EG and MLE were administered orally using a gastric tube for 21 days. The parameters observed included SOD and MDA activity, calcium oxalate crystals, and kidney histopathology. Results: MLE inhibited calcium oxalate crystal formation, reduced MDA levels, increased SOD activity, and prevented tubular epithelial cell degeneration, epithelial necrosis, and interstitial inflammation in the kidneys. Conclusion: MLE demonstrated calcium oxalate crystal dissolution activity, antioxidant effects by reducing MDA content, enhanced SOD activity, and improved kidney conditions, thereby contributing to good health and well-being. Keywords: Antioxidant, Degeneration, Good health and well-being, Necrosis, Urinary crystals. IntroductionEthylene glycol (EG) is a colorless, sweet-tasting toxic alcohol compound commonly found in household items, antifreeze agents, and automotive and industrial solvents (Song et al., 2017). Accidental or intentional ingestion of EG poses severe health risks due to its toxicity, and prompt and appropriate medical intervention. The kidneys are particularly vulnerable to EG toxicity because it promotes the formation of calcium oxalate crystals, a major factor contributing to kidney failure (Koda et al., 2017). Moreover, the presence of calcium oxalate crystals generates free radicals, leading to the formation of reactive oxygen species (ROS), which induce oxidative stress and tissue damage in the kidneys (Khan, 2014). This damage manifests as cellular degeneration, necrosis, and inflammatory cell infiltration. The imbalance between free radicals and antioxidants in the body results in oxidative stress (Chaudhary et al., 2023), characterized by lipid peroxidation that elevates malondialdehyde (MDA) levels and decreases superoxide dismutase (SOD) activity (Pomierny et al., 2014). Innovative preventive measures include inhibiting free radical activity and protecting kidney cells using compounds found in Moringa oleifera L., a novel approach yet to be explored in depth (Akter et al., 2021). Moringa leaves contain active ingredients such as alkaloids, flavonoids, polyphenols, polysaccharides, saponins, phenolics, and tannins, which are known for their antioxidant and diuretic properties (Khalid et al., 2024a; Khalid et al., 2024b). Flavonoids like quercetin reduce free radicals by inhibiting xanthine oxidase and Nicotinamide Adenine Dinucleotide Phosphate oxidase enzymes, enhancing endogenous antioxidant secretion, and scavenging hydrogen atoms from hydroxyl groups, thereby breaking the chain reaction of free radicals and preventing oxidative stress (Kashyap et al., 2022). Additionally, flavonoids act as diuretics (Nayak et al., 2017) by increasing the glomerular filtration rate, facilitating rapid excretion of nephrotoxic substances, and preventing crystal formation. Polyphenols serve as antioxidants and anti-inflammatory agents (Guerreiro et al., 2022), whereas polysaccharides prevent stone adhesion to cell membranes (Menon et al., 2022). This study aimed to pioneer the investigation into the potential of M. oleifera leaf extract (MLE) in preventing calcium oxalate crystal formation, reducing MDA levels, enhancing SOD levels, and mitigating kidney cell damage, such as degeneration, necrosis, and inflammatory cell infiltration, in EG-induced rats. Materials and MethodsEthical approvalThe Animal Care and Use Committee of Airlangga University, Surabaya, Indonesia (No. 1119/HRECC.FODM/IX/2023). Animal treatment was conducted with minimum pain or discomfort according to the guidelines established by the Institutional Animal Ethics Committee. Chemicals and reagentsEG was purchased from PT. Brataco Chemicals. MDA tissue samples were obtained from NWLSS (USA, Cat. No. NWK-MDA01). SOD tissue activity was determined using a kit [Cayman Chemicals (USA, Cat. No. 706002)]. Carboxymethylcellulose sodium (CMC Na 0.5%) was used as the vehicle for EG. M. oleifera leaf extractionEthanolic extraction of M. oleifera leaves was conducted based on the report of Ngizzah et al. (2023). Phytochemical analysis of M. oleifera leaf extract was conducted using the thinthin layer chromatography method (Shafiq et al., 2024). Doses of EG and MLEKidney toxicity was induced using a single dose of EG at a dose of 265 mg/kg body weight per day, based on previous studies (Ghannoum et al., 2023). The doses of the ethanol extract of M. oleifera leaf were determined based on the method of Ngizzah et al. (2023). Experimental animalsMale Wistar rats (Rattus norvegicus) weighing 202.43 ± 30.39 g and aged 3 months were obtained from Universitas Airlangga, Surabaya, Indonesia for experimental purposes. Rats were housed in plastic cages in a temperature-controlled room (26°C ± 2°C) with a 12-hour light-dark cycle. They had ad libitum access to tap water and standard commercial rat food. Experimental designFifty male Wistar rats were randomly divided into five groups. The control (C) group received distilled water and CMC Na daily at 4-hour intervals (at 07.00 before feeding and at 11.00 am). The T0, T1, T2, and T3 groups received 265 mg/kg BW of EG daily, followed by distilled water (T0), MLE at doses of 200 mg (T1), 316 mg (T2), and 500 mg/kg BW (T3), respectively. EG was dissolved in distilled water and orally administered at a volume of 0.5-ml volume at 07.00 am before being fed. MLE was dissolved in 0.5% CMC Na given 0.5 ml orally at 11.00 am. All treatments were performed via gastric gavage daily for 21 days. On day 22, all rats were euthanized after ketamine anesthesia was administered at a dose of 40 mg/kg BW intraperitoneally. Blood was collected directly from the heart via subcostal laparotomy for testosterone hormone examination. The aorta was severed, and the left kidney was extracted. Kidney samples were placed in pots filled with 10% neutral buffered formalin for histopathological examination. The right kidney tissue was homogenized in 50 mM sodium phosphate buffer on ice (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA). The supernatants were separated by centrifugation at 1,000 g for 20 minutes at 4°C and used for MDA and SOD analysis. The left kidney tissue was placed in pots filled with 10% neutral buffered formalin for subsequent histopathological preparation. MDA levelsMDA levels were measured using the thiobarbituric acid reactive substances method. A total of 100 μl of serum was mixed with 1 ml of 0.9% NaCl and centrifuged at 8,000 rpm for 20 minutes. To this, 550 μl of distilled water and 100 μl of Thiobarbituric Acid was added. After vortexing, 250 μl of HCl was added and vortexed again. Subsequently, 100 μl of Na-Thio was added, vortexed, and centrifuged at 500 rpm for 15 minutes. The resulting supernatant was transferred to a new microtube and heated in a water bath at 100°C for 30 minutes. Absorbance was measured using a UV-1601 spectrophotometer at a wavelength of 535 nm (Fogarasi et al., 2016; Steensen et al., 2020). Measurement of SOD activityA total of 0.06 ml of kidney specimen was reacted with a mixture consisting of 2.70 ml of 50 mM sodium carbonate buffer containing 0.1 mM EDTA (pH 10); 0.06 ml of 10 mM xanthine; 0.03 ml of 0.5% bovine serum albumin; and 0.03 ml of 2.5 mM nitro blue tetrazolium. Xanthine oxidase (0.04 units) was then added, and a solution without specimen was used as the control: PBS containing 11.5 g/l KCl was used in sample preparation. Absorbance was measured after 30 minutes at a wavelength of 550 nm (Weydert and Cullen, 2010). SOD activity (%) was calculated using the following equation:
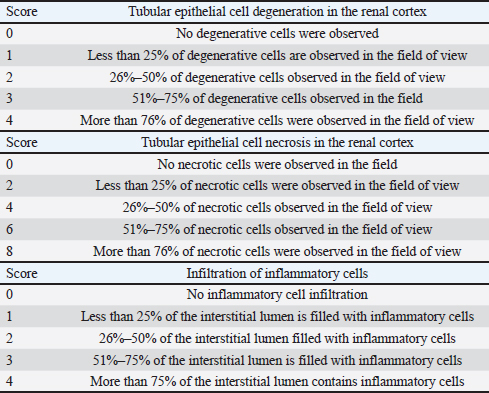
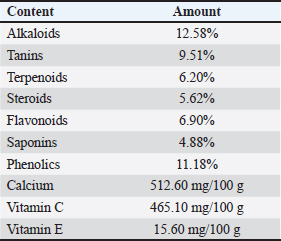
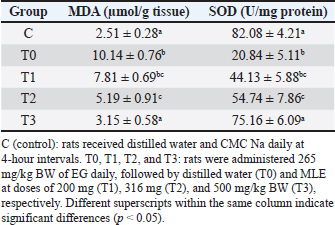
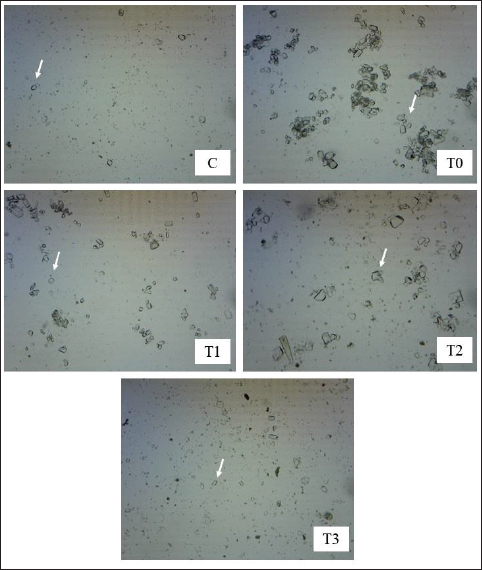
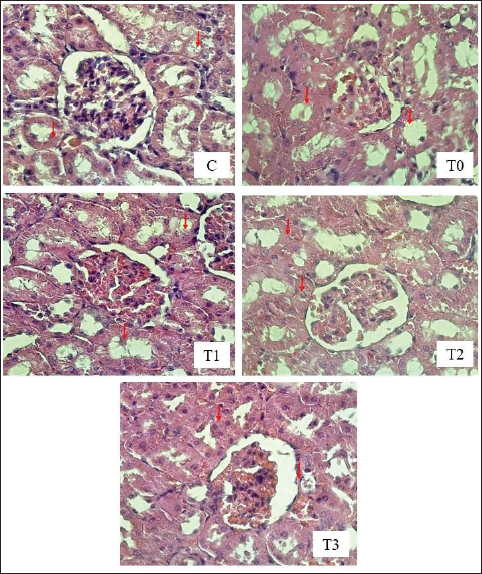
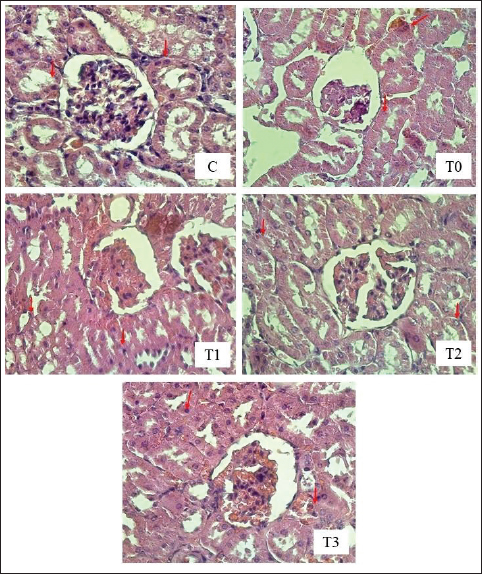
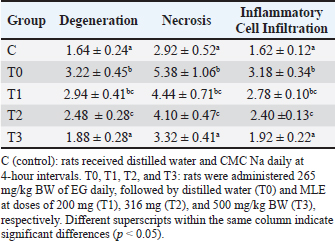
A=Absorbance of the sample solution. B=Absorbance of the control solution. Examination of calcium oxalate crystals in urineUrine was collected on day 21. Crystals were observed in urine using a light microscope. Fresh urine samples were centrifuged for 10 minutes, and the supernatants were separated. The sediment was mixed and used for examination. Urine samples were pipetted onto object glass, covered with a cover glass, and examined under a light microscope at magnifications of 100× and 400× (Yamaguchi et al., 2005; Kaleeswaran et al., 2018). Urine crystals were examined using the native method. The crystals were examined five times in different fields of view, and the average number of crystals found was calculated. The results were categorized based on crystallographic severity levels using the method described by Lee et al. (2022): (–) no crystals, (+) 1–5 crystals, (++) 6–20 crystals, and (+++) >20 crystals per high-power field. Histopathological examination of the kidneyKidney samples were collected and placed in pots containing 10% neutral buffered formalin solution. Histopathological preparations of kidney samples were then made using the Hematoxylin and Eosin staining method. Histopathological variables included tubular epithelial cell degeneration, necrosis, and interstitial infiltration of inflammatory cells in the kidney. Each variable was specifically described from low to high severity using a scoring method modified by Klopfleisch (2013). Tissue damage, which was observed as tissue changes, was recorded and scored based on the average tissue change. The histopathological preparations of kidney samples were examined using a Nikon Eclipse E100® light microscope at 400× magnification. Observations were conducted in five fields of view: upper, lower, right, left, and middle. Scoring of kidney histopathological featuresHistopathological scoring is a structured method for evaluating tissue changes in experimental studies. These scores allow for an objective assessment of the severity of kidney damage, including tubular epithelial cell degeneration, necrosis, and inflammatory cell infiltration. The scoring system ensures consistency and reliability in quantifying histopathological alterations, thereby aiding in the comprehensive analysis of renal tissue responses to experimental conditions. The scoring criteria for kidney histopathological features are presented in Table 1. ResultPhytochemical analysis using thin-layer chromatography showed that MLE contained alkaloids, tannins, terpenoids, steroids, flavonoids, saponins, phenolics, calcium, vitamin C, and vitamin E (Table 2). MDA and SOD levelsRats exposed to EG (T0) showed higher (p < 0.05) MDA and SOD levels in the kidney than control (C) rats. Administration of MLE to rats exposed to EG followed by decreases (p < 0.05) MDA levels and increases SOD levels. A dose of 500 mg/kg BW (T3) did not result in any significant difference (p > 0.05) in MDA and SOD levels between the control rats (C) (Table 3). Calcium oxalate crystals in urineRats exposed to EG (T0) showed a higher (p < 0.05) number of crystal calcium oxalate in the kidneys than control (C) rats. Administration of MLE to rats exposed to EG followed by decreases (p < 0.05) in the number of crystal calcium oxalates. However, compared with the control rats (C), the highest dose of MLE (T3) (500 mg/kg BW) resulted in a larger number of crystals of calcium oxalate (p > 0.05) (Table 4). The most common crystal type identified was calcium oxalate monohydrate (Fig. 1). Degeneration and necrosis of tubular epithelial cells and infiltration of inflammatory cells in the renal interstitiumRats exposed to EG (T0) showed higher (p < 0.05) scores of degeneration (Fig. 2) and necrosis (Fig. 3) as well as inflammatory cell infiltration (Fig. 4) in the kidney than control (C) rats. Administration of MLE on rats exposed to EG followed by decreases (p < 0.05) score of degeneration and necrosis of tubular epithelial cells, as well as the infiltration of inflammatory cells. The dose of 500 mg/kg BW (T3) resulted in no significant difference (p > 0.05) of degeneration and necrosis of tubular epithelial cells, as well as the infiltration of inflammatory cells scores in the control rats (C) (Table 5). The control group (C), which received only 1% CMC Na suspension and aquadest without M. oleifera leaf extract and EG, exhibited the lowest level of inflammatory cell infiltration in the renal interstitium. This finding can be attributed to the inherent factors of the rats, such as stress and uncontrollable variables during the study. The results indicated that the administration of EG at a dose of 265 mg/kg BW in the T0 group resulted in the highest level of inflammatory cell infiltration in the renal interstitium. This increased infiltration was correlated with higher necrosis levels in the T0 group. Table 1. Histopathological lesions of the kidney (Klopfleisch, 2013).
Table 2. Results of the phytochemical analysis of MLE using thinthin layer chromatography method.
Table 3. Levels of MDA and SOD in the kidneys of Wistar Rats exposed to EG with or without treatment with MLE.
Table 4. MDA and SOD levels in the kidneys of Wistar rats exposed to EG with or without treatment with MLE.
Fig. 1. Urinary crystals cCalcium oxalate monohydrate) of rats exposed to EG with or without treatment with MLE. C (control): rats received distilled water and CMC Na daily at 4-hour intervals. T0, T1, T2, and T3: rats were administered 265 mg/kg BW of EG daily, followed by distilled water (T0) and MLE at doses of 200 mg (T1), 316 mg (T2), and 500 mg/kg BW (T3), respectively. The T1 and T2 treatment groups, with doses of 200 and 316 mg/kgBW, respectively, demonstrated a reduction in inflammatory cell infiltration in the renal interstitium, although there was a significant difference (p < 0.05) compared with the T3 group. This significant difference might be due to insufficient flavonoid content, which acts as an anti-inflammatory agent, thus failing to prevent inflammatory cell infiltration caused by increased ROS production and calcium oxalate crystal accumulation due to EG induction. Conversely, the T3 treatment group, which received 500 mg/kgBW of M. oleifera leaf extract, did not differ significantly from the control group. This suggests that the flavonoid content in M. oleifera leaf extract is sufficient to inhibit ROS production, prevent inflammatory cell infiltration, and facilitate optimal cell regeneration. DiscussionMoringa oleifera leaf samples in this study were obtained from Puri Kelorina Village, Ngawenombo, Kunduran, Blora, Central Java, Indonesia, the same as in a study by Nugraha et al. (2023), in which 2,2-diphenyl-2-picrylhydrazyl scavenging activity, nitric oxide scavenging activity, and inhibition of lipid peroxidation indicated that moringa leaves have antioxidant potential. Moringa leaves have been characterized as antioxidants of alkaloids, tannins, terpenoids, steroids, flavonoids, saponins, phenolics, calcium, vitamin C, and vitamin E (Pareek et al., 2023). MDA and SOD levelsWhen diethylene glycol and EG enter the body, these compounds undergo oxidation by enzymes, leading to the formation of glycolaldehyde, which is subsequently oxidized to glycolic acid and eventually forms calcium oxalate (Patel et al., 2020). Calcium oxalate crystallizes into needle-like structures (Teichert et al., 2019). Oxalic acid forms insoluble salts when it encounters calcium, depositing in organs such as the gallbladder and kidneys, where it can lead to kidney stone formation (Chen et al., 2023). The sharp crystals of calcium oxalate can damage the kidneys, with potentially severe consequences in children due to their smaller kidney size (Schott et al., 2022).
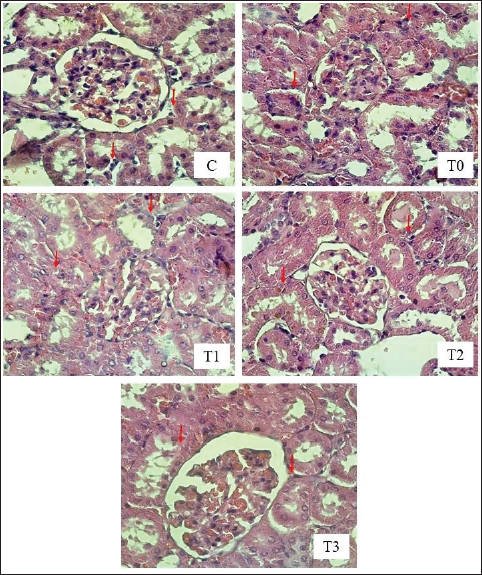
Fig. 2. Tubular epithelial cell degeneration in the kidney of rats exposed to EEG with or without treatment with MLE. HE Staining, under a Light Microscope (Nikon Eclipse E100 LED) at 400× magnification. The red arrows indicate tubular epithelial cell degeneration. C (control): rats received distilled water and CMC Na daily at 4-hour intervals. T0, T1, T2, and T3: rats were administered 265 mg/kg BW of EG daily, followed by distilled water (T0) and MLE at doses of 200 mg (T1), 316 mg (T2), and 500 mg/kg BW (T3), respectively. The influence of EG on other antioxidant enzymes, such as catalase (CAT), glutathione peroxidase (GPx), and SOD, is notable. The GPx enzyme requires selenium for its antioxidant function. EG exhibits high reactivity toward selenium, which can lead to selenium binding and subsequent reduction in GPx enzyme activity (Pomierny et al., 2014; Musalov et al., 2023). Excessive free radicals can overwhelm antioxidant defenses, leading to decreased total antioxidant status and increased lipid peroxidation. MDA is a biomarker of lipid peroxidation caused by excessive free radicals (Al-Assaf et al., 2020). In the group receiving EG without the extract, there was an increase in MDA levels and a decrease in SOD levels. This outcome can be attributed to the EG-induced formation of free radicals in the body, resulting in oxidative stress. Free radicals are highly reactive because of the presence of unpaired electrons in their outer orbitals. EG is known for its high reactivity with cell membranes, potentially compromising their integrity by reacting with unsaturated fatty acids, which are key components of cell membranes (Itagaki et al., 2015). Moreover, EG can alter the chain length of unsaturated fatty acids. Excessive EG intake increases the reaction with unsaturated fatty acids, leading to lipid peroxidation (Urbanovich et al., 2023). This lipid peroxidation results in the production of MDA, thereby increasing lipid peroxidation levels within cells. The entry of EG into the body at certain concentrations can lead to changes in several body molecules, ultimately disrupting bodily functions. One such organ affected is the liver, which plays a crucial role in detoxifying toxic compounds ingested through food or medicine. EG-induced liver damage can induce free radical formation and reduce the activity and production of endogenous antioxidants, resulting in oxidative stress (Cordiano et al., 2023). Oxidative stress occurs when there is an imbalance between ROS and antioxidant defenses, leading to excessive lipid peroxidation. MDA forms via the reaction of free radicals with unsaturated fatty acids in cell membranes. An imbalance in which free radicals outnumber endogenous antioxidants results in oxidative stress (Martemucci et al., 2022). Additionally, increased free radicals can cause imbalances in other molecules. MDA is a biological marker of oxidative stress. EG induction increases free radical production in the body, leading to oxidative stress (Khoubnasabjafari et al., 2015).
Fig. 3. Tubular epithelial cell necrosis in kidney rats exposure to EEG with or without treatment with MLE. HE Staining, under a light microscope (Nikon Eclipse E100 LED) at 400× magnification. The red arrows indicate tubular epithelial cell necrosis. C (control): rats received distilled water and CMC Na daily at 4-hour intervals. T0, T1, T2, and T3: rats were administered 265 mg/kg BW of EG daily, followed by distilled water (T0) and MLE at doses of 200 mg (T1), 316 mg (T2), and 500 mg/kg BW (T3), respectively. In the EG-treated group, decreased SOD levels may occur because EG leads to the formation of ROS such as H2O2, RO–, NO–, ONOO, and OH–. Continuous increases in free radicals can deplete intracellular antioxidant enzyme activity (Collin, 2019). The results indicate that SOD levels in this group were lower compared to the MLE treatment group. Reduced antioxidant levels may result from disrupted endogenous antioxidant synthesis due to EG. Glutathione is the most important endogenous antioxidant capable of capturing free radicals. However, EG exhibited high affinity and reactivity toward the sulfhydryl groups of glutathione. The binding of sulfhydryl groups to EG can render glutathione unable to function as an antioxidant (Santos et al., 2016). The effect of EG on GPx enzymes occurs because of EG’s high reactivity toward selenium, an essential component of GPx enzyme function. Increased EG levels in the body can bind with selenium, thereby reducing selenium levels. The unavailability of selenium in GPx prevents it from functioning as an antioxidant (Xia et al., 2018). EG’s indirect impact on CAT enzyme antioxidant activity occurs because of its interference with heme synthesis. Heme is a component required for CAT enzyme synthesis. EG disrupts heme synthesis (Huang et al., 2003). The administration of MLE aims to increase antioxidant activity to counteract free radicals caused by EG. The antioxidant compounds in MLE synergistically neutralize free radicals, thereby reducing free radicals and lipid peroxidation. MLE contains several antioxidants, including vitamin C, E, and beta-carotene. MLE is known to contain specific antioxidant compounds, such as flavonoids. MLE’s chemical compounds are polar and semipolar. Semipolar chemical compounds have a complex double-bond structure with more hydroxyl groups, making them more potent at neutralizing free radicals. Flavonoids are secondary metabolites produced by plants that function as antioxidants and belong to the phenolic compound group, which is polar and water-soluble. Flavonoids have antioxidant activities as free radical scavengers and can mitigate singlet oxygen (O–) formation. Structurally, flavonoids have more than one phenol (–OH and aromatic) group and conjugated double bonds, which can neutralize free radicals (Hassanpour and Doroudim, 2023). Flavonoids in MLE inhibit lipid peroxidation by donating hydrogen atoms from phenolic hydroxyl groups when reacting with free radicals. Flavonoids in MLE play a role in scavenging and neutralizing free radicals (scavenger) (Poluan et al., 2023). The mechanism by which flavonoids act as antioxidants occurs directly by donating hydrogen ions to neutralize the toxic effects of free radicals. In addition, an indirect mechanism is by increasing the mechanism of endogenous antioxidant gene expression, such as increasing antioxidant gene expression through nuclear factor erythroid 2-related factor 2 (Nrf2). This gene participates in the synthesis of endogenous antioxidant enzymes such as SOD (Hassanpour and Doroudim, 2023). Increased antioxidant levels in the body due to MLE reduce free radical formation and consequently decrease lipid peroxidation and MDA levels. The study results show that administration of MLE in the T3 group significantly reduced MDA levels and increased SOD levels. Based on the study results, MLE effectively increases SOD levels and reduces MDA levels in combating free radicals caused by various pollutants.
Fig. 4. Histopathological image of inflammatory cell infiltration in the renal interstitium of rats exposed to EG with or without treatment with MLE. HE staining under a light microscope (Nikon Eclipse E100 LED) at 400× magnification. The red arrows indicate inflammatory cell infiltration in the renal interstitium. C (control): rats received distilled water and CMC Na daily at 4-hour intervals. T0, T1, T2, and T3: rats were administered 265 mg/kg BW of EG daily, followed by distilled water (T0) and MLE at doses of 200 mg (T1), 316 mg (T2), and 500 mg/kg BW (T3), respectively. Table 5. Scores for tubular epithelial cell degeneration, necrosis, and inflammatory cell infiltration in the renal interstitium of rats exposed to EG with or without treatment with MLE.
Calcium oxalate crystals in urineThe formation of calcium oxalate crystals in urine can be influenced by various factors, leading to either normal or abnormal crystallization, which can cause acute or chronic conditions (Prabhu et al., 2015). Nearly all treatment groups exhibited the same type of crystals, which are inorganic urinary sediments classified as normal crystals influenced by acidity or pH (Brunzel, 2013). Urine supersaturation is a critical process in the initial stages of urolithiasis and is characterized by microcrystal retention. Damaged renal epithelial cells provide an attachment medium for microcrystals to develop into stones (Yamaguchi et al., 2005). Urinary supersaturation can lead to urinary tract obstruction, hydronephritis, infection, and bleeding. Crystals can grow larger and form stones if retained in the urinary tract. Stone formation involves four stages: nucleation, growth, aggregation, and retention (Kaleeswaran et al., 2018). The most common component of urolithiasis is calcium oxalate (CaOx) stones, predominantly in monohydrate or dihydrate forms. Calcium oxalate crystals form due to electrostatic interactions between calcium and oxalate ions at high concentrations in renal tubular fluid. According to Verdesca et al. (2011) and Kaleeswaran et al. (2018), crystal formation can be influenced by pH changes. Calcium oxalate crystals form at pH 5.0–6.5 (Kishore et al., 2013). Previous studies have reported that administering 0.75% EG for 28 days in drinking water increases urinary oxalate concentration and can cause kidney stone formation, particularly CaOx (Afzal et al., 2021). The content of flavonoids, tannins, and saponins in MLE contributes to the dissolution of calcium oxalate. Menon et al. (2022) noted that polyphenols act as antioxidants and antiinflammatory agents, and flavonoids, triterpenes, and tannins can enhance the solubility of CaOx crystal deposits and restore kidney stones. Karadi et al. (2009) reported that plants containing flavonoids, glycosides, polysaccharides, phenols, steroids, and tannins have diuretic activity. Flavonoids, in particular, inhibit calcium oxalate crystallization in human and animal models of urolithiasis (Ali et al., 2021; Menon et al., 2022). According to Kashyap et al. (2022), the flavonoids in MLE that effectively dissolve CaOx are apigenin 7-glucoside and luteolin 7-glucoside. Additionally, saponins can disperse mucoprotein suspensions that promote crystallization, conferring saponin anticrystalline properties (Gãrocak and Kãpeli, 2006). Flavonoid and polyphenol activities as antioxidants prevent calcium oxalate crystal deposition in the kidneys by preventing oxidative damage to renal tubule membranes caused by EG administration. The hydroxyl groups of flavonoids stabilize ROS (Panche et al., 2016). Degeneration and necrosis of tubular epithelial cells and infiltration of inflammatory cells in the renal interstitiumThe highest average values for renal damage, including tubular epithelial cell degeneration, necrosis, and inflammatory cell infiltration, were observed in the T0 group, which received EG at 265 mg/kg BW. This increase in damage is attributed to EG’s ability to elevate ROS levels, leading to oxidative stress and tubular epithelial cell damage (Hovda et al., 2010). The formation of ROS can disrupt mitochondrial function by impairing oxidative phosphorylation, resulting in decreased Adenosine Triphosphate (ATP) levels (Bahadoran et al., 2016). An imbalance between reduced ATP and excessive ROS production can disturb the sodium-potassium pump membrane function. The sodium-potassium pump is crucial for regulating cell volume; however, impaired ATP reduces sodium (Na+) transport out of the cell, causing cytoplasmic concentration and water influx into the cell. Consequently, potassium (K+) and magnesium (Mg2+) move out of the cell, leading to cell degeneration characterized by cloudy, enlarged cells with cytoplasm filled with water vacuoles (Liu et al., 2017). The treatment groups receiving MLE (herbal extract) showed a dose-dependent reduction in tubular epithelial cell degeneration, demonstrating milder microscopic degeneration compared with the group that only received EG. This is likely due to the antioxidant properties of MLE’s secondary metabolites, which prevent fluid retention and decrease capillary permeability. Normalized capillary permeability ensures fluid does not return to the capillaries, thereby preventing cell degeneration (Claesson-Welsh et al., 2021). MLE has a protective effect as an antioxidant because the flavonoid quercetin in MLE can reduce free radicals by preventing and eliminating oxidative damage to target molecules. This is achieved by reducing the levels of free radical-forming enzymes and stimulating internal antioxidant enzymes. Quercetin, a flavonoid, scavenges and sequesters ROS by chelating free radicals through hydrogen atom donation or single electron transfer (Liu et al., 2017). Necrosis is a condition that follows degeneration, in which cells experience severe injury reaching a “point of no return,” making the damage irreversible (Miller and Zachary, 2017). The rats given only 1% CMC Na and distilled water without any MLE or EG administration showed the lowest level of tubular epithelial cell necrosis. This group exhibited almost normal kidney cell conditions, with only a few cells undergoing necrosis. The necrosis observed might have been due to uncontrollable external factors during the study, such as suboptimal health conditions or stress. These factors can lead to ATP depletion and increase intracellular calcium levels, thereby activating proteases, endonucleases, and phospholipases, which cause lipid peroxidation and free radical formation, resulting in permanent cellular damage (Sun et al., 2018). The reduction in tubular epithelial cell necrosis can be attributed to the antioxidants and diuretics in MLE, such as flavonoids. The antioxidant mechanism of flavonoids involves the direct scavenging of ROS and chelating free radicals by donating hydrogen atoms or electron transfers, as well as increasing endogenous antioxidant secretion through the activity of Nrf2 (Ndlovu et al., 2023). Furthermore, MLE administration can dissolve calcium oxalate deposits in the kidneys by forming bonds with the calcium of kidney stones, thereby creating calcium–flavonoid complexes. These complexes are more water-soluble and can be easily excreted through urine (Karadi et al., 2006; Menon et al., 2022). The T0 group exhibited the highest level of cell necrosis compared with the other treatment groups. This is because the T0 group was not administered any M. oleifera leaf extract and was only administered 265 mg/kg BW EG. EG can enhance the activity of oxalate synthesis enzymes like lactate dehydrogenase. High levels of oxalate cause apoptosis and necrosis of renal epithelial cells because of their toxic effects, which can corrode cells (Tsujihata et al., 2006). This condition can also cause crystal deposition, activating angiotensin II, and stimulating the formation of NADPH oxidase, which produces ROS, leading to oxidative stress. Oxidative stress can release proapoptotic factors and cause mitochondrial dysfunction (Deepika et al., 2013). Kidney damage results from elevated calcium oxalate crystal levels, prompting epithelial cells to produce ROS (Wientarsih et al., 2012). The accumulation of calcium oxalate crystals in the kidneys triggers an inflammatory response characterized by increased leukocyte infiltration, especially neutrophils, exceeding normal values as part of the body’s defense response to various stimuli, such as infectious agents, toxic compounds, chemicals, and physical factors (Lovrić et al., 2007). Pro-inflammatory cytokines (TNF-α, IL-1, and IL-18) released by monocytes and macrophages at the inflammation site stimulate the release of other cytokines and activate inflammatory cells. For instance, TNF-α, a potent proinflammatory cytokine, stimulates mononuclear phagocytic cells to produce additional proinflammatory cytokines (IL-1, IL-6, IL-18) (Schindler et al., 2001). The administration of M. oleifera leaf extract (MLE) reduced inflammatory cell infiltration in the renal interstitium, indicating an anti-inflammatory effect likely due to secondary metabolites such as flavonoids. Flavonoids decrease inflammatory stimuli, preventing the release of IKKα from the IKK complex. Consequently, reduced IKKα phosphorylation to IkB inhibits IkB proteasomal degradation and diminishes NF-B activation for nuclear transcription. Inhibition of monocytes also affects the activity of protein tyrosine kinase (PTK) p56, resulting in inactive PTK. Inactive PTK maintains NFBB bound to its inhibitor, preventing the transcription and translation of proinflammatory cytokines. A lack of proinflammatory cytokine formation reduces inflammatory cell activity, preventing ROS release and oxidative stress (Yilmaz et al., 2014). ConclusionThe administration of M. oleifera L. leaf extract has significant potential in mitigating oxidative stress and renal damage induced by EG in rats (R. norvegicus). Our findings indicate that M. oleifera L. leaf extract effectively reduces MDA levels and enhances SOD activity, highlighting its antioxidative properties. Furthermore, the extract dissolves calcium oxalate crystals, which primarily contribute to kidney damage in this model. Additionally, M. oleifera L. leaf extract prevents epithelial tubular cell degeneration, renal epithelial cell necrosis, and inflammatory infiltration in the renal interstitium of EG-induced rats. These protective effects can be attributed to the extract’s antiinflammatory and antioxidative components, particularly flavonoids. M. oleifera L. leaf extract is a promising therapeutic agent for managing and preventing renal pathologies associated with oxidative stress and crystal formation. AcknowledgmentsThe researchers would like to thank the Faculty of Veterinary Medicine, Airlangga University, for all the facilities provided during the research. Conflict of interestThe authors declare no conflict of interest. FundingThe authors acknowledge Universitas Airlangga, which funded this study (contract no. 179/UN3.15/PT/2023. The authors thank Agil Ramadan Ahmad for providing technical support. Author’s contributionsDKM, ARK, WW, AGR, and IM: conceived the idea and manuscript drafting. ZNAR, KR, ANH, and NS: acquisition, analysis, and interpretation of data. RIM, RZA, AOA, KHPR, and FE: critically read and revised the manuscript for intellectual content. All authors have read and approved the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAfzal, M., Kazmi, I., Quazi, A.M., Ahmad, A., Al-Abaasi, F.A., Imam, F., Alharbi, K.S., Alzarea, S.I. and Zafar, A. 2021. 6-Shogaol attenuated ethylene glycol and aluminium chloride induced urolithiasis and renal injuries in rodents. Saudi J. Biol. Sci. 28(6), 3418–3423. Akter, T., Rahman, M.A., Moni, A., Apu, M.A.I., Fariha, A., Hannan, M.A. and Uddin, M.J. 2021. Prospects for protective potential of Moringa oleifera against kidney diseases. Plants (Basel) 10(12), 2818. Al-Assaf, I.N., Al-Dabbagh, A.G.A., Al-Abachi, R.Y.Q. and Al-Bajari, S.A. 2020. Protective role of Urtiea dicica on the pathological alteration induced by ethylene glycol in male rabbits. Sys. Rev. Pharm. 11(12), 1180–1183. Ali, H., Nadeem, A., Anwaar, M. and Jabeen, Q. 2021. Evaluation of antiurolithiatic potential of Moringa oleifera seed extract. Biomedica. Biomed. J. Sci. Tech. Res. 36(5), 28889–28895. Bahadoran, H., Naghii, M.R., Mofid, M., Asadi, M.H., Ahmadi, K. and Sarveazad, A. 2016. Protective effects of boron and vitamin E on ethylene glycol-induced renal crystal calcium deposition in rat. Endocr. Regul. 50(4), 194–206. Brunzel, N.A. 2013. Fundamentals of urine and body fluid analysis, third edition. St. Louis, MO: Elsevier Saunders, pp: 189–191. Chaudhary, P., Janmeda, P., Docea, A.O., Yeskaliyeva, B., Razis, A.F.A., Modu, B., Calina, D. and Sharifi-Rad, J. 2023. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front. Chem. 11(1), 1158198. Chen, T., Qian, B., Zou, J., Luo, P., Zou, J., Li, W., Chen, Q. and Zheng, L. 2023. Oxalate as a potent promoter of kidney stone formation. Front. Med. (Lausanne) 10(1), 1159616. Claesson-Welsh, L., Dejana, E. and McDonald, D.M. 2021. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27(4), 314–331. Collin, F. 2019. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 20(10), 2407. Cordiano, R., Di Gioacchino, M., Mangifesta, R., Panzera, C., Gangemi, S. and Minciullo, P.L. 2023. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules 28(16), 5979. Deepika, A., Sharma, M. and Surindr, K.S. 2013. The role of natural antioxidants as potential therapeutic agent in ephrolithiasis. Asian J. Pharm. Clin. Res. 6(3), 48–53. Fogarasi, E., Croitoru, M.D., Fãlöp, I., Nagy, E.N., Tripon, R.G., Szabo, Z.S. and Muntean, D.L. 2016. Malondialdehyde levels can be measured in serum and saliva by using a fast HPLC method with visible detection. Rev. Romana Med. Lab. 24(3), 319–326. Ghannoum, M., Gosselin, S., Hoffman, R.S., Lavergne, V., Mégarbane, B., Hassanian-Moghaddam, H., Rif, M., Kallab, S., Bird, S., Wood, D.M., Roberts, D.M. and EXTRIP Workgroup. 2023. Extracorporeal treatment for ethylene glycol poisoning: systematic review and recommendations from the EXTRIP workgroup. Crit. Care 27(1), 56. Guerreiro, ã, Ferreira-Pãgo, C., Carregosa, D., Santos, C.N., Menezes, R., Fernandes, A.S. and Costa, J.G. 2022. Polyphenols and their metabolites in renal diseases: an overview. Foods 11(7), 1060. Gãrocak, S. and Kãpeli, B. 2006. Consumption of historical and current phytotherapeutic agents for urolithiasis: a critical review. J. Urol. 176(2), 450–455. Hassanpour, S.H. and Doroudi, A. 2023. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 13(4), 354–376. Hovda, K.E., Guo, C., Austin, R. and McMartin, K.E. 2010. Renal toxicity of ethylene glycol results from internalization of calcium oxalate crystals by proximal tubule cells. Toxicol. Lett. 192(3), 365–372. Huang, Q., Al-Azzam, W., Griebenow, K. and Schweitzer-Stenner, R. 2003. Heme structural perturbation of PEG-modified horseradish peroxidase C in aromatic organic solvents probed by optical absorption and resonance Raman dispersion spectroscopy. Biophys J. 84(5), 3285–3298. Itagaki, T., Arima, Y., Kuwabara, R., Kitamura, N. and Iwata, H. 2015. Interaction between cells and poly(ethylene glycol)-lipid conjugates. Colloids Surf. B Biointerfaces 135(1), 765–773. Kaleeswaran, B., Ramadevi, S., Murugesan, R., Srigopalram, S., Suman, T. and Balasubramanian, T. 2018. Evaluation of anti-urolithiatic potential of ethyl acetate extract of Pedalium murex L. on struvite crystal (kidney stone). J. Tradit. Complement. Med. 9(1), 24–37. Karadi, R.V., Gadge, N.B., Alagawadi, K.R. and Savadi, R.V. 2006. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J. Ethnopharmacol. 105(1-2), 306–311. Karadi, R., Palkar, M.B., Gaviraj, E.N. and Gadge, N.B. 2009. Antiurolithiatic property of Moringa oleifera root bark. Pharm. Biol. 46(12), 861–865. Kashyap, P., Kumar, S., Riar, C.S., Jindal, N., Baniwal, P., Guiné, R.P.F., Correia, P.M.R., Mehra, R. and Kumar, H. 2022. Recent advances in drumstick (Moringa oleifera) leaves bioactive compounds: composition, health benefits, bioaccessibility, and dietary applications. Antioxidants (Basel) 11(2), 402. Khalid, F., Azmat, H., Khan, N. and Saima. 2024a. Ameliorative effects of Moringa oleifera leaf extract against arsenic induced histo-biochemical alterations in Labeo rohita. Ecotoxicol. Environ. Saf. 287(1), 117258. Khalid, F., Azmat, H., Khan, N. and Saima. 2024b. Ameliorative potential of Moringa oleifera leaf extract against arsenic toxicity in Labeo rohita. J. Anim. Plant Sci. 34(3), 614–625. Khan, S.R. 2014. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 3(3), 256–276. Khoubnasabjafari, M., Ansarin, K. and Jouyban, A. 2015. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 5(3), 123–127. Kishore, D.V., Moosavi, F. and Varma, D.R.R.K. 2013. Effect of ethanolic extract of Portulaca oleracea (Linn.) on ethylene glycol and ammonium chloride induced urolithiasis. Int. J. Pharm. Pharm. Sci. 5(2), 134–140. Klopfleisch, R. 2013. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology - a systematic review. BMC Vet. Res. 9(1), 123. Koda, R., Watanabe, H. and Iino, N. 2017. Calcium oxalate monohydrate crystals in ethylene glycol poisoning. Clin. Exp. Nephrol. 21(4), 741–742. Lee, A.J., Yoo, E.H., Bae, Y.C., Jung, S.B. and Jeon, C.H. 2022. Differential identification of urine crystals with morphologic characteristics and solubility test. J. Clin. Lab. Anal. 36(11), e24707. Liu, J., Yan, Y., Nie, Y. and Shapiro, J.I. 2017. Na/K-ATPase signaling and salt sensitivity: the role of oxidative stress. Antioxidants (Basel) 6(1), 18. Lovrić, M., Granić, P., Čubrilo-Turek, M., Lalić, Z. and Sertić, J. 2007. Ethylene glycol poisoning. Forensic Sci. Int. 170(2–3), 213–215. Martemucci, G., Costagliola, C., Mariano, M., D’andrea, L., Napolitano, P. and D’Alessandro, A.G. 2022. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2(2), 48–78. Menon, S., Al-Saadi, A.S., Al-Aamri, N.J., Al-Jaradi, A.H., Al-Mamari, H.K., Al-Haddabi, L.H., Jayachandran, V.P. and Shinisha, C.B. 2022. Inhibition of crystallization of calcium oxalate monohydrate using leaves from different species of Moringa – experimental and theoretical studies. J. Cryst. Growth 598(1), 126859. Miller, M.A. and Zachary, J.F. 2017. Mechanisms and morphology of cellular injury, adaptation, and death. Pathol. Basis Vet. Dis. 2017(1), 2–43.e19. Musalov, M.V., Kapustina, I.S., Spiridonova, E.V., Ozolina, N.V., Amosova, S.V., Borodina, T.N. and Potapov, V.A. 2023. Transannular selenocyclofunctionalization of 1,5-cyclooctadiene: the antioxidant properties of 9-selenabicyclo[3.3.1]nonane derivatives and the discovery of increasing both GPx and GR activities. Inorganics 11(7), 304. Nayak, B.S., Ellaiah, P., Dinda, S.C. and Moharana, B.P. 2017. Diuretic activity of flavonoid compound isolated from Gmelina arborea fruits extract. Eur. J. Pharm. Med. Res. 4(2), 616–622. Ndlovu, S.S., Chuturgoon, A.A. and Ghazi, T. 2023. Moringa oleifera Lam leaf extract stimulates NRF2 and attenuates ARV-induced toxicity in human liver cells (HepG2). Plants (Basel) 12(7), 1541. Ngizzah, N., Wurlina, W., Hastutiek, P., Hestianah, E.P., Mafruchati, M., Hamid, I.S. and Yustinasari, L.R. 2023. Effect of ethanolic extract of Moringa oleifera leaves on the number of spermatogenic cells and Leydig cells of Gentamicin-induced rats. Ovozoa J. Anim. Reprod. 12(2), 99–106. Nugraha, A.P., Triwardhani, A., Sitalaksmi, R.M., Ramadhani, N.F., Luthfi, M., Ulfa, N.M., and Noor, T.N.E.B.T.A. 2023. Phytochemical, antioxidant, and antibacterial activity of Moringa oleifera nanosuspension against peri-implantitis bacteria: an in vitro study. J. Oral Biol. Craniofac. Res. 13(6), 720–726. Panche, A.N., Diwan, A.D. and Chandra, S.R. 2016. Flavonoids: an overview. J. Nutr. Sci. 5(1), e47. Pareek, A., Pant, M., Gupta, M.M., Kashania, P., Ratan, Y., Jain, V., Pareek, A. and Chuturgoon, A.A. 2023. Moringa oleifera: an updated comprehensive review of its pharmacological activities, ethnomedicinal, phytopharmaceutical formulation, clinical, phytochemical, and toxicological aspects. Int. J. Mol. Sci. 24(3), 2098. Patel, R., Mistry, A.M. and Mistry, C.M. 2020. Unintentional ethylene glycol poisoning in an adolescent. Cureus 12(11), e11521. Poluan, J.C., Zubair, M.S., Ramadani, A.P. and Hayati, F. 2023. Narrative review: potential of flavonoids from moringa (Moringa oleifera Lam.) leaves as immunomodulators. Galenika J. Pharm. 9(2), 270–283. Pomierny, B., Krzyanowska, W., Smaga, I., Pomierny-Chamioło,, L., Stankowicz, P. and Budziszewska, B. 2014. Ethylene glycol ethers induce oxidative stress in the rat brain. Neurotox Res. 26(4), 422–429. Prabhu, N., Marzuki, S.M.M., Banthaviz, S.P., Sundhararajan, A., Uma, A. and Saradaz, V. 2015. Prevalence of crystalluria and its association with Escherichia coli urinary tract infections. Int. J. Res. Med. Sci. 3(5), 1085–1090. Santos, M.C., Seabra, A.B., Pelegrino, M.T. and Haddad, P.S. 2016. Synthesis, characterization and cytotoxicity of glutathione- and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl. Surf. Sci. 367(1), 26–35. Schindler, H., Lutz, M.B., Röllinghoff, M. and Bogdan, C. 2001. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J. Immunol. 166(5), 3075–3082. Schott, C., Pourtousi, A. and Connaughton, D.M. 2022. Monogenic causation of pediatric nephrolithiasis. Front. Urol. 2(1), 1075711. Shafiq, N.E., Mahdee, A.F. and Hasan, Z.Y.M. 2024. Leaf extracts of Moringa oleifera cultivated in Baghdad: characterization and antimicrobial potential against endodontic pathogens. Sci. World J. 2024(1), 6658164. Song, C.H., Bae, H.J., Ham, Y.R., Na, K.R., Lee, K.W. and Choi, D.E. 2017. A case of ethylene glycol intoxication with acute renal injury: successful recovery by fomepizole and renal replacement therapy. Electrolyte Blood Press 15(2), 47–51. Steffensen, I.L., Dirven, H., Couderq, S., David, A., D’Cruz, S.C., Fernãndez, M.F., Mustieles, V., Rodríguez-Carrillo, A. and Hofer, T. 2020. Bisphenols and oxidative stress biomarkers-associations found in human studies, evaluation of methods used, and strengths and weaknesses of the biomarkers. Int. J. Environ. Res. Public Health 17(10), 3609. Sun, M.S., Jin, H., Sun, X., Huang, S., Zhang, F.L., Guo, Z.N. and Yang, Y. 2018. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2018(1), 3804979. Teichert, J., Block, T., Pöttgen, R., Doert, T. and Ruck, M. 2019. Tin and lead alkoxides of ethylene glycol and glycerol and their decomposition to oxide materials. Eur. J. Inorg. Chem. 2019(34), 3799–3882. Tsujihata, M., Tsujikawa, K., Tei, N., Yoshimura, K. and Okuyama, A. 2006. Urinary macromolecules and renal tubular cell protection from oxalate injury: comparison of normal subjects and recurrent stone formers. Int. J. Urol. 13(3), 197–201. Urbanovich, O.V., Davydenko, A.I., Sverdlov, R.L., Bekish, A.V., Lastovskii, S.B. and Tochilin, E.V. 2023. Radiation-chemical transformations of ethylene glycol and its deuterated derivative in deaerated aqueous solutions. High Energy Chem. 57(1), 404–409. Verdesca, S., Fogazzi, G.B., Garigali, G., Messa, P. and Daudon, M. 2011. Crystalluria: prevalence, different types of crystals and the role of infrared spectroscopy. Clin. Chem. Lab. Med. 49(3), 515–520. Weydert, C.J. and Cullen, J.J. 2010. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 5(1), 51–66. Wientarsih, I., Madyastuti, R., Prasetyo, B.F. and Aldobrata, A. 2012. Antilithiasis of avocado (Persea americana Mill) leaves extract in white male rats. HAYATI J. Biosci. 19(1), 49–52. Xia, J., Li, T., Lu, C. and Xu, H. 2018. Selenium-containing polymers: perspectives toward diverse applications in both adaptive and biomedical materials. Macromolecules 51(19), 7435–7455. Yamaguchi, S., Wiessner, J.H., Hasegawa, A.T., Hung, L.Y., Mandel, G.S. and Mandel, N.S. 2005. Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int. J. Urol. 12(3), 290–298. Yilmaz-Ersan, L., Ozcan, T., Akpinar-Bayizit, A. and Delikanli, B. 2014. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 5(5), 393–396. | ||
| How to Cite this Article |
| Pubmed Style Meles DK, Mustofa I, Wurlina W, Rosyada ZNA, Khairullah AR, Rachmawati K, Hidayah AN, Suwasanti N, Mustofa RI, Akintunde AO, Ahmad RZ, Ekawasti F, Riwu KHP, Riwu AG. Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Vet. J.. 2025; 15(4): 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 Web Style Meles DK, Mustofa I, Wurlina W, Rosyada ZNA, Khairullah AR, Rachmawati K, Hidayah AN, Suwasanti N, Mustofa RI, Akintunde AO, Ahmad RZ, Ekawasti F, Riwu KHP, Riwu AG. Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. https://www.openveterinaryjournal.com/?mno=237362 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.25 AMA (American Medical Association) Style Meles DK, Mustofa I, Wurlina W, Rosyada ZNA, Khairullah AR, Rachmawati K, Hidayah AN, Suwasanti N, Mustofa RI, Akintunde AO, Ahmad RZ, Ekawasti F, Riwu KHP, Riwu AG. Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Vet. J.. 2025; 15(4): 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 Vancouver/ICMJE Style Meles DK, Mustofa I, Wurlina W, Rosyada ZNA, Khairullah AR, Rachmawati K, Hidayah AN, Suwasanti N, Mustofa RI, Akintunde AO, Ahmad RZ, Ekawasti F, Riwu KHP, Riwu AG. Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 Harvard Style Meles, D. K., Mustofa, . I., Wurlina, . W., Rosyada, . Z. N. A., Khairullah, . A. R., Rachmawati, . K., Hidayah, . A. N., Suwasanti, . N., Mustofa, . R. I., Akintunde, . A. O., Ahmad, . R. Z., Ekawasti, . F., Riwu, . K. H. P. & Riwu, . A. G. (2025) Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Vet. J., 15 (4), 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 Turabian Style Meles, Dewa Ketut, Imam Mustofa, Wurlina Wurlina, Zulfi Nur Amrina Rosyada, Aswin Rafif Khairullah, Kadek Rachmawati, Astri Nur Hidayah, Niluh Suwasanti, Rheza Imawan Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Fitrine Ekawasti, Katty Hendriana Priscilia Riwu, and Audrey Gracelia Riwu. 2025. Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Veterinary Journal, 15 (4), 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 Chicago Style Meles, Dewa Ketut, Imam Mustofa, Wurlina Wurlina, Zulfi Nur Amrina Rosyada, Aswin Rafif Khairullah, Kadek Rachmawati, Astri Nur Hidayah, Niluh Suwasanti, Rheza Imawan Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Fitrine Ekawasti, Katty Hendriana Priscilia Riwu, and Audrey Gracelia Riwu. "Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol." Open Veterinary Journal 15 (2025), 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 MLA (The Modern Language Association) Style Meles, Dewa Ketut, Imam Mustofa, Wurlina Wurlina, Zulfi Nur Amrina Rosyada, Aswin Rafif Khairullah, Kadek Rachmawati, Astri Nur Hidayah, Niluh Suwasanti, Rheza Imawan Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Fitrine Ekawasti, Katty Hendriana Priscilia Riwu, and Audrey Gracelia Riwu. "Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol." Open Veterinary Journal 15.4 (2025), 1734-1746. Print. doi:10.5455/OVJ.2025.v15.i4.25 APA (American Psychological Association) Style Meles, D. K., Mustofa, . I., Wurlina, . W., Rosyada, . Z. N. A., Khairullah, . A. R., Rachmawati, . K., Hidayah, . A. N., Suwasanti, . N., Mustofa, . R. I., Akintunde, . A. O., Ahmad, . R. Z., Ekawasti, . F., Riwu, . K. H. P. & Riwu, . A. G. (2025) Impact of Moringa oleifera leaf extract on renal histopathology, malondialdehyde, superoxide dismutase, and calcium oxalate crystals in rats (Rattus norvegicus) exposed to ethylene glycol. Open Veterinary Journal, 15 (4), 1734-1746. doi:10.5455/OVJ.2025.v15.i4.25 |