| Research Article | ||
Open Vet. J.. 2025; 15(5): 2016-2020 Open Veterinary Journal, (2025), Vol. 15(5): 2016-2020 Research Article First detection of West Nile virus seropositivity in horses in southern IraqHayder Mohammad Al-Rammahi1*, Rahman Kadhum Mohsen1 and Rasha Monther Othman21Department of Internal and Preventive Medicine, College of Veterinary Medicine, Basrah University, Basrah, Iraq 2Department of Microbiology, College of Veterinary Medicine, Basrah University, Basrah, Iraq *Corresponding Author: Hayder Mohammad Al-Rammahi. Department of Internal and Preventive Medicine, College of Veterinary Medicine, Basrah University, Basrah, Iraq. Email: hayderm.alrammahi [at] uokufa.edu.iq Submitted: 07/01/2025 Revised: 15/03/2025 Accepted: 02/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
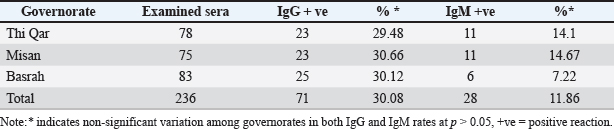
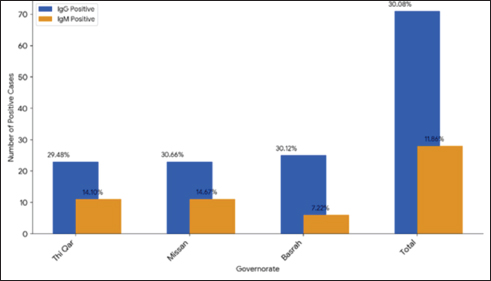
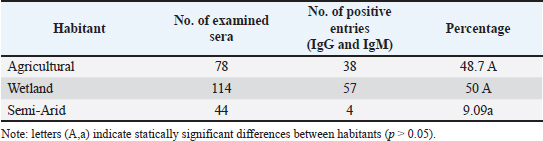
ABSTRACTBackground: West Nile virus (WNV) circulates between birds and mosquitoes, with horses and humans as incidental dead-end hosts. Despite documented cases in neighboring countries and human cases in southern Iraq, no previous studies have investigated WNV in Iraqi horses. Aim: To determine the seroprevalence of WNV in horses from three southern Iraqi governorates (Thi-Qar, Misan, and Basrah) between March and June 2024. Methods: Blood samples were collected from 236 horses regardless of age, sex, or health status. Serum samples were analyzed using commercial competitive enzyme-linked immunosorbent assay kits to detect specific anti-Pr E IgG and IgM antibodies. Results: The overall IgG seropositivity rate was 30.08%, with similar rates reported for Misan (30.66%), Basrah (30.12%), and Thi-Qar (29.48%). IgM seropositivity was 11.86% overall, with 14.67%, 7.22%, and 14.1% in Misan, Basrah, and Thi-Qar, respectively. No statistically significant differences were observed among governorates (p > 0.05). Females had a slightly increased positivity rate (42.7%) when placed alongside males (38%), and with respect to age, the positivity rates for three distinct age categories: 1 to 2 years (no.64), 2 to 5 years (no.135), and above 5 years (no.37) were recorded as 45.31%, 41.48%, and 37.83%, respectively. Conclusion: This is the first study to demonstrate WNV seropositivity in Iraqi horses, revealing active virus circulation in southern Iraq, particularly in marshland areas suitable for mosquito vectors and migratory birds. The findings highlight the need for surveillance programs and preventive measures to control WNV transmission throughout the region. Keywords: West Nile virus, Equine, ELISA, Iraq, Seroprevalence. IntroductionWest Nile fever (WNF) is a viral disease known as an arboviral disease, caused by West Nile virus (WNV), which is a single-stranded RNA virus with a positive sense, and belongs to the flaviviridaees between birds and ornithophily mosquitoes (Eybpoosh et al., 2019); however, humans and horses may become infected as incidental dead-end hosts (Pealer et al., 2003). The illness was first documented in Uganda in 1937. Subsequently, it manifested in the form of localized outbreaks in various regions of Africa, the Middle East, and Asia, resulting in mild symptoms or isolated instances; following the year 1999, the WNV made its way into the western hemisphere, leading to significant outbreaks among humans, horses, birds, and other wildlife in the United States and Europe (Sejvar, 2003). The WNV has been established as an endemic presence in most regions across the world, specifically in Africa, Asia, Australia, Europe, and North America (Nash et al., 2001). Although the WNF has been documented among equines in most neighboring countries to Iraq, including Iran (Paquette et al., 2023), Turkey (Taskin et al., 2023), Jordan (Obaidat et al., 2019), and Saudi Arabia (Alkharsah and Al-Afaleq, 2021), the seropositivity of WNV has only been documented among humans in the southern region of Iraq in 2015 (Barakat et al., 2015). The lack of prior research on WNV in Iraqi animals motivated this study, which aimed to determine the seroprevalence of WNV in horses from three southern Iraqi governorates (Thi-Qar, Misan, and Basrah) between March and June 2024. Materials and MethodsThe study was conducted between March and June 2024 in the southern governorates of Iraq (Thi-Qar, Misan, and Basrah), which cover an area of 49.416 Km2 between 32°N and 46°E. These governorates include the main marshlands of the country with many rural villages (Khalif, 2002); therefore, many farmers raise domestic animals in proximity to their habitations, especially after the obvious effects of climate change (Al-Muhyi and Aleedani, 2022). A total of 236 blood samples were collected from horses regardless of age, sex, and health status, placed in labeled plain tubes, and submitted immediately to the laboratory under cooling conditions. In the laboratory, the sera were separated by centrifugation (200×g for 10 minutes). Each obtained serum sample was divided into two portions; the first was examined with a commercial competitive ELISA kit (ID.vet/France/0012660/WNIGM ver0919EN) to detect IgG, while the rest was examined by IgM Antibody Capture ELISA (MAC) kit designed for the detection of anti-prE IgM antibodies in horse serum and plasma according to guideline outlined by manufacturers for assay procedure and interpretation. All obtained data were statistically analyzed using the SPSS version 12 chi-square test and the t-test. Ethical approvalAll procedures performed in this study were approved by the Ethics Committee of the University of Basrah (reference number: 47-37-2024) and were conducted in accordance with applicable guidelines and regulations for animal research. ResultsTable 1 shows that out of the 236 analyzed samples, 71 were seropositive for IgG, representing a percentage rate of 30.08%. These samples included samples from the Misan, Thi-Qar, and Basrah regions, with positivity rates of 30.66%, 29.48%, and 30.12%. The IgG response rate was 30.8%. According to the specific IgM test, the seropositivity was 11.86%, including 11%, 11%, and 6% in Thi Qar, Misan, and Basrah, respectively (Table 1 and Fig. 1). Table 2 outlines several demographic parameters; the aggregate positivity rate across both genders was 41.9% (99/236). Females had a slightly increased positivity rate (42.7%) when placed alongside males (38%), but this gap was not statistically noteworthy. On the other hand, with respect to age, the positivity rates for three distinct age categories: 1 to 2 years (no.64), 2 to 5 years (no.135), and above 5 years (no.37) were 45.31%, 41.48%, and 37.83%, respectively. According to habitat, the analysis of IgM and IgG seropositivity rates across different habitats revealed distinct statistical groups at p > 0.05. In contrast to the seropositivity rate of semi-arid environments (9.09%), there were no significant differences between wetland (50%) and agricultural environment (48.7%), whereas both environments maintain similarly high seropositivity rates, Table 3. Table 1. IgG and IgM seropositivity in the examined samples.
Fig. 1. Demonstrate the seropositivity of IgG and IgM among the governorates included in the study. Table 2. Distribution of IgG and IgM seropositivity rates according to sex and age.
Table 3. Distribution of IgG and IgM seropositivity rates among the study population.
DiscussionThe present study is the first report of an immune reaction to WNV in the southern region of Iraq. The findings were predictable due to previous disease occurrences in human populations documented in the same area (Barakat et al., 2015), particularly in the study area harboring the most southern wetlands in Iraq. Consequently, the inhabitants serve as common hosts for mosquitoes, alongside a substantial population of migrant and domestic birds suspected to be reservoirs of WNV, in addition to these areas characterized by interconnected marshlands that overlap with endemic regions of Khuzestan province, Iran (Amin et al., 2020). The seropositivity rate documented here aligns with similar rates found in various neighboring countries, such as 31.6% in Turkey (Ozkul et al., 2013) and 24.9% in Jordan (Obaidat et al., 2019), and 27.33% in southern Iran (Amin et al., 2020). Nevertheless, this prevalence is lower than the rate of 70.3% reported in Saudi Arabia (Alkharsah and Al-Afaleq, 2021). Most studies indicate that the synthesis of IgG in horses commences 14 days post-infection and persists for at least 15 months after infection (Alkharsah and Al-Afaleq, 2021). Thus, the present findings suggest the potential stability of the disease in the southern regions of Iraq, where a suitable habitat for the vector (Culex spp.) is present (Thamer and Abdulsamad, 2005; Laftah and Najim, 2023), which is responsible for some viral insect-borne diseases in the area (Shantasinbat and Hasony, 2021). The statement is further supported by documented cases of the disease in southern Iraq among humans during 2015, whereas Barakat et al. (2015) reported seroprevalence rates of IgG and IgM of 9.88% and 0.82%, respectively. In comparison with IgG, IgM antibodies are more specific to WNV and typically become detectable within a period of 6–7 days following the onset of illness and remain present for a duration of up to 90 days or more (Alkharsah and Al-Afaleq, 2021). Thus, according to a specific IgM test, the seropositivity was 11.86%, which may indicate active circulating of WNV between birds and mosquitoes in the study site during the season of insect activity. The absence of statistical significance in the disparities between IgG and IgM rates, as indicated in the findings, may possibly be explained by the timing of the study, which was conducted in the early summer when IgM levels typically surge in response to ongoing active cases, while IgG levels remain relatively steady. The present study revealed significant variation in WNF seropositivity among horses across different habitats in the southern region of Iraq. The high seropositivity rates observed in both agricultural and wetland areas, with no significant difference between them, suggest that these environments provide similarly favorable conditions for WNV transmission. These findings align with studies by Garecia-Bcanerga et al. (2018), who reported comparable seropositivity patterns in horses from irrigated agricultural regions and natural wetlands. These elevated rates could be attributed to several ecological factors common to both habitats, including the presence of standing water bodies that serve as breeding sites for mosquito vectors, abundant vegetation providing resting sites for adult mosquitoes, and the presence of wild birds that act as natural reservoirs for the virus (Tran et al., 2014). The significantly lower seropositivity rate detected in semi-arid regions likely reflects less favorable conditions for virus maintenance and transmission. This finding supports observations by Durand et al. (2017) in Mediterranean regions, where WNV seroprevalence showed a strong correlation with habitat moisture levels. The limited availability of water bodies in semiarid areas restricts mosquito breeding sites and potentially reduces vector populations, as documented by Ciota and Kramer (2013) in similar ecological contexts. ConclusionThis investigation constitutes the initial assessment of the immune response to WNV in equines from the southern region of Iraq. The presence of both IgG and IgM antibodies indicates active WNV circulation in the study area. This study has several limitations that should be considered. The cross-sectional study design limits our ability to infer causality or establish temporal relationships between risk factors and WNV infection. Additionally, single-time-point sampling prevents observation of seasonal variations in infection rates. Our findings have important implications for public health and veterinary practice in Iraq. Given that WNV vaccination is not available in the country, we recommend focusing on vector control strategies, including environmental management of mosquito breeding sites and appropriate chemical control methods. For horses, practical protective measures such as the use of mosquito nets during peak vector activity periods should be implemented. These findings also highlight the need for enhanced clinical surveillance and case reporting systems among veterinary practitioners in Iraq to better monitor and respond to WNV infections in horses. AcknowledgmentsThe authors thank the veterinary clinics and horse owners in southern Iraq for their cooperation in sample collection. The authors also acknowledge the laboratory staff at the College of Veterinary Medicine for their technical assistance in conducting the serological tests. Conflict of interestThe authors declare no conflicts of interest. FundingThe authors receive no specific funding for this research. This study was conducted using the authors’ institutional resources. Author contributionsConceptualization: Hayder M Al-Rammai and Rahman Mohsen. Methodology: Hayder M Al-Rammai, Rahman Mohsen, and Rasha M. Othman. Investigation: Hayder M Al-Rammai, Rasha M. Othman Data collection: Hayder M Al-Rammai and Rasha Al-Othman. Laboratory analysis: Rahman K. Mohsen and Rasha M. Othman. Data analysis: Hayder M Al-Rammai, and Rahman Mohsen. Writing-original draft: Hayder M Al-Rammai Writing-review & editing: Hayder M Al-Rammai, Rahman Mohsen, and Rasha M. Othman. Supervision: Rahman K. Mohsen. Project administration: Hayder M Al-Rammai Data availabilityThe complete dataset and supplementary materials related to this study are available upon request from the corresponding author. ReferencesAlkharsah, K.R. and Al-Afaleq, A.I. 2021. Serological evidence of West Nile virus infection among humans, horses, and pigeons in Saudi Arabia. Infect. Drug Resist. 14, 5595–5601. Al-Muhyi, A.A. and Aleedani, F.Y. 2022. Impacts of global climate change on temperature and precipitin in Basra city, Iraq. Bas. J. Sci. 40(1), 215–230. Amin, M., Zaim, M., Edalat, H., Basseri, H.R., Yaghoobi-Ershadi, M.R., Rezaei, F., Azizi, K., Salehi-Vaziri, M., Ghane, M., Yousefi, S., Dabaghmanesh, S., Kheirandish, S., Najafi, M.E. and Mohammadi, J. 2020. Seroprevalence study of West Nile virus (WNV) infection: a hidden viral disease in Fars Province, Southern Iran. J. Arthropod. Borne Dis. 14(2), 173–184. Barakat, A.M., Olli Vapalahti, M.D. and Hassan, J. 2015. West Nile virus (WNV) in Southern Iraq. Int. J. Sci. Eng. Res. 6(4), 1176–1189. Ciota, A.T. and Kramer, L.D. 2013. Vector-virus interaction and transmission dynamics of West Nile virus. Viruses 5(12), 3021–3047. Durand, B., Tran, A., Balanca, G. and Chevalier, V. 2017. Geographic variations of the bird borne structural risk of West Nile virus circulation in Europe. PLoS One12(10), e0185962. Eybpoosh, S., Fazlalipour, M., Baniasadi, V., Pouriayevali, M.H., Sadeghi, F., Ahmadi Vasmehjani, A., Karbalaie Niya, M.H., Hewson, R. and Salehi-Vaziri, M. 2019. Epidemiology of West Nile virus in the Eastern Mediterranean region: a systematic review. PLoS Negl. Trop. Dis. 13(1), 16–26. Garecia-Bcanerga, I., Belkhiria, J., Napp, S., Cano-Terriza, D., Jimenez-Ruiz, S. and Martínez-López, B. 2018. Epidemiology and spatio-temporal analysis of West Nile virus in horses in Spain between 2010 and 2016. Transbound. Emerg. Dis. 65(2), 567–577. Khalif, E.M. 2002. Marsh dwellers: a historical and demographic study. Karbala Univ. Sci. J. 5(2), 296–307 (in Arabic). Laftah, Z.A. and Najim, S.A. 2023. Diversity of aquatic insect in eastern Al-Hammar marsh, Basra province, Iraq. Marsh Bulletin 1, 10–25. Nash, D., Mostashari, F., Fine, A., Miller, J., O’Leary, D., Murray, K., Huang, A., Rosenberg, A., Greenberg, A., Sherman, M., Wong, S. and Layton, M. 2001. The outbreak of West Nile virus infection in New York City area in 1999. N. Engl. J. Med. 344, 1807–1814. Obaidat, M.M., Stringer, A.P. and Roess, A.A. 2019. Seroprevalence, risk factors and spatial distribution of West Nile virus in Jordan. Trans. R. Soc. Trop. Med. Hyg. 113(1), 24–30. Ozkul, A., Ergunay, K., Koysuren, A., Alkan, F., Arsava, E.M., Tezcan, S., Emekdas, G., Hacioglu, S., Turan, M. and Us, D. 2013. Concurrent occurrence of human and equine West Nile virus infections in Central Anatolia, Turkey: the first evidence for circulation of lineage 1 viruses. Int. J. Infect. Dis. 17, 546–551. Paquette, S.J., Simon, A.Y., Xiii, A., Kobinger, G.P. and Shahhosseini, N. 2023. Medically significant vector-borne viral diseases in Iran. Microorganisms 11(12), 3006. Pealer, L.N., Marfin, A.A., Petersen, L.R., Lanciotti, R.S., Page, P.L., Stramer, S.L., Stobierski, M.G., Signs, K., Newman, B., Kapoor, H., Goodman, J.L., Chamberland, M.E. and West Nile Virus Transmission Investigation Team. 2003. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 349, 1236–1245. Sejvar, J.J. 2003. West Nile virus: an historical overview. Ochsner J. Summer. 5(3), 6–10. Shantasinbat, A.K. and Hasony, H.J. 2021. Seroepidemiology of Zikavirus in Basrah, Southern Iraq. Ann. Rom. Soc. Cell Biol. 25(6), 12546–12553. Taskin, M.H., Tamer, C., Muftuoglu, B., Ozan, E., Kilic, S.S., Akkoyunlu, G.K., Kurucay, H.N., Albayrak, H., Igde, M., Mesquita, J.R., Elhag, A.E., Gumusova, S. and Yazici, Z. 2023. First serological detection of West Nile virus infection among residents living in northern Turkey. J. Vector Borne Dis. 60(1), 101–105. Thamer, N.K. and Abdulsamad, S. 2005. The effect of different NaCl and pH levels on the survival of Culex sp. (Diptera; Culicidae) Larvae in Basrah. J. Basrah Res. Sci. 31(2), 31–36. Tran, A., Sudre, B., Paz, S., Rossi, M., Desbrosse, A., Chevalier, V. and Semenz, J.C. 2014. Environmental predictors of West Nile fever risk in Europe. Int. J. Health Geogr. 13, 26. | ||
| How to Cite this Article |
| Pubmed Style Al-rammahi HM, Mohsen RK, Othman RM. First detection of West Nile virus seropositivity in horses in southern Iraq. Open Vet. J.. 2025; 15(5): 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 Web Style Al-rammahi HM, Mohsen RK, Othman RM. First detection of West Nile virus seropositivity in horses in southern Iraq. https://www.openveterinaryjournal.com/?mno=236506 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.17 AMA (American Medical Association) Style Al-rammahi HM, Mohsen RK, Othman RM. First detection of West Nile virus seropositivity in horses in southern Iraq. Open Vet. J.. 2025; 15(5): 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 Vancouver/ICMJE Style Al-rammahi HM, Mohsen RK, Othman RM. First detection of West Nile virus seropositivity in horses in southern Iraq. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 Harvard Style Al-rammahi, H. M., Mohsen, . R. K. & Othman, . R. M. (2025) First detection of West Nile virus seropositivity in horses in southern Iraq. Open Vet. J., 15 (5), 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 Turabian Style Al-rammahi, Hayder Mohammad, Rahman Kadhum Mohsen, and Rasha Monther Othman. 2025. First detection of West Nile virus seropositivity in horses in southern Iraq. Open Veterinary Journal, 15 (5), 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 Chicago Style Al-rammahi, Hayder Mohammad, Rahman Kadhum Mohsen, and Rasha Monther Othman. "First detection of West Nile virus seropositivity in horses in southern Iraq." Open Veterinary Journal 15 (2025), 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 MLA (The Modern Language Association) Style Al-rammahi, Hayder Mohammad, Rahman Kadhum Mohsen, and Rasha Monther Othman. "First detection of West Nile virus seropositivity in horses in southern Iraq." Open Veterinary Journal 15.5 (2025), 2016-2020. Print. doi:10.5455/OVJ.2025.v15.i5.17 APA (American Psychological Association) Style Al-rammahi, H. M., Mohsen, . R. K. & Othman, . R. M. (2025) First detection of West Nile virus seropositivity in horses in southern Iraq. Open Veterinary Journal, 15 (5), 2016-2020. doi:10.5455/OVJ.2025.v15.i5.17 |