| Research Article | ||
Open Vet. J.. 2025; 15(5): 1982-1989 Open Veterinary Journal, (2025), Vol. 15(5): 1982-1989 Short Communication Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus)Amung Logam Saputro1*, Ragil Angga Prastiya1, Muhammad Thohawi Elziyad Purnama1, Ratih Novita Praja1, Wiwik Misaco Yuniarti2, Salipudin Tasil Maslamama3, Azhar Burhanuddin1, Dilla Chelsea Aziizahrani Santoso1, Evelyn Zaalfa Winni Kusuma1, Jihan Annisa1, Shifa Salsabilla Praja1, Wayan Ari Wijaya1, and Wira Tirta Jaladara11Program Study of Veterinary Medicine, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 2Department of Veterinary Clinic, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Agricultural Biotechnology, Faculty of Agriculture, Eskişehir Osmangazi Üniversitesi, Eskişehir, Turkey *Corresponding Author: Amung Logam Saputro. Veterinary Medicine, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: amunglogamsaputro [at] fkh.unair.ac.id Submitted: 02/01/2025 Revised: 07/04/2025 Accepted: 26/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
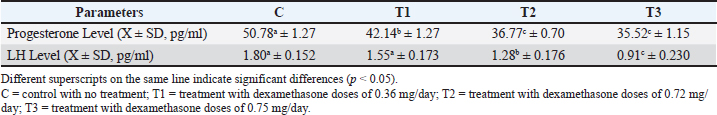
ABSTRACTBackground: Misuse of dexamethasone as a synthetic glucocorticoid anti-inflammation drug is prevalent because of its affordability and therapeutic benefits, despite frequent disregard for proper usage guidelines. Aim: This study investigated the effects of dexamethasone administration during the second trimester of pregnancy, which is the success or failure factor of final stage fetal development on progesterone, luteinizing hormone (LH), and endometrial thickness in Wistar rats. Methods: The study involved 120 pregnant female Wistar rats divided into four treatment groups as follows: C as the control without injection, T1 receiving dexamethasone doses of 0.36 mg/day, T2 receiving dexamethasone doses of 0.72 mg/day, and T3 receiving dexamethasone doses of 0.75 mg/day, which received injection treatment for seven consecutive days (days 8–14 of pregnancy). At the end of the study period, the following parameters were measured: progesterone, LH, and endometrial thickness. Results: Dexamethasone significantly decreased progesterone levels, LH, and endometrial thickness (p < 0.05). Progesterone levels in the control group differed significantly from all treatment groups (T1, T2, and T3). T1 was distinct from the control, T2, and T3 and no significant difference between T2 and T3. The LH levels of the control group were not significantly different from those of T1, but they differed significantly from those of T2 and T3. Similarly, T1 was comparable to the control but significantly different from T2 and T3. The endometrial thickness analysis revealed significant differences across all groups, with the control group consistently differing from T1, T2, and T3. Conclusion: Dexamethasone administration during the second trimester caused adverse hormonal imbalances and disrupted endometrial development in pregnant rats. These findings underscore the risks of glucocorticoid misuse during pregnancy. Proper adherence to medical guidelines is crucial for minimizing the detrimental effects on reproductive function and fetal development. This study emphasizes the importance of monitoring and regulating dexamethasone use, particularly in pregnant individuals, to safeguard maternal and fetal health. Keywords: Dexamethasone, Corticosteroids, Hormone balance, Reproduction, Responsible Consumption and Production. IntroductionThe public often lacks awareness regarding the appropriate usage guidelines, indications, contraindications, and side effects of drugs, with the mistaken assumption that all adverse effects are the therapeutic outcomes of drug use (Apsari, 2022). The widespread and uncontrolled use of dexamethasone is largely due to its affordability, ease of access, and reputation as a “miracle” drug (Faggiano et al., 2022). Dexamethasone is an anti-inflammatory and analgesic drug. This drug is classified as a corticosteroid that contains synthetic glucocorticoids (Indayani, 2015). The administration of dexamethasone is often misused, including its use beyond the recommended indication, dose, and duration of treatment, which can result in harmful adverse effects (Blackwell et al., 2020). The effects of any medication depend on its dose and duration of use. Medicines should always be administered under proper medical supervision and accurate diagnosis. Misuse of dexamethasone, especially with regard to dose and duration, can lead to adverse side effects (Polderman et al., 2018). Long-term use of corticosteroids such as dexamethasone at high doses can negatively affect hormone production and balance (Ramamoorthy and Cidlowski, 2016). Chronic dexamethasone use can alter hormone levels in the reproductive system (Ahmadabad et al., 2016). Progesterone and estrogen are the main reproductive hormones in mammals that are classified as steroid hormones (DeMayo et al., 2002). Progesterone plays an important role in reproduction, particularly in supporting pregnancy. Its main functions during pregnancy include stimulating endometrial thickening to create an optimal environment for embryo implantation, reducing uterine contractions after implantation to prevent premature labor, and supporting fetal development during pregnancy (Chen and Khalil, 2017). Dexamethasone interferes with the secretion of progesterone and estradiol by suppressing the regulation of the anterior pituitary gland follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Wang et al., 2021). LH is a glycoprotein hormone secreted along with FSH by gonadotropin cells in the adenohypophysis (Nedresky and Singh, 2022). Progesterone is important for the onset and maintenance of pregnancy; LH stimulates this hormone. LH also encourages the ovaries to release steroids, such as estrogen and progesterone (Raju et al., 2013). Progesterone and estrogen levels increase as pregnancy progresses (Jee and Sawal, 2024). The corpus luteum is important for maintaining early pregnancy in animals, such as cows, by producing the hormone progesterone (Skovorodin et al., 2020). Estrogen prepares the uterus by stimulating the growth and development of the endometrial lining. The endometrium plays an important role in successful pregnancy by providing a favorable environment for the fetus, facilitating morphological changes, and supplying essential nutrients (Butt et al., 2023). Exposure to glucocorticoids during pregnancy can affect fetal development, leading to abnormal morphological and functional changes in the fetus, potentially causing long-term or permanent damage (Chen et al., 2019). Despite extensive research on the endocrine effects of dexamethasone, its impact on reproductive hormones during pregnancy remains insufficiently explored. Although glucocorticoids disrupt hormonal balance, studies on dexamethasone’s effects on progesterone and estrogen are limited. Understanding these interactions is essential for safer corticosteroid use and better reproductive health. This study examined the influence of estrogen replacement therapy on reproductive hormone dynamics during pregnancy. Materials and MethodsPreparationThe implementation of the research and data collection was performed in the Experimental Animal Laboratory, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi. The research materials consisted of animal cages, 1 cc tuberculin syringes (Onemed®), 3 cc syringes (Terumo®), cotton swabs, 75% alcohol, plain vacuum tubes (Vaculab®), microcentrifuge tubes, sterile cotton swabs (Onemed®), trinocular microscope (Olympus® CX43), objective lens, cover glass, Giemsa staining kit, EBA200 centrifuge (Hettich®), cool box, refrigerator, fan, masks, gloves, and containers for eating and drinking. The total number of rats used as the object of this study was 120 female and four male Wistar-strain white rats, Bernofarm® dexamethasone injection, sawdust, commercial rat food pellets, unlimited drinking water, enzyme-linked immunosorbent assay (ELISA) reagents, physiological saline (NaCl), and formalin. The mating process of the ratsThe Wistar rat must adapt 7 days before mating. The mating process of white rats began by placing male and female rats in separate cages for 1 day. The initial step involved placing three female white rats, which had undergone the estrus phase, in a vaginal plug test. Take a male white rat in a box cage measuring 30 × 35 × 25 cm. The mating process typically occurs during nighttime and extends over 5 days. Research designThe research type was a laboratory-based experimental study utilizing 30 pregnant female Wistar rats for each group to test the impact of dexamethasone injection on days 8 to 14 on progesterone levels, LH levels, and endometrial thickness. In this study, the experimental animals were divided into four groups: C: control without treatment T1: treatment with dexamethasone doses of 0.36 mg/day T2: treatment with dexamethasone doses of 0.72 mg/day T3: treatment with dexamethasone doses of 0.75 mg/day All treatments were administered via intramuscular injection for 7 consecutive days from days 8 to 14 of pregnancy. Necroption and blood sampling were performed on day 15. Hormone sample analysisProgesterone and LH hormone levels were measured using the ELISA method. Blood serum was obtained by drawing blood from the lateral vein of rats using sterile vacuum tubes, and then centrifuged at 3,000 rpm for 15 minutes to separate the serum, which was analyzed with ELISA reagents according to the manufacturer’s protocol. Histopathological examinationEndometrial preparations were prepared using the hematoxylin and eosin (HE) staining method. The uterus from each rat was taken on the 15th day of pregnancy, fixed in 10% formalin solution, and processed through dehydration, clearing, and embedding in paraffin. Tissue sections 5-μm thick were made using a microtome, fixed on a glass slide, and stained with HE. Endometrial length measurement Histopathology of the endometrial uterine n was performed under an Olympus CX43 trinocular microscope at 100× magnification. Observations were made by measuring the length of six points from the shortest to the longest thickness of the endometrium using the Olympus EPView 50 application in Windows. Statistical analysisData were gathered and analyzed using SPSS software (version 20) for Windows with normality for quantitative variables assessed by the Kolmogorov−Smirnov test. All data are normal with one-way ANOVA (p < 0.05) and Duncan post hoc tests to evaluate treatment effects and intergroup differences. Ethical approvalAn ethical permit has been issued under No. (2.KEH.189.09.2024 in 27/9/2023) by the Animal Ethics Commission, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya. ResultsSignificant differences (p < 0.05) in progesterone levels between the control group (C) and the treatment groups (T1, T2, and T3) have been shown through the results of Duncan’s multiple range test, which also show that drug effects can vary based on dose and duration of treatment, as evidenced by the absence of significant differences in progesterone levels between groups T2 and T3. Compared with the control group, LH levels were also significantly decreased in the treatment group, indicating that dexamethasone administration may affect LH synthesis through inhibitory mechanisms in the hypothalamus and anterior pituitary gland. Table 1 presents the mean progesterone levels in pregnant Wistar rats treated with dexamethasone during the second trimester of pregnancy. Table 1 presents the levels of progesterone and LH across different treatment groups. For progesterone levels, the control group (C) exhibited the highest concentration (50.78 ± 1.27 pg/ml), which was significantly higher than all treatment groups (p < 0.05). Among the treatments, T1 (42.14 ± 1.27 pg/ml) had a lower but still significantly different progesterone levels compared with T2 (36.77 ± 0.70 pg/ml) and T3 (35.52 ± 1.15 pg/ml), which did not differ significantly from each other. The control group again showed the highest LH concentration (1.80 ± 0.152 pg/ml), followed by T1 (1.55 ± 0.173 pg/ml), which was not significantly different from the control. However, T2 (1.28 ± 0.176 pg/ml) and T3 (0.91 ± 0.230 pg/ml) exhibited progressively lower LH levels, with significant differences between all groups (p < 0.05). These data indicate a consistent trend of hormonal reduction in response to treatment, with T3 showing the most pronounced decrease in both progesterone and LH levels. Measurement of endometrial thickness showed a significant decrease in the treatment group compared with the control group, with the highest dose (0.75 mg/day) producing the greatest decrease in endometrial thickness. This indicates that dexamethasone may interfere with processes that support the growth and development of the endometrial lining, which is important for successful implantation and fetal maintenance. Table 2 shows that the thickness of the endometrial layer varied significantly among the groups. The control group (C) had the highest endometrial thickness (48.96 ± 0.80 μm), which was significantly greater than that of all treatment groups (p < 0.05). Among the treatments, T1 (20.64 ± 0.96 μm) displayed a thicker endometrium than T2 (16.32 ± 0.85 μm), while T3 (10.73 ± 0.49 μm) exhibited the thinnest endometrium layer. All differences between groups were statistically significant (p < 0.05), demonstrating a clear reduction in endometrial thickness with the treatments, with T3 having the most pronounced effect. DiscussionOverview of the progesterone levelThe findings obtained are the results reported according to Ahmadabad et al. (2016), who reported that the administration of dexamethasone in pregnancy can reduce progesterone and estrogen levels, which play an important role in maintaining pregnancy and immune modulation Shah et al. (2019) also stated that the decrease in progesterone levels can be attributed to the negative impact of dexamethasone on the placenta. Progesterone concentrations increase rapidly between days 1 and 4 of pregnancy, peaking between days 8 and 15. This production depends on placental syncytiotrophoblast cells and human chorionic gonadotropin (hCG) in the mother’s blood (Lee et al., 2015). Administration of dexamethasone via intramuscular injection allows dexamethasone to enter the mother’s bloodstream and cross the placenta, binding to glucocorticoid receptors in the mother and fetus (Shah et al., 2018; Wan et al., 2018). This interaction interferes with placental hCG and progesterone synthesis, thereby contributing to decreased progesterone levels. In addition, dexamethasone exposure can trigger apoptosis and necrosis in placental cells, further reducing progesterone production (Liu et al., 2022). Further analysis revealed a significant decrease in the LH level in the dexamethasone group. Table 1. Mean progesterone and LH levels in pregnant Wistar rats treated with dexamethasone.
Table 2. Mean thickness of the endometrium layer in pregnant Wistar rats treated with dexamethasone.
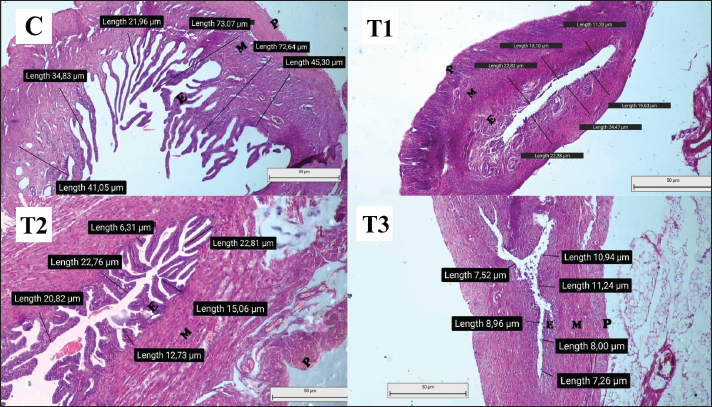
Overview of LHLH is a glycoprotein hormone secreted by gonadotropin cells in the anterior pituitary along with FSH (Nedresky and Singh, 2022). Progesterone is an important hormone that supports the early stages of pregnancy and maintains pregnancy. LH-stimulated corpus luteum produces the hormone. LH is important in stimulating steroidogenesis, which produces estrogen and progesterone in the ovaries. LH also triggers ovulation and supports the formation of the corpus luteum, which produces progesterone to sustain early pregnancy (Przygrodzka et al., 2021). LH levels fluctuate during pregnancy (Chen et al., 2016). During the estrous phase, LH levels in the anterior pituitary gland increase to stimulate follicles. When LH levels reach their peak, this triggers ovulation of the follicle, leading to the release of mature oocytes from the ovary. The corpus luteum, which originates from the remaining follicles after ovulation, is stimulated by LH to produce progesterone (Duncan, 2021). In the early trimester of pregnancy, LH levels decrease gradually because of the negative feedback from increased progesterone production (Choi and Smitz, 2014). On days 8–12 of pregnancy, there is a slight increase in LH levels due to an increase in the pulsatile frequency of LH (Mauvais-Jarvis et al., 2013). The corpus luteum depends on LH to maintain its function until the 11th day of pregnancy, after which the placenta starts producing lactogen prolactin, which takes over the supporting role of the corpus luteum (Przygrodzka et al., 2021). As pregnancy progresses, especially in the third trimester, LH levels decrease and are suppressed, with hormonal regulation shifting from the anterior pituitary to the placenta (Choi and Smitz, 2014). The findings of this study showed a significant decrease in mean LH levels in pregnant Wistar rats treated with dexamethasone at doses of 0.72 and 0.75 mg/day during the second trimester compared with the control group and treatment group 1. The observed decrease in LH levels may be attributed to reduced LH signaling induced by gonadotropin cells, which are reduced in number (Eng et al., 2024). Glucocorticoids, such as dexamethasone, reduce gonadotropin releasing hormone (GnRH) mRNA expression in the hypothalamus and alter the number of gonadotropin-producing cells (Boomsma et al., 2022). This finding is consistent with the statement of Bhaumik et al. (2023), who stated that increased exposure to glucocorticoids, such as dexamethasone, affects the hypothalamus and pituitary gland by inhibiting the release of GnRH. Inhibition of GnRH release by dexamethasone also interferes with LH and FSH, which GnRH normally stimulates from the pituitary gland (Marques et al., 2022). Dexamethasone administration suppresses the pituitary gland’s response to GnRH. It inhibits GnRH pulsatility in the hypothalamus, leading to a decrease in gonadotropin cell numbers and an overall decrease in pituitary gland volume upon GnRH promoter activity in hypothalamic cells via gonadotropin receptor signaling (Mbiydzenyuy and Qulu, 2024). Increased glucocorticoid exposure leads to changes in anterior pituitary axis activity and regulation induced by basal and stressful conditions (Burford et al., 2017). The anterior pituitary, which GnRH normally stimulates, is unable to secrete LH due to glucocorticoid modulation of the GnRH receptor signaling mechanism, resulting in reduced hormone release (Zhang et al., 2022). Impact of prenatal endometrial conditionData for this study were obtained from uterine preparations of pregnant Wistar rats (Rattus norvegicus) stained with HE. The endometrial thickness in these rats was measured and is shown in Figure 1. The results of statistical analysis of mean endometrial length are presented in Figure 1. The control group (C) exhibited marked differences compared with treatment groups T1, T2, and T3, which could be attributed to the varying doses administered to each group. The mean endometrial thickness was higher in the control group than in the dexamethasone-treated group. Previous studies by McDonald et al. (2003) and Anwar (2005) have shown a greater reduction in mean endometrial thickness compared with the dexamethasone-treated group, which in turn interferes with the production of estrogen and progesterone, both hormones that are important for maintaining pregnancy. Thinning of the endometrial lining can lead to pregnancy-related complications, mainly due to inadequate nutritional support for fetal development. Insufficient endometrial thickness is associated with an increased risk of abortion because of inadequate fetal support (Karavani et al., 2021). Treatment group 3 (T3) received 0.75 mg/day dexamethasone and had the lowest mean endometrial thickness among the treatment groups. This was due to the dose of 0.75 mg/day, which is a toxic level of dexamethasone, resulting in the abortion of pregnant rats in this group. This observation is consistent with the findings of Yahi et al. (2017) that high-dose dexamethasone can cause abortion in pregnant animals.
Fig. 1. Histopathology of uterine preparation, which was observed under an Olympus CX43 trinocular microscope. Data were collected according to the mean endometrial thickness in each treatment group. C=control with no treatment; T1=treatment with dexamethasone doses of 0.36 mg/day; T2=treatment with dexamethasone doses of 0.72 mg/day; T3=treatment with dexamethasone doses of 0.75 mg/day. Treatment group two (T2), which received dexamethasone at a dose of 0.72 mg/day, showed signs of preterm labor on the 13th day of pregnancy. Treatment group 1 (T1), which received dexamethasone at a dose of 0.36 mg/day, had a decreased mean endometrial thickness compared with the control group but had a significantly higher thickness than groups T2 and T3. This suggests that even therapeutic doses of dexamethasone can affect endometrial thickness during pregnancy compared with the control group (C), which did not receive dexamethasone treatment. During pregnancy, the fetus is protected from the detrimental effects of glucocorticoids by the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) (Hunter and Bailey, 2015; Wang et al., 2022). This enzyme plays an important role in regulating the passage of glucocorticoids through the placenta (Konstantakou et al., 2017). However, as a synthetic glucocorticoid, dexamethasone cannot be inactivated by 11β-HSD in the placenta (Yahi et al., 2017). Leading to several adverse effects during pregnancy, including changes in hormone levels (Yahi et al., 2017). Estrogen and progesterone, which are steroid hormones, are the main reproductive hormones in mammals, and their levels increase as pregnancy progresses (Chang and Lubo, 2008). Both hormones are produced by the corpus luteum and placenta during pregnancy (Marquardt et al., 2019). Estrogen helps prepare the uterus by stimulating the growth and development of the uterine lining (endometrium), which plays an important role in successful pregnancy by creating an optimal environment for fetal growth and undergoing morphological changes to nourish the fetus (Parisi et al., 2023). Insufficient endometrial thickness can lead to complications because it inhibits the ability to provide adequate nutrition to the fetus, thereby increasing the risk of abortion (Karavani et al., 2021). McDonald et al. (2003) and Anwar (2005) showed that dexamethasone interferes with placental function, thereby interfering with the production of estrogen and progesterone, the main regulators of pregnancy. Decreased progesterone levels can affect uterine contractions because progesterone regulates prostaglandin production. Low progesterone levels can inhibit uterine relaxation, leading to increased prostaglandin concentrations, which can trigger contractions and potentially lead to abortion (Lubis, 2023). ConclusionAdministration of dexamethasone at doses of 0.36, 0.72, and 0.75 mg/day for 7 days between days 8 and 14 of pregnancy resulted in adverse effects on hormone balance and endometrial development in pregnant rats (R. norvegicus). Specifically, dexamethasone treatment caused a decrease in progesterone and LH levels, as well as a reduction in endometrial thickness, indicating its potential to interfere with the physiological processes essential for maintaining pregnancy. These findings underscore the importance of exercising caution when administering glucocorticoids during pregnancy, given their potential negative impact on reproductive function and fetal development. AcknowledgmentsWe sincerely thank to Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, for the support through the Airlangga Research Fund programme (ARF) 2024 that has provided facilities and funding for the smooth running of this research. We also appreciate the entire FIKKIA research team for their cooperation, dedication, and contribution to this research. We hope that the results of this research will provide benefits and positive contributions to science and society. Conflict of interestThe authors declare that they have no conflicts of interest. FundingAirlangga Research Fund programme (ARF) 2024. Authors’ contributionsAll authors contributed to the present study. Data availabilityAll data were provided in the manuscript. ReferencesAhmadabad, H.N., Jafari, S.K., Firizi, M.N., Abbaspour, A.R., Gharib, F.G., Ghobadi, Y. and Gholizadeh, S. 2016. Pregnancy outcomes following the administration of high doses of dexamethasone in early pregnancy. Clin. Exp. Reprod. Med. 43(1), 15–25. Anwar, R. 2005. Adrenal gland function and its abnormalities. Bandung, EJ: Faculty of Medicine, Padjajaran University. Apsari, A.D. 2022. Overview of community knowledge and behavior towards the use of dexamethasone tablets in Sumur Batu Village, North Teluk Betung District, Bandar Lampung in 2022. Doctoral Dissertation, Tanjungkarang Poltekkes, Tanjung Karang, Indonesia. Bhaumik, S., Lockett, J., Cuffe, J. and Clifton, V.L. 2023. Glucocorticoids and their receptor isoforms: roles in female reproduction, pregnancy, and foetal development. Biology 12(8), 1104. Blackwell, J., Selinger, C., Raine, T., Parkes, G., Smith, M.A. and Pollok, R. 2020. Steroid use and misuse: a key performance indicator in the management of IBD. Front. Gastro. 12(3), 207–213. Boomsma, C.M., Kamath, M.S., Keay, S.D. and Macklon, N.S. 2022. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 6(6), CD005996. Butt, Z., Tinning, H., O’Connell, M.J., Fenn, J., Alberio, R. and Forde, N. 2023. Understanding conceptus–maternal interactions: what tools do we need to develop? Reprod. Fertil. Dev. 36(2), 81–92. Burford, N.G., Webster, N.A. and Cruz-Topete, D. 2017. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int. J. Mol. Sci. 18(10), 2150. Chang, K. and Lubo, Z. 2008. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod. Sci. 15(4), 336–348. Chen, C.D., Chiang, Y.T., Yang, P.K., Chen, M J., Chang, C.H., Yang, Y.S. and Chen, S.U. 2016. Frequency of low serum LH is associated with increased early pregnancy loss in IVF/ICSI cycles. Reprod. Biomed. Online. 33(4), 449–457. Chen, J. and Khalil, R.A. 2017. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 148, 87–165. Chen, J., Wang, Z.Z., Zhang, S., Chu, S.F., Mou, Z. and Chen, N.H. 2019. The effects of glucocorticoids on depressive and anxiety-like behaviors mineralocorticoid receptor-dependent cell proliferation regulates anxiety-like behaviors. Behav. Brain Res. 362, 288–298. Choi, J. and Smitz, J. 2014. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol. Cell Endocrinol. 383(1–2), 203–213. DeMayo, F.J., Zhao, B., Takamoto, N. and Tsai, S.Y. 2002. Mechanisms of action of estrogen and progesterone. Ann. N. Y. Acad. Sci. 955, 48–59. Duncan, W.C. 2021. The inadequate corpus luteum. Reprod. Fertil. 2(1), C1–C7. Eng, P.C., Phylactou, M., Qayum, A., Woods, C., Lee, H., Aziz, S., Moore, B., Miras, A.D., Comninos, A.N., Tan, T., Franks, S. Dhillo, W.S. and Abbara, A. 2024. Obesity-related hypogonadism in women. Endocr. Rev. 45(2), 171–189. Faggiano, A., Mazzilli, R., Natalicchio, A., Adinolfi, V., Argentiero, A., Danesi, R., D’Oronzo, S., Fogli, S., Gallo, M., Giuffrida, D., Gori, S., Montagnani, M., Ragni, A., Renzelli, V., Russo, A., Silvestris, N., Franchina, T., Tuveri, E., Cinieri, S., Colao, A., Giorgino, F. and Zatelli, M.C. 2022. Oncological Endocrinology research group of the Italian Society of Endocrinology. Corticosteroids in oncology: use, overuse, indications, contraindications. An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit. Rev. Oncol. Hematol. 180, 103826. Hunter, R.W. and Bailey, M.A. 2015. Glucocorticoids and 11β-hydroxysteroid dehydrogenases: mechanisms for hypertension. Curr. Opin. Pharmacol. 21, 105–114. Indayani, N.S. 2015. Effect of dexamethasone administration on hepar damage in male rats (Ratus norvegicus) Wistar breed. J. Online UM Life Sci. 1(1), 1–7. Jee, S.B. and Sawal, A. 2024. Physiological changes in pregnant women due to hormonal changes. Cureus 16(3), e55544. Karavani, G., Alexandroni, H., Sheinin, D., Dior, U.P., Simon, A., Ben-Meir, A. and Reubinoff, B. 2021. Endometrial thickness following early miscarriage in IVF patients-is there a preferred management approach? Reprod. Biol. Endocrinol. 19(1), 93. Konstantakou, D., Heussner, A. and Wu, Y. 2017. Glucocorticoids and their metabolism in the placenta. Front. Physiol. 8, 471. Lee, B., Park, T.C. and Lee, H.J. 2015. Maternal age and serum concentration of human chorionic gonadotropin in early pregnancy: influence of gonadotropin-releasing hormone. Acta Obstet. Gynecol. Scand. 94(4), 443–444. Liu, Y., Ding, H., Yang, Y., Liu, Y., Cao, X. and Feng, T. 2022. Progesterone induces apoptosis and steroidogenesis in porcine placental trophoblasts. Animals 12(19), 2704. Lubis, P.N. 2023. Effectiveness of oral and vaginal progesterone in the management of abortus imminens. Mirr. World Med. 50(6), 339–341. Marquardt, R.M., Kim, T.H., Shin, J.H. and Jeong, J.W. 2019. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int. J. Mol. Sci. 20(15), 3822. Marques, P., Skorupskaite, K., Rozario, K.S., Anderson, R.A. and George, J.T. 2022. Physiology of gnRH and gonadotropin secretion. Dartmouth, MA: Endotext. Mauvais-Jarvis, F., Clegg, D.J. and Hevener, A.L. 2013. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34(3), 309–338. Mbiydzenyuy, N.E. and Qulu, L.A. 2024. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 39(8), 1613–1636. McDonald, T.J., Franko, K.L., Brown, J.M., Jenkins, S L., Nathanielsz, P.W. and Nijland, M.J. 2003. Betamethasone in the last week of pregnancy causes fetal growth retardation but not adult hypertension in rats. J. Soc. Gynecol. Investig. 10(8), 469–473. Nedresky, D. and Singh, G. 2022. Physiology, luteinizing hormone luteinizing hormone. Treasure Island, FL: StatPearls. Parisi, F., Fenizia, C., Introini, A., Zavatta, A., Scaccabarozzi, C., Biasin, M. and Savasi, V, 2023. The pathophysiological role of estrogens in the initial stages of pregnancy: molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update. 29(6), 699–720. Polderman, J.A., Farhang-Razi, V., Van Dieren, S., Kranke, P., DeVries, J.H., Hollmann, M.W., Preckel, B. and Hermanides, J. 2018. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 8(8), CD011940. Przygrodzka, E., Plewes, M.R. and Davis, J.S. 2021. Luteinizing hormone regulation of inter-organelle communication and fate of the corpus luteum. Int. J. Mol. Sci. 22(18), 9972. Raju, G.A., Chavan, R., Deenadayal, M., Gunasheela, D., Gutgutia, R., Haripriya, G., Govindarajan, M., Patel, N.H. and Patki, A.S. 2013. Luteinizing hormone and follicle stimulating hormone synergy: a review of role in controlled ovarian hyper-stimulation. J. Hum. Reprod. Sci. 6(4), 227–234. Ramamoorthy, S. and Cidlowski, J.A. 2016. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. North Am. 42(1), 15–31. Shah, T.J., Conway, M.D. and Peyman, G.A. 2018. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: design, development, and place in therapy. Clin. Ophthalmol. 12, 2223–2235. Shah, N.M., Lai, P.F., Imami, N. and Johnson, M.R. 2019. Progesterone-related immune modulation of pregnancy and labor. Front. Endocrinol (Lausanne). 10, 198. Skovorodin, E., Bogolyuk, S., Bazekin, G., Sharipov, A. and Khokhlov, R. 2020. Morphology and histochemistry of the corpus luteum (CL) of ovaries of pregnant and infertile cows. Am. J. Anim. Vet. Sci. 15(4), 257–265. Wan, J., Hum Z., Zengm K., Yin, Y., Zhao, M., Chen, M. and Chen, Q. 2018. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 11, 18–25. Wang, H.Q., Zhang, W.D., Yuan, B. and Zhang, J.B. 2021. Advances in the regulation of mammalian follicle-stimulating hormone secretion. Animals (Basel) 11(4), 1134. Wang, J., Chen, F., Zhu, S., Li, X., Shi, W., Dai, Z., Hao, L. and Wang, X. 2022. Adverse effects of prenatal dexamethasone exposure on fetal development. J. Reprod. Immunol. 151, 103619. Yahi, D., Ojo, N.A. and Mshelia, G.D. 2017. Influence of dexamethasone on some reproductive hormones and uterine progesterone receptor localization in pregnant yankasa sheep in semiarid zones of Nigeria. J. Vet. Med. 2017, 9514861. Zhang, X., Wei, Y., Li, X., Li, C., Zhang, L., Liu, Z., Cao, Y., Li, W., Zhang, X., Zhang, J., Shen, M. and Liu, H. 2022. The corticosterone–glucocorticoid receptor–AP1/CREB axis inhibits the luteinizing hormone receptor expression in mouse granulosa cells. Int. J. Mol. Sci. 23(20), 12454. | ||
| How to Cite this Article |
| Pubmed Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Santoso DCA, Kusuma EZW, Annisa J, Praja SS, Wijaya WA, Jaladara WT. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Vet. J.. 2025; 15(5): 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 Web Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Santoso DCA, Kusuma EZW, Annisa J, Praja SS, Wijaya WA, Jaladara WT. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). https://www.openveterinaryjournal.com/?mno=235690 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.12 AMA (American Medical Association) Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Santoso DCA, Kusuma EZW, Annisa J, Praja SS, Wijaya WA, Jaladara WT. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Vet. J.. 2025; 15(5): 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 Vancouver/ICMJE Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Santoso DCA, Kusuma EZW, Annisa J, Praja SS, Wijaya WA, Jaladara WT. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 Harvard Style Saputro, A. L., Prastiya, . R. A., Purnama, . M. T. E., Praja, . R. N., Yuniarti, . W. M., Maslamama, . S. T., Burhanuddin, . A., Santoso, . D. C. A., Kusuma, . E. Z. W., Annisa, . J., Praja, . S. S., Wijaya, . W. A. & Jaladara, . W. T. (2025) Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Vet. J., 15 (5), 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 Turabian Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Dilla Chelsea Aziizahrani Santoso, Evelyn Zaalfa Winni Kusuma, Jihan Annisa, Shifa Salsabilla Praja, Wayan Ari Wijaya, and Wira Tirta Jaladara. 2025. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Veterinary Journal, 15 (5), 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 Chicago Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Dilla Chelsea Aziizahrani Santoso, Evelyn Zaalfa Winni Kusuma, Jihan Annisa, Shifa Salsabilla Praja, Wayan Ari Wijaya, and Wira Tirta Jaladara. "Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus)." Open Veterinary Journal 15 (2025), 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 MLA (The Modern Language Association) Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Dilla Chelsea Aziizahrani Santoso, Evelyn Zaalfa Winni Kusuma, Jihan Annisa, Shifa Salsabilla Praja, Wayan Ari Wijaya, and Wira Tirta Jaladara. "Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus)." Open Veterinary Journal 15.5 (2025), 1982-1989. Print. doi:10.5455/OVJ.2025.v15.i5.12 APA (American Psychological Association) Style Saputro, A. L., Prastiya, . R. A., Purnama, . M. T. E., Praja, . R. N., Yuniarti, . W. M., Maslamama, . S. T., Burhanuddin, . A., Santoso, . D. C. A., Kusuma, . E. Z. W., Annisa, . J., Praja, . S. S., Wijaya, . W. A. & Jaladara, . W. T. (2025) Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus norvegicus). Open Veterinary Journal, 15 (5), 1982-1989. doi:10.5455/OVJ.2025.v15.i5.12 |