| Research Article | ||
Open Vet. J.. 2025; 15(5): 1990-1997 Open Veterinary Journal, (2025), Vol. 15(5): 1990-1997 Research Article The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickensWidia Siska1*, Hadri Latif 2 and Trioso Purnawarman21Graduate Program in Veterinary Public Health and Epidemiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 2Division of Veterinary Public Health and Epidemiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia *Corresponding Author: Widia Siska. Graduate Program in Veterinary Public Health and Epidemiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia. E-mail: siskawidia [at] apps.ipb.ac.id Submitted: 01/01/2025 Revised: 18/03/2025 Accepted: 22/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
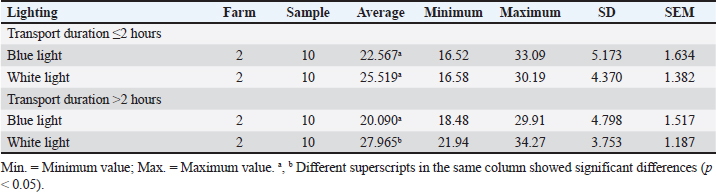
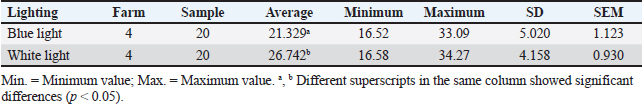
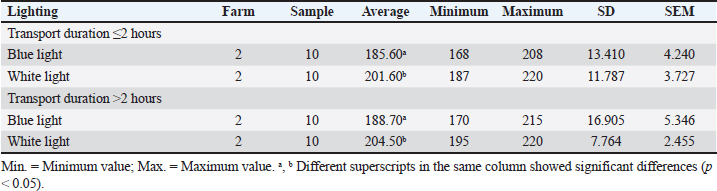
ABSTRACTBackground: The preslaughter process is a critical point in implementing animal welfare principles in poultry slaughterhouses. The duration of transportation from the farm to the poultry slaughterhouse and differences in lighting in the preslaughter room can affect animal welfare. Aim: This study aimed to evaluate animal welfare indicators by measuring corticosterone hormone concentration, bleeding time, and carcass quality in broiler chickens considering differences in transport duration and lighting conditions in the preslaughter room. Methods: This study used a completely randomized design with four treatments and two replications. A total of 40 chickens were sampled. The study was conducted across four treatments: (1) transport duration from the farm to the poultry slaughterhouses ≤2 hours; (2) transport duration from the farm to the poultry slaughterhouses >2 hours; (3) blue lighting; and (4) white lighting. For treatments (1) and (2), 10 chickens from different farms were used for each replicate. Treatment groups (1) and (2) received treatments (3) and (4) using blue light-emitting diode (LED) lights with an intensity of 18 lux and white LED lights with an intensity of 321 lux for 15 seconds. Treatment groups (3) and (4) each consisted of 5 chickens per replication exposed to blue or white light in the preslaughter room. Results: The average concentration of corticosterone hormone in blue and white light was 21.329 ng/ml and white light was 26.742 ng/ml. The average bleeding time under blue lighting was 187.15 seconds, and under white lighting, it was 203.05 seconds. Blue-light lighting can maintain carcass quality by 100% compared to white-light lighting, which produces varying quality. Conclusion: Corticosterone hormone concentration, bleeding time, and carcass quality were influenced by lighting. The use of blue light preslaughter significantly improved the corticosterone hormone concentration, bleeding time, and carcass quality compared with white light. Overall, blue lighting resulted in better animal welfare indicators for broiler chickens in poultry slaughterhouses. Blue lighting is recommended for implementation at poultry slaughterhouses. Keywords: Animal welfare, Broiler chickens, Lighting differences, Transport duration. IntroductionAnimal welfare in Indonesia is being implemented in every livestock production unit. The implementation of animal welfare, particularly in animal product enterprises, aims to produce safe and quality animal products for the public (Latif et al., 2023). This welfare system encourages livestock production systems to apply animal welfare principles. According to Law Number 18 of 2009 in conjunction with Law Number 41 of 2014 concerning Livestock and Animal Health, animal welfare is everything related to the physical and mental condition of animals according to natural animal behavior standards, which must be applied and enforced to protect animals from inappropriate treatment by humans (Yulianto et al., 2023). Animal welfare applies to all types of animals that can feel pain. Animal welfare practices can be implemented in various livestock activities according to Government Regulation Number 95 of 2012 concerning Veterinary Public Health and Animal Welfare, including capture, placement, maintenance, transportation, use, treatment, and slaughter (GR, 2012). Animal welfare in the slaughter process at poultry slaughterhouses is one of the stages that can determine the quality and safety of meat in the meat supply chain. Poultry Slaughterhouses are complexes of buildings with equipment designed to meet the requirements for slaughtering poultry for public consumption (Latif et al., 2023). One of the highlighted systems regarding animal welfare at poultry slaughterhouses is the human treatment of animals during preslaughter. The preslaughter process at poultry slaughterhouses is a critical control point for the implementation of animal welfare principles. The implementation of animal welfare is not only limited to maintenance but also extends to the slaughter process at poultry slaughterhouses. However, not all poultry slaughterhouses adhere to the basic principles of animal welfare. Several aspects of animal welfare that must be considered in the slaughter process at poultry slaughterhouses include transportation, receiving poultry from transportation, resting, shackling, lighting exposure in the preslaughter area, stunning preslaughter, slaughter, and handling postslaughter before the chickens are killed. These factors can contribute to stress in chickens, affecting their welfare. This stress can influence the corticosterone hormone concentration, bleeding time, and carcass quality. To date, limited scientific studies have been conducted in Indonesia to determine animal welfare indicators at poultry slaughterhouses. Based on these findings, the current research aimed to evaluate animal welfare indicators in broiler chickens by measuring corticosterone hormone concentration, bleeding time, and carcass quality, which are influenced by transport duration and lighting differences in the preslaughter room. Materials and MethodsTime and location of studyThe study was conducted from February to June 2024 at poultry slaughterhouses in Cianjur Regency, West Java. Sampling was carried out at the Division of Veterinary Public Health and Epidemiology Laboratory and the Microbiology and Immunology Laboratory of the Primate Animal Research Center, LPPM-IPB, Bogor. Samples collectionThis study used broiler chickens with an average body weight of 2.0 ± 0.2 kg, which were healthy. The chicken samples were rested for approximately 2 hours at the poultry slaughterhouses. Antemortem examinations were performed on each sample under the same conditions. The chicken samples were placed on an automatic moving shackle (conveyor) in an inverted position with the chicken breast facing the slaughterer. This study used a completely randomized design with four treatments and two replications. Based on the Federer method, 40 broiler chickens were used as a sample. The study was conducted with four treatments: (1) transport duration from the farm to poultry slaughterhouses ≤2 hours, (2) transport duration from the farm to poultry slaughterhouses >2 hours, (3) blue lighting, and (4) white lighting in the preslaughter room. For treatments (1) and (2), 10 chickens from different farms were used for each replicate. Treatment groups (1) and (2) received treatments (3) and (4), using blue light-emitting diode (LED) lights with an intensity of 18 lux and white LED lights with an intensity of 321 lux for 15 seconds. Treatment groups (3) and (4) each consisted of 5 chickens per replication. Chickens that received lighting treatment were immediately stunned. Stunning was performed using an electrical water bath stunning method with a voltage of 220 V, a frequency of 50 Hz, and an electric current of 0.10.15 A by dipping the chicken heads in water for 5 seconds. Slaughtering was carried out immediately after stunning using a knife. Corticosterone hormone concentrationBlood samples from each chicken were collected immediately after slaughter, with 3 ml of each chicken collected in a vacutainer tube without an anticoagulant. The samples were left at room temperature during transportation, approximately 45 hours, and then placed in a refrigerator for 46 hours. The formed serum was collected and centrifuged at 2,000 rpm for 20 minutes to separate the blood components. The serum was stored at −18ºC and prepared for further testing. The serum concentration of corticosterone hormone was measured using the enzyme-linked immunosorbent assay (ELISA) method. The ELISA product used was the Chicken CORT (Corticosterone) ELISA kit (Elabscience® 96) with a detection range of 0.3925 ng/ml. The results were read using an ELISA Microtiter Plate Reader with an optical density of 450 nM. Bleeding timeThe bleeding time was recorded for all chicken samples immediately after slaughter. The slaughtered chickens were invertedly placed on an automatic moving shackle (conveyor), and the bleeding time was measured. The measurement started when the blood began to flow out until it stopped using a stopwatch, and the time was recorded. Carcass qualityA sample examination was conducted using physical testing. The assessment of physical quality levels for carcasses was based on the Indonesian National Standard for Carcasses and Broiler Chicken Meat Number 3924:2023, conducted by 3 trained panelists (Indonesian National Standardization Board (BSN), 2023). The quality factors assessed included carcass integrity, color changes (other than bruising), and cleanliness. Carcass integrity was evaluated based on the condition of the carcass in terms of the presence or absence of broken or missing bones, dislocated joints, torn or missing skin, or meat. Color changes were assessed based on deviations caused by bruising, blood splashing, reddened wing blood vessels, freeze burns, ruptured gall bladders, and/or other discolorations. The assessment used quality levels IIII, with higher quality levels indicating lower carcass quality. Data analysisData on transport duration and lighting differences were analyzed using two-way analysis of variance. Treatments that showed significant differences at the probability level of p < 0.05 were compared using pairwise t-tests on corticosterone hormone concentration and bleeding time and descriptive analysis on carcass quality. Ethical approvalThis study was approved by the Animal Ethics Committee School of Veterinary Medicine and Biomedical Sciences (SVMBS) at IPB University number 192/KEH/SKE/III/2024 (March 28, 2024). ResultsCorticosterone hormone concentrationCorticosterone hormone concentration in the group of chickens with a transport duration from the farm to poultry slaughterhouses ≤2 hours, using blue light and white light, showed no significant difference (p > 0.05). The group of chickens with a transport duration from the farm to poultry slaughterhouses >2 hours using blue light had a significantly different corticosterone hormone concentration compared with those using white light (p < 0.05). The results of corticosterone hormone concentration measurements according to transport duration and lighting are presented in Table 1. Corticosterone hormone concentration in the group of chickens with transport durations from the farm to poultry slaughterhouses ≤2 and >2 hours was generally lower under blue light than under white light (Table 2). The difference in the intensity of blue light compared with white light significantly affected the corticosterone hormone concentration in chickens (p < 0.05). Bleeding timeBleeding times in groups of chickens with transport durations from the farm to poultry slaughterhouses ≤2 and >2 hours under blue and white light were significantly different (p < 0.05). The bleeding time occurred faster under blue light than under white light. The results of bleeding time measurements based on transport duration and different lighting conditions are presented in Table 3. Table 1. Corticosterone hormone concentration (ng/ml) in chickens with different transport durations and lighting conditions.
Table 2. Corticosterone hormone concentration (ng/ml) under different lighting conditions.
Table 3. Bleeding time (seconds) in chickens with different transport durations and lighting conditions.
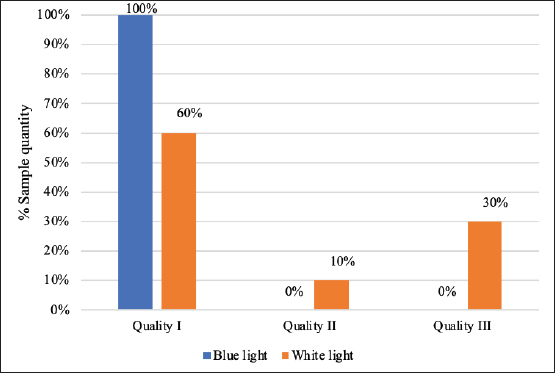
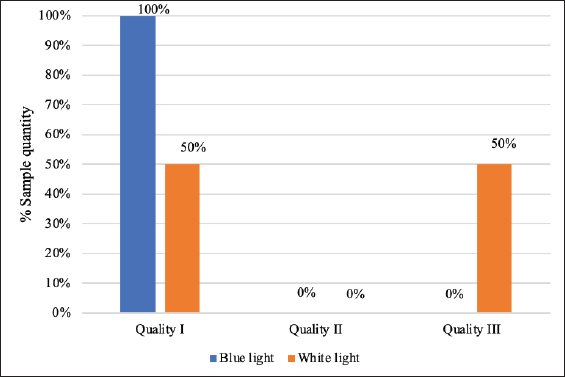
Carcass qualityThe carcass quality evaluation results using blue and white lighting for transport durations ≤2 hours are presented in Figure 1. The percentage of carcass quality using blue lighting consistently showed quality grade I for all samples. White lighting preslaughter resulted in varying carcass quality grades. Figure 2 presents a comparison of carcass quality using blue and white lighting for transport durations of >2 hours. The percentage of carcass quality for transport duration >2 hours under blue lighting showed quality grade I in all samples. White lighting resulted in 50% grade I quality and 50% grade III quality. In general, carcass quality in this study was not influenced by the duration of transportation but was influenced by lighting differences (p < 0.05).
Fig. 1. Carcass quality for transport duration ≤2 hours.
Fig. 2. Carcass quality for transport duration >2 hours. DiscussionCorticosterone hormone concentrationThe corticosterone hormone concentration in the group of chickens with a transport duration from the farm to poultry slaughterhouses ≤2 hours, which showed no significant difference, might be due to the stress experienced by the chickens during the initial harvesting process at the farm. Short transport duration induces acute stress in chickens, which are sensitive to acute stress (Tamzil et al., 2019). The transport of chickens from farm to poultry slaughterhouses triggers stress that affects body metabolism and homeostasis. Zheng et al. (2020) reported that hormone secretion in chickens increases by 2 hours of transportation. Upon arrival at poultry slaughterhouses, the chickens are still stressed from transportation and are then rested. The process of unloading chickens from the transport vehicle after resting and then shackling them in the preslaugher area causes repeated stress events. This means that chickens that are not fully recovered from stress are then exposed to different lighting treatments, which results in no significant differences in the corticosterone hormone concentration. Corticosterone hormone concentrations can vary depending on environmental stimuli, causing variations in each chicken. Corticosterone concentrations in the blood reflect recent fluctuations after the animal is exposed to acute stress (Hillebrecht et al., 2024). Chickens transported from the farm to poultry slaughterhouses for >2 hours under white light had higher corticosterone hormone concentrations than those transported under blue light. Elevated hormone levels might occur because chickens on longer transport recover from the stress experienced during transit. Initially stressed from harvesting to transportation, chickens gradually recover during extended transport, reducing stress levels. Wicaksono et al. (2020) reported that early morning transportation on quiet roads with cool air helps chickens recover their physical condition when transported for longer periods. The chickens were rested after transportation. During the rest period before slaughter, the body’s physiology adjusts, and corticosterone hormone concentrations gradually decrease. The 2-hour resting period at poultry slaughterhouses for chickens transported >2 hours significantly impacts their physical recovery. Chickens recovering from stress will experience a decrease in corticosterone hormone concentration. If these chickens are shackled and exposed to white light in the preslaughter area, they will experience a significant increase in stress, leading to higher corticosterone levels. Conversely, under blue light, the chickens remain calmer, and their corticosterone concentrations are significantly lower (Tan et al., 2023) than under white light. In general, this study showed that the corticosterone hormone concentration in chickens is influenced by lighting. Using blue lighting for chickens for longer periods of time shows a lower corticosterone concentration. Blue light significantly reduces corticosterone levels in chicken compared with white light (Bhatt et al., 2021; Zeng et al., 2022). Broiler chickens are quieter under blue light, which helps to reduce stress, fear, and anxiety (Mohamed et al., 2020). Bleeding timeThe bleeding time for chickens transported from the farm to poultry slaughterhouses ≤2 and >2 hours, possibly due to higher stress levels. Stressed chickens exhibit increased blood pressure, heart rate, and respiration. Exposure to stress during transportation makes the chicken’s body fatigue. Stress causes fatigue (Sutherland et al., 2023). If slaughter is performed, while the livestock is still recovering and fatigued, the heart cannot pump blood effectively, resulting in an incomplete bleeding time (Ulfa et al., 2019). Acute stress causes blood vessel constriction, leading to reduced blood volume output and longer bleeding times (Anwar 2018; Kim et al., 2019). Constricted blood vessels affect the blood output volume (Sabow et al., 2016; Kim et al., 2019). Prolonged stress can cause weakness, breathing difficulties, and trauma. Extended stress forces the body to work harder (Sutherland et al., 2023), causing chickens to become lethargic, resulting in slower blood output and longer bleeding times. The blood drainage process during slaughter is influenced by severed blood vessels, vasodilation or vasoconstriction, muscle contractions pressing on blood vessels, and the carcass orientation in a horizontal or vertical position (Sabow and Majeed 2019). A perfectly slaughtered chicken is marked by the cessation of blood drainage from the body after slaughter. A chicken is considered killed after slaughter when bleeding stops (Bostami et al., 2021; Supratikno et al., 2022). The bleeding time can affect the halal aspect of slaughtering. The halal aspect ensures that slaughtered chicken is completely slaughtered before further handling (Fuseini et al., 2022). One of the requirements for halal slaughter is that killing must not be caused by anything other than the neck incision (Hakim et al., 2020). The bleeding time also affects carcass quality. Incomplete bleeding time reduces carcass quality (Sasidharan et al., 2022) because it is a suitable growth medium for microorganisms (Santos et al., 2019). Efficient bleeding time during slaughtering can improve carcass quality during storage (Sabow et al., 2016). Efficient bleeding time in chickens benefits poultry slaughterhouses by improving production efficiency and producing high-quality products. Carcass qualityExposure to blue light preslaughter can better maintain carcass quality than white lights. Blue light exposure during transportation ≤2 and >2 hours resulted in 100% quality grade I carcasses. Carcasses of quality grade I exhibit no abnormalities or discoloration, indicating that blue lighting maintains high carcass quality. Blue light calms and comforts chickens, minimizing movement and thrashing, which can affect carcass quality. Preslaughter blue light exposure at poultry slaughterhouses can provide comfort to poultry, preserving meat quality (Barbosa et al., 2013). Evaluation of grade II carcass quality under white light occurs due to deviations in the carcass, such as torn skin. White light has a greater impact on behavioral expression in chickens (Franco et al., 2022). Skin tears can result from movements like wing flapping or pecking caused by stress under white light. The evaluation of grade III carcass quality is based on changes in carcass integrity and color. Integrity changes can include torn skin, broken bones, and dislocated joints, while color changes can include bruising, bleeding, ruptured wing blood vessels, freeze burn, ruptured gall bladders, and/or other discolorations caused by microorganism activity and/or the use of hazardous chemicals (BSN, 2023). Carcass quality grade III in this study generally occurred due to ruptured wing blood vessels (reddened wing) and bruising of the breast or thigh. The percentage of grade III quality was found under white light transport durations ≤2 and >2 hours. White light exposure causes chickens to move constantly to free themselves from shackling. Bright light exposure can increase wing flapping, pecking, aggression, and cannibalism in chickens (Pal et al., 2019). Active movement in chickens can cause them to bump into each other, leading to ruptured blood vessels in the wings, thighs, or breasts (Novoa et al., 2019; Saraiva et al., 2020). Carcass quality degradation can affect consumer acceptance. Color is a crucial sensory attribute for evaluating product quality and is the first criterion consumers use to assess carcass or meat quality (Mihafu et al., 2020; Cappellozza and Marquez, 2021). In general, transport duration using good techniques did not significantly affect carcass quality. Transportation in this study was carried out in the early morning and generally complied with good transportation systems and practices. Research conducted by Nielsen et al. (2019) and Gholamreza et al. (2019) showed that the transportation process, which is carried out with care and good control, can minimize damage to the carcasses. Early morning transportation can maintain carcass quality because chickens are more comfortable when transported (Iftitah et al., 2022). ConclusionCorticosterone hormone concentration, bleeding time, and carcass quality were not affected by transport duration but by lighting conditions. Blue light lighting preslaughter significantly outperforms white light in terms of corticosterone hormone concentration, bleeding time, and carcass quality. Overall, blue lighting resulted in better animal welfare indicators for broiler chicken slaughter at poultry slaughterhouses. Blue lighting is recommended for implementation at poultry slaughterhouses. AcknowledgmentsConflict of interestThe authors declare no conflict of interest. FundingThis research did not receive external funding. Author’s contributionsWS, HL, and TP drafted, revised, and edited the manuscript. All authors have read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAnwar, D. 2018. Stress index of broiler chickens slaughtered with and without stunning. Thesis, IPB University, Bogor, Indonesia. Barbosa, C.F., de Carvalho, F.H., Rossa, A., Soares, A.L., Coro, F.A.G., Shimokomaki, M. and Ida, E.I. 2013. Commercial preslaughter blue light ambience for controlling broiler stres and meat qualities. Braz. Arch. Biol. Technol. 56(5), 817821. Bhatt, N., Singh, N.P., Mishra, A.K., Kandpal, D., Rajneesh and Jamwal, S. 2021. A detailed review of transportation stress in livestock and its management technique. Int. J. Livest. Res. 11, 3041. Bostami, A.B.M.R., Mun, H.S., Dilawar, M.A., Baek, K.S. and Yang, C.J. 2021. Carcass characteristics, proximate composition, fatty acid profile, and oxidative stability of pectoralis major and flexor cruris medialis muscle of broiler subjected to with or without level of electrical stunning, slaughter, and subsequent bleeding. Animals 11, 1679. [BSN] Badan Standarisasi Nasional. 2023. Standar Nasional Indonesia: Carcasses and Broiler Chicken Meat Number 3924:2023. Jakarta, Indonesia: Badan Standarisasi Nasional. Cappellozza, B.I. and Marques, R.S. 2021. Effects of pre-slaughter stress on meat characteristics and consumer experience. In: Meat and nutrition. Ed., Ranabhat, C.L. London, UK: IntechOpen Limited, pp: 115. Franco, B.R., Shynkaruk, T., Crowe, T., Francer, B., French, N., Gilingham, S. and Schwean-Lardner, K. 2022. Light color and the commercial broiler: effect on behavior, fear, and stress. Poult. Sci. 101(11), 19. Fuseini, A., Teye, M. and Lever, J. 2022. An update on halal slaughter: current methods and ongoing research on halal meat production techniques and their implication for animal welfare. Int. Food. Res. J. 31, 269276. Gholamreza, Z., Xi, H., Xi, F. and U, A.D. 2019. How can heat stress affect chicken meat quality? a review. Poult. Sci. 98, 15511556. [GR] Government Regulation of the Republic of Indonesia. 2012. Chapter III (animal welfare), part one. Vet. Public Health Anim. Welfare. No. 95, article 83. Hakim, L.I., Isa, N.M.M., Tahir, S.M. and Ibitoye, E.B. 2020. Effect of halal and nonhalal slaughtering methods on the bacterial contamination of poultry meat. Agr. Food Sci. 49, 19471950. Hillebrecht, T., Korbel, R., Rinder, M. and Gahr, M. 2024. Cicardian corticosterone profile in laying hens (Gallus galus domesticus). Animals 14(873), 118. Iftitah, D., Arisandi, B., Widyani, R.R.R. and Juniah, J. 2022. Physiological conditions of broiler chickens during transportation with vitamin treatment and distance difference. JIIP. 32(3), 313327. Kim, J.H., Almuwaqqat, Z., Hammadah, M., Liu, C., Ko, Y., Lima, B., Sullivan, S., Alkhoder, A., Abdulbaki, R., Ward, L., Bremmer, J. D., Sheps, D. S., Raggi, P., Sun, Y.V., Shah, A.J., Vaccarino, V. and Quyyumi, A.A. 2019. Peripheral vasoconstriction during metal stress and adverse cardiovascular outcomes in patien with coronary artery disease. Circ. Res. 125, 874883. Latif, H., Purnawarman, T., Supratikno., Yulianto, H., Jaelani, A., Wahyudi, P., Sutanto, Y.C., Fitrianti, A.T. and Surbakti, J.A. 2023. Guidance on the implementation of animal welfare in animal product business units. Jakarta, Indonesia: Kementerian Pertanian. Law of the Republic of Indonesia. 2014. Amendment to Law Number 18 of 2009 Concerning animal husbandry and animal health. Jakarta, Indonesia: People’s Representative Council of the Republic of Indonesia. Mihafu, F.D., Issa, J.Y. and Kamiyango, M.W. 2020. Implication of sensory evaluation and quality assessment in food product development: a review. Curr. Res. Nutr. Food Sci. J. 8(3), 690702. Mohamed, R., Abou-Elnaga, A., Ghazy, E., Mohammed, H., Shukry, M., Farrag, F., Mohammed, G. and Bahattab, O. 2020. Effect of different monochromatic led light colour and intensity on growth performace, physiological response and fear reaction in broiler chicken. Ital. J. Anim. Sci. 19(1), 10991107. EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S., Alvarez, J., Bicout, D.J., Calistri, P., Depner, K., Drewe, J.A., Garin-Bastuji, B., Gonzales Rojas, J.L., Gortázar Schmidt, C., Miranda Chueca, M.Á., Roberts, H.C., Sihvonen, L.H., Spoolder, H., Stahl, K., Velarde Calvo, A., Viltrop, A., Winckler, C., Candiani, D., Fabris, C., Van der Stede, Y. and Michel, V. 2019. Slaughter of animals: poultry. EFSA J. 17(11), e05849. Novoa, M., Vazquez, L., Lage, A., Gonzalez-Torres, I., Perez-Garcia, L.F., Cobas, N. and Lorenzo, J.M. 2019. Water-bath stunning process in broiler chickens: effect of voltage and intensity. SJAR. 17(2), 18. Pal, P., Dey, D., Sharma, B., Choudhary, S., Jyotimala, S., Kumar, S. and Ghosh, S. 2019. Effect of light management in broiler production: a review. JEZS 7(3), 437441. Sabow, A.B. and Majeed, S.J. 2019. Bleeding eficiency and keeping quality in broiler chicken meat subjected to two slaughtering method. J. Univ. Duhok. 22(2), 152158. Sabow, A.B., Zulkifli, I., Goh, Y.M., Kadir, M.Z.A.A., Kaka, U., Imlan, J.C., Abubakar, A.A., Adeyemi, K.D. and Sazili, A.Q. 2016. Bleeding efficiency, microbiological quality, and oxidative stability of meat from goats subjected to slaughter without stunning in comparison with different method of preslaughter electrical stunning. PLoS One. 11(4), e0152661. Santos, T.C.D., Gates, R.S., Souza, C.D.F., Tinoco, I.D.F.F., Candido, M.G.L. and Freitas, L.C.D.S.R. 2019. Meat quality parameters and the effects of stress: a riview. J. Agric. Sci. Technol. B. 9, 305315. Saraiva, S., Esteves, A., Oliveira, I., Mitchell, M. and Stilwell, G. 2020. Impact of pre-slaughter factors on walfare of broiler. Vet. Anim Sci. 10, 17. Sasidharan, V., Vasudevan, V.N., Sunil, B. and Sathu, T. 2022. Evaluation of colour and bleeding efficiency of imperfectly bled, scientifically slaughtered and cold slaughtered beef carcasses. J. Vet. Anim. Sci. 53(3), 385391. Supratikno., Setijanto, H., Nuraini, H. and Agungpriyono, S. 2022. Bleeding time and false aneurysm incidence on cattle slaughtering using non-penetrative pre-slaughter stunning in Indonesia. Trop. Anim. Sci. J. 45(4), 482490. Sutherland, C., Smallwood A., Wooten T. and Redfern, N. 2023. Fatigue and its impact on performance and health. Br. J. Hosp. Med. 84(2), 18. Tamzil, M.H., Indarsih, B. and Jaya, I.N.S. 2019. Rest before slaughtering alleviates transportation stress and improves meat quality in broiler chickens. Int. J. Poult. Sci. 18(2), 585590. Tan, Z., Zhou, C., Shi, X., Wang, L. and Wang, S. 2023. Evaluation of light-emitting diode colors and intensities on slaughter performance, meat quality and serum antioxidant capacity in caged broilers. Anim. Biosci. 36, 731739. Ulfa, F., Rastina., Farsyi, T.R., Jalaluddin, M., Ismail. and Harris, A. 2019. Perfection of blood removal in meugang beef at lambaro central market aceh besar. Jimvet 3(2), 3741. Wicaksono, M.A., Afnan, R. and Suryati, T. 2020. Oxidative stress, physiological responses and performance of broilers transported with different transportation duration. JIPTP. 8, 137143. Yulianto, H., Wahyudi, P. and Jaelani, A. 2023. Guidelines animal welfare in layer farms. Jakarta, Indonesia: Kementerian Pertanian. Zeng, L., Liu, Q., Wang, T., Yang, Y., Jado, A., Elhadidi, Y., Lin, W., Li, J. and Pan, J. 2022. Effect of monochromatic blue light on reducing the adverse impact of induced cyclic chronic heat stress during the thermal manipulation of broiler embryos. Oxid. Med. Cell. Longev. 2022, 9898311. Zheng, A., Cai, H., Lin, S., Pirzado, S.H., Chen, Z., Chang, W. and Liu, G. 2020. Stress associated with simulated transport, changes serum biochemistry, postmortem muscle metabolism, and meat quality of broilers. Animals 10, 112. | ||
| How to Cite this Article |
| Pubmed Style Siska W, Latif H, Purnawarman T. The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Vet. J.. 2025; 15(5): 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 Web Style Siska W, Latif H, Purnawarman T. The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. https://www.openveterinaryjournal.com/?mno=235586 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.13 AMA (American Medical Association) Style Siska W, Latif H, Purnawarman T. The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Vet. J.. 2025; 15(5): 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 Vancouver/ICMJE Style Siska W, Latif H, Purnawarman T. The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 Harvard Style Siska, W., Latif, . H. & Purnawarman, . T. (2025) The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Vet. J., 15 (5), 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 Turabian Style Siska, Widia, Hadri Latif, and Trioso Purnawarman. 2025. The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Veterinary Journal, 15 (5), 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 Chicago Style Siska, Widia, Hadri Latif, and Trioso Purnawarman. "The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens." Open Veterinary Journal 15 (2025), 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 MLA (The Modern Language Association) Style Siska, Widia, Hadri Latif, and Trioso Purnawarman. "The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens." Open Veterinary Journal 15.5 (2025), 1990-1997 . Print. doi:10.5455/OVJ.2025.v15.i5.13 APA (American Psychological Association) Style Siska, W., Latif, . H. & Purnawarman, . T. (2025) The evaluation of animal welfare indicators based on transport duration and lighting differences in the slaughter of broiler chickens. Open Veterinary Journal, 15 (5), 1990-1997 . doi:10.5455/OVJ.2025.v15.i5.13 |