| Research Article | ||
Open Vet. J.. 2025; 15(4): 1713-1718 Open Veterinary Journal, (2025), Vol. 15(4): 1713-1718 Research Article Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, IraqAli Hamzah Makttoof Al-Taee1* and Mansour Jadaan Ali21Department of Parasitology, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq 2Department of Microbiology, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq *Corresponding Author: Ali Hamzah Makttoof Al-Taee. Department of Parasitology, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq. Email: alihamzahvet [at] gmail.com Submitted: 29/12/2024 Accepted: 17/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
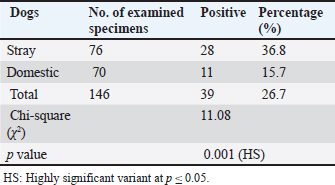
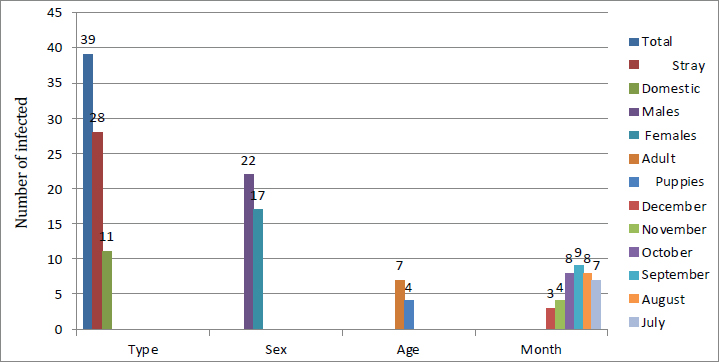
AbstractBackground: Neospora caninum is a significant intracellular protozoan that invades dogs as definitive and intermediate hosts, particularly farm animals as intermediate animal hosts. This parasitic infection leads to extreme financial losses due to reproductive distress and neurological illnesses in dogs. Aim: The current study aimed to identify N. caninum in domestic and stray dogs from different areas of Babylon province and to evaluate the influence of risk factors like sex, age, and months, on the ratio of infection. Methods: Based on serological tests Indirect Enzyme-Linked Immunosorbent assay, (iELISA), a total of 146 blood samples were collected from dogs, with 76 and 70 samples collected from stray and domestic dogs, respectively, from July to December (2024). Results: The overall prevalence rate was 26.7% (39/146), whereas the infection rate in stray dogs was 36.8% (28/76), which was higher than that in domestic dogs at 15.7% (11/70), with a highly significant difference (p≤0.05). No significant difference was observed (p ≥ 0.05) between the sexes; the proportion of males was 29.3% (22/75) and that of females was 23.9% (17/71). The higher ratio of infection in domestic dogs was investigated in the adult age group, 17.5% (7/41), in comparison with the lower infection rate of 13.7% (4/29) in the puppies, with no significant statistical difference (p ≥ 0.05). A monthly analysis demonstrated a higher infection rate in stray dogs and domestic dogs (34.6%) in September, followed by October (32%), whereas a lower infection rate was found in November (18.1%), followed by December (13%). Conclusion: The present study used serological diagnostic examination methods, particularly ELISA, to identify the critical role of stray and domestic dogs in the epidemiological cycle of neosporrosis and dissemination of N. caninum infection among animals in Babylon Province, Iraq. Keywords: Neosporosis, Dogs, Babylon, ELISA, Blood. IntroductionThe apicomplexan protozoan Neospora caninum is widely distributed geographically and causes significant financial strain on farmers and the livestock industry (Huaman et al., 2023; Nazari et al., 2023). Dogs in Norway were first identified with this infection in 1984 (Bjerkas et al., 1984). The parasite shares a large number of morphologic and biological characteristics with its close relative, Toxoplasma gondii (Dubey and Schares, 2006). N. caninum has an advanced life cycle, and its sexual cycle occurs exclusively in canids, which are recognized as the protozoa’s definitive hosts, such as domestic and wild dogs (Yang et al., 2022) and coyotes (Gharekhani et al., 2022). Asexual reproduction occurs within intermediate hosts (Dubey and Schares, 2007). Neospora can spread vertically in dogs during the last stages of pregnancy or after birth through milk (King et al., 2011), while horizontal transmission involves consumption of infected tissues from intermediate hosts harboring tissue cysts (Khan et al., 2020). Infected dogs exhibit neuromuscular symptoms, including encephalomyelitis and myositis, which can cause paralysis and premature puppy death (Al-Qassab et al., 2010). Depending on the location where the parasite multiplies in the central nervous system, adult dogs may exhibit a variety of neurological symptoms, with the most common manifestations including rigid hyperextension and paralysis of the pelvic limbs (Reichel et al., 2007; Dubey et al., 2017). The diagnosis of neosporosis can be made using a variety of serological methods, including the Indirect Hemagglutination Assay, Neospora Agglutination Test, Latex Agglutination Assay ELISA Enzyme-Linked Immunosorbent Assay (ELISA), and indirect fluorescence antibody test. ELISA has high sensitivity and specificity and, is the most crucial serological test used in the laboratory diagnostics of neosporosis (Gao and Wang, 2019). The lack of knowledge on the application of Elisa for detecting N. caninum in dog blood samples and the effect of specific risk agents on the infection rate in Babylon province served as the impetus for this study. Materials and MethodsCollection of samplesFrom July to December 2024, 146 blood samples were drawn from the jugular or cephalic veins of randomly selected domestic and stray dogs of both sexes and ages from various locations in Babylon Province. This exercise was performed using sterile tubes without anticoagulant, a sterile syringe, and a disposable glove to form serum. After being chilled during transit to the parasitological laboratory at the University of Al-Qadisiyah’s College of Veterinary Medicine, the blood samples were centrifuged for 10 minutes at 3,000 rpm to extract the serum, which was then stored in 1.5 ml Eppendorf tubes at –20°C until subjected to indirect ELISA (Fanokh and Al-Rubaie, 2022). Indirect ELISACompared with other serological techniques, the ELISA method offers numerous benefits that make it the best option for a wide range of scientific and medical applications. These benefits include high sensitivity, flexibility, simplicity, speed, accuracy, and dependability; reasonable cost; and the ability to conduct multiple experiments simultaneously on multiple samples. In accordance with the manufacturer’s protocol (Company, SUNLONG, and Origin, China), indirect ELISA was used in this study to detect anti-N. Caninum antibodies in dog serum. A microtiter plate reader (BioBase, China) was used to measure the optical density (O.D.) at 450 nm. The cutoff value was determined using the following formula: Critical=average of the negative control well + 0.15. The results were interpreted as follows: Negative results=sample O.D. < calculated critical (cutoff). Positive results=sample ≥ calculated critical (cutoff). Statistical analysisData were collected, tabulated, and statistically analyzed using a personal computer with the “Statistical Package for the Social Sciences version 26 program” (IBM Corporation, Armonk, NY, USA). The chi-square test was used to evaluate qualitative data. A value of p < 0.05 was considered statistically significant (Rahman, 2015). ResultsThe overall incidence rate of N. caninum in the blood specimens of the examined dogs was 26.7% (39/146). Table 1 and Figure 1 show a 36.8% (28/76) incidence rate of N. caninum in stray dogs, which is considerably higher than that in domestic dogs, 15.7% (11/70), with a highly significant statistical variance at p ≤ 0.05. Table 1. Whole incidence rate and distribution of Neosporosis according to canine type.
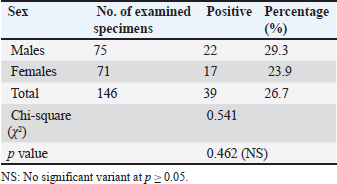
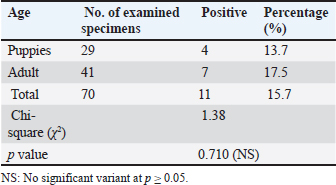
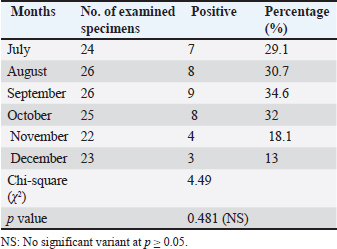
Infection rate according to sexMale dogs exhibited a higher rate of infection at 29.3% (22/75), whereas female dogs had a lower infection rate of 23.9% (17/71). Table 2 reflects that this variance has no statistical significance at p ≥ 0.05. Infection rate in household dogs according to ageAmong domestic dogs, the infection rate was lowest in the puppies (young age group) at 13.7% (4/29), whereas the infection rate was highest in the adult age group at 17.5% (7/41) without significant statistical variance at p ≥ 0.05 Table 3. Infection rate in monthsTable 4 shows that the incidence of N. caninum was highest in September (34.6%), followed by October (32%), whereas there was a lower incidence in November (18.1%), followed by December (13%) without significant statistical variance at p ≥ 0.05. DiscussionNeospora caninum is a pathogenic intracellular parasite that specifically affects cattle and causes abortion or neonatal death in a variety of animal species (Amdouni et al., 2022). Dogs play an important role in sustaining the infection and facilitating a storm of abortion because of the horizontal spread of N. caninum to domestic animals (Gharekhani et al., 2020a). The current study’s findings indicate that the overall seroprevalence of N. caninum infection in dogs is 26.7% (39/146), which is higher than the infection rates recorded in Baghdad province, Iraq at 24.17% (Fanokh and Al-Rubaie, 2022), Turkey at 10% (Coskun et al., 2000), Iran at 10.6% (Khanmohammadi and Fallah, 2011), and Germany at 7.33% (Villagra-Blanco et al., 2018). However, it is lower than the rates found in Wasit province, Iraq, 46.74% (Altaee et al., 2022), in the central Zagros region of Iran, 43.3% (Beki et al., 2023), in Turkey, 55.5% (ã–cal et al., 2014), and in Italy 32% (Robbe et al., 2016). The seroprevalence of N. caninum in dogs varies globally based on the study methodologies, number of animals studied, study location, serological technique, sensitivity, sample, technician, and laboratory, as well as direct exposure to possible infection sources and feeding practices (Gharekhani et al., 2020b).
Fig. 1. The relationship between the infection rate and the type of dog, sex, age, and months of the year using ELISA. In this study, the infection rate recorded in stray dogs was higher than that in domestic dogs; this result is in agreement in Iraq (Fanokh and Al-Rubaie, 2022; Altaee et al., 2022), in Hamedan province (Iran) (Gharekhani et al., 2014), and in Korea (Nguyen et al., 2012). Neospora were more common in stray dogs than in domestic dogs, which could be attributed to the consumption of raw meat containing parasite cysts as well as variations in living conditions and welfare (Fanokh and Al-Rubaie, 2022). According to this study, male dogs are more likely to be infected than female dogs, which is consistent with findings in Iraq (Fanokh and Al-Rubaie, 2022; Altaee et al., 2022) and Iran (Adhami et al., 2020). In contrast, these results are in disagreement in Hamedan province (West of Iran) (Gharekhani et al., 2014) and Indonesia (Dwinata et al., 2018). The difference in incidence rates between males and females may be attributed to hormone levels in both sexes and the fact that male dogs are typically found on farms. In addition, male dogs are typically more common than female dogs (Khanmohammadi and Fallah, 2011). The infection rate was lowest in the puppies’ age group, whereas the infection rate was highest in the adult age group. This is consistent with the findings, in Iraq (Fanokh and Al-Rubaie, 2022; Altaee et al., 2022), in the central Zagros region (Iran) (Beki et al., 2023), and in Indonesia (Dwinata et al., 2018), whereas it was different in Fars province, Iran (Khordadmehr et al., 2012). The increased seroprevalence rate in adult dogs indicates postpartum exposure to N. caninum through horizontal transmission and accumulation of additional oocysts excreted by the final host. High infection rates were attributed to a high probability of acquiring Neospora oocysts over time (Hosseininejad and Hosseini, 2011). Table 2. Infection rate of canine neosporosis according to sex.
Table 3. Infection rate of Neosporosis in household dogs according to age.
Table 4. Infection rate of canine Neosporosis as recognized per month.
The highest infection rates were observed in September and October, whereas the lowest rates were observed in November and December. This study’s findings were in agreement with findings from Iraq (Fanokh and Al-Rubaie, 2022, Al-Ubaidy, and Alsultan, 2023), which was recorded in September (25.00%), followed by October (22.22%), July, and August (25.00%). AL-Jumaily and Al-Rubaie (2019) in Iraq studied neosporosis in the local bread chickens and reported an infection rate in July, August, and December (5%). Ibrahim (2013) observed variations in the infection rates in Egypt during all seasons, such as 22.62% in Spring, 21.28% in Autumn, 17.54% in Summer, and 9.83% in Winter. Nasir et al. (2011) observed similar seasonal effects in Pakistan and explained that the parasite in buffaloes is affected by the seasons of the year, with the highest prevalence in the hot season and the lowest in cold season. The limited increase in temperature for a limited degree may be favored for faster sporulation of oocysts of the parasites in the environment. Some epidemiological factors, such as optimal temperature and humidity, accelerate sporulation, enhance the survival of N. caninum oocysts in the climate, and increase the risk of postparturient infection during postparturient periods (Dubey and Schares, 2007; Al-Saadi et al., 2023). ConclusionThis result showed that N. caninum is considered prevalent in Babylon province due to its high incidence in dogs (stray, male, adult) as well as in specific months, which confirms the requirement for routine monitoring of this parasite to avert economic losses across dissemination of its occurrence to farm animals. Stopping horizontal transmission between definitive and intermediate hosts, thereby disrupting the parasite’s life cycle, helps control it. Cattle should only be fed covered food and water to minimize the risk of oocyst infection. AcknowledgmentThe authors extend special thanks and appreciation to the head and all staff members of the Microbiology branch, College of Veterinary Medicine, University of Al-Qadisiyah, for facilitating all the facilities and support available in the department. Conflict of interestThe authors declare no conflict of interest. FundingThis study did not receive any external funding and was independently financed by the authors. Ethical approvalThe study was conducted in compliance with ethical guidelines for animal use and care, as approved by the University of Al-Qadisiyah College of Veterinary Medicine. On November 17, 2024, this study was approved under Approval Number. 4953. Author contributionsThe authors have contributed significantly to the study and validated it for publication. Data availabilityThe manuscript contains all the necessary information. ReferencesAdhami, G.H., Dalimi, A., Hoghooghi-Rad, N. and Fakour, S.H. 2020. Molecular and serological study of Neospora caninum infection in dogs and foxes in Sanandaj, Kurdistan Province, Iran. Arch. Razi. Inst. 75(2), 267–274. Al-Ubaidy, Y. and Alsultan, A., 2023. Using of integrons as biomarker to assess sissemination and diversity of antimicrobial resistance genes in farm animal manure. J. Pure Appl. Microbiol. 17(3), 1708–1714. AL-Jumaily, A.I. and Al-Rubaie, H.M. 2019. Seroprevalence of Neospora caninum in local breed chickens in AL Fallujah District, Iraq. Al-Anbar J. Vet. Sci. 12 (2), 97–104. Al-Qassab, S.E., Reichel, M.P. and Ellis, J.T. 2010. The biological and genetic diversity in Neospora caninum . Divers. 2(3), 411–438. Al-Saadi, M., Al-Sallami, D. and Alsultan, A. 2023. Molecular identification of Anaplasma platys in cattle by nested PCR. Iran. J. Microbiol. 15(3),433–438. Altaee, M.N.K., Sray, A.H. and Ashur, K.H. 2022. First serological investigation of Neospora Caninum in stray dogs, IRAQ. Biochem. Cell. Arch. 22(1), 3007–3012. Amdouni, Y., abedennebi, I., Amairia, S., Abdelkader, A., Chandoul, W. and Gharbi, M. 2022. First molecular detection of Neospora caninum naturally infected slaughtered camels in Tunisia. Vet. Med. Sci. 8(5),2241–2247. Beki, F.H.K., Azizi, H., Kojouri, G.A., Pirali, Y. and Hoseininezhad, M. 2023. Seroprevalence of neosporosis in goats and farm dogs at the central Zagros region (Iran). Iran. Vet. J. 19(3), 41–44. Bjerkas, I., Mohn, S. and Presthus, J. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasit. 70(2), 271–274. Coskun, S.., Aydyn, L. and Bauer, C. 2000. Seroprevalence of Neospora caninum infection in domestic dogs in Turkey. Vet. Rec. 146(22), 649. Dubey, J. and Schares, G. 2006. Review diagnosis of bovine neosporosis. J. Vet. Parasitol. 140(1-2),1–34. Dubey, J.P., Schares, G. and Ortega-Mora, L.M. 2007. Epidemiology and control of Neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20(2), 323–367. Dubey, J.P., Hemphill, A., Calero-Bernal, R. and Schares, G. 2017. Neosporosis in animals. CRC Press. J. Clean. Produc. 142, 1119–1130. Dwinata, M., Oka, I.B., Agustina, K.K. and Damriyasa, M. 2018. Seroprevalence of Neospora caninum in local Bali dog. Vet. World. 11(7), 926–929. Fanokh, M.A. and Al-Rubaie, H.M. 2022. Seroprevalence of Neospora caninum of domestic and stray dogs in Baghdad city. AL-Qadisiyah J. Vet. Medi. Sci. 21(1), 27–33. Gao, X. and Wang, H. 2019. Seroprevalence and risk factors for Neospora caninum infection in dogs in rural northeastern mainland China. Parasite. 26, 32. Gharekhani, J., Tavoosidana, G.R. and Akbarein, H. 2014. Serological study of Neospora caninum infection in dogs and cattle from west of Iran. Comp. Clin. Path. 23(5), 1203–1207. Gharekhani, J., Yakhchali, M. and Berahmat, R. 2020a. Neospora caninum infection in Iran (2004–2020): a review. J. Parasit. Dis. 44(4), 671–686. Gharekhani, J., Yakhchali, M. and Heidari, R. 2022. Molecular detection and phylogenetic analysis of Neospora caninum in various hosts from Iran. Comp. Immunol. Microbiol. Infect. Dis. 80, 101737. Gharekhani, J., Yakhchali, M. and Khaltabadi-Farahani, R. 2020b. Prevalence and risk factors associated to Neospora caninum (Apicomplexa: Toxoplasmatidae) in pet dogs from Hamadan, West of Iran, 2016. Avicenna J. Clin. Microbiol. Infect. 7(1), 22–26. Hosseininejad, M. and Hosseini,F. 2011. Seroprevalence of Neospora caninum and toxoplasma gondii infection in dogs from west and central parts of Iran using two indirect ELISA tests and assessment of associate risk factors. Iran. J. Vet. Res. 12(1), 46–51. Huaman, J.L., Pacioni, C., Doyle, M., Forsyth, D.M., Helbig, K.J. and Carvalho, T.G. 2023. Evidence of Australian wild deer exposure to N. caninum infection and potential implications for the maintenance of N. caninum sylvatic cycle. BMC Vet. Res. 19,153. Ibrahim, H.M. 2013. Seroprevalence of Neospora caninum antibodies in chicken samples from Delta Egypt using a recombinant NcSAG1 protein-based ELISA. Egypt J. Immunol. 20(1), 29–37. Khan, A., Shaik, J.S., Sikorski, P., Dubey, J.P. and Grigg, M.E. 2020. Neosporosis: an overview of its molecular epidemiology and pathogenesis. Engineering. 6(1), 10–19. Khanmohammadi, M. and Fallah, E. 2011.Prevalence of Neospora caninum antibodies in shepherd dogs in Sarab district, East Azarbaijan province, Iran. Afr. J. Micro. Res. 5(28), 5062–5066. Khordadmehr, M., Hosseini, S.M.H., Mohsenifar, E. and Namavari, M.M. and Khordadmehr, S. 2012. Seroprevalence of Neospora caninum in farm and household dogs determined by ELISA. Online J. Vet. Res. 16(4), 172–181. King, J.S., Jenkins, D.J., Ellis, J.T., Fleming, P., Windsor, P.A. and Alapeta, J. 2011. Implications of wild dog ecology on the sylvatic and domestic life cycle of Neospora caninum in Australia. Vet. J. 188(1), 24–33. Nasir, A., Ashraf, M., Khan, M.S., Yaqub, T., Javeed, A., Avais, M. and Akhtar, F. 2011. Seroprevalence of Neospora caninum in Dairy Buffaloes in Lahore District, Pakistan. J. Parasitol. 97(3), 541–543. Nazari, N., Khodayari, M.T., Hamzavi, Y., Raeghi, S., Karamati, S.A., Falahi, S., Bozorgomid, A. and Sajedi, M.T. 2023. Systematic review and meta-analysis of role of felids as intermediate hosts in the life cycle of Neospora caninum based on serological data. Acta. Parasitol. 68(1), 266–76. Nguyen, T., Choe, S., Byun, J., Bumkoh, H., Soolee, H. and Womkang, S. 2012. Seroprevalence of T. gondii and N. caninum in dogs from Korea. Acta. Parasitol. 57(1), 7–12. ã–cal, N., Atmaca, H.T., Albay, M.K., Deniz, A., Kalender, H., Yildiz, K. and Kul, O. 2014. A new approach to Neospora caninum infection epidemiology: neosporosis in integrated and rural dairy farms in Turkey. Turk. J. Vet. Anim. Sci. 38(2), 161–168. Reichel, M.P., Ellis, J.T. and Dubey, J.P. 2007. Neosporosis and Hammondiosis in dogs. J. Small Anim. Pract. 48(6), 308–312. Robbe, D., Passarelli, A., Gloria, A., Di Cesare, A., Capelli, G., Iorio, R. and Traversa, D. 2016. Neospora caninum seropositivity and reproductive risk factors in dogs. Exp. Parasitol. 164, 31–35. Rahman, J. 2015. Brief guidelines for methods and statistics in medical research. 1st edition. New York, NY: Springer Singapore Heidelberg. Villagra-Blanco, R., Angelova, L., Conze, T., Schares, G., Bãrwald, A., Taubert, A. and Wehrend, A. 2018. Seroprevalence of Neospora caninum specific antibodies in German breeding bitches. Parasites Vectors. 11, 96. Yang, J., Ai, J., Qi, T., Ni, X., Xu, Z., Guo, L., Sun, Y., Li, Y., Kang, M. and Li, J. 2022. Toxoplasma gondii and Neospora caninum Infections in Stray cats and dogs in the Qinghai-Tibetan Plateau Area, China. Animals 12(11), 1390. | ||
| How to Cite this Article |
| Pubmed Style Al-taee AHM, Ali MJ. Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Vet. J.. 2025; 15(4): 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 Web Style Al-taee AHM, Ali MJ. Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. https://www.openveterinaryjournal.com/?mno=235147 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.23 AMA (American Medical Association) Style Al-taee AHM, Ali MJ. Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Vet. J.. 2025; 15(4): 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 Vancouver/ICMJE Style Al-taee AHM, Ali MJ. Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 Harvard Style Al-taee, A. H. M. & Ali, . M. J. (2025) Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Vet. J., 15 (4), 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 Turabian Style Al-taee, Ali Hamzah Makttoof, and Mansour Jadaan Ali. 2025. Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Veterinary Journal, 15 (4), 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 Chicago Style Al-taee, Ali Hamzah Makttoof, and Mansour Jadaan Ali. "Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq." Open Veterinary Journal 15 (2025), 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 MLA (The Modern Language Association) Style Al-taee, Ali Hamzah Makttoof, and Mansour Jadaan Ali. "Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq." Open Veterinary Journal 15.4 (2025), 1713-1718. Print. doi:10.5455/OVJ.2025.v15.i4.23 APA (American Psychological Association) Style Al-taee, A. H. M. & Ali, . M. J. (2025) Serological diagnosis of Neospora caninum in domestic and stray dogs in Babylon province, Iraq. Open Veterinary Journal, 15 (4), 1713-1718. doi:10.5455/OVJ.2025.v15.i4.23 |