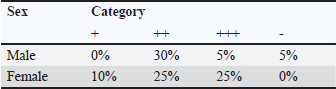| Research Article | ||
Open Vet. J.. 2025; 15(4): 1702-1712 Open Veterinary Journal, (2025), Vol. 15(4): 1702-1712 Research Article Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in IndonesiaMuhammad Hanafiah1*, Amalia Sutriana2, Teuku Zahrial Helmi3, Ruhul Fajriah4, Happy Mulyana4, Cut Nanda Sabila5 and Nadhira Dewantara51Laboratory of Parasitology and Coordinator of One Health Research and Veterinary Economics, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Laboratory of Pharmacology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Laboratory of Biochemistry, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 4Functional Veterinary Medical Laboratory Bacteriology, UPTD Veterinary Laboratory Aceh, Banda Aceh, Indonesia 5Students of the Veterinary Education Study Program, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Muhammad Hanafiah. Department of Parasitology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: hanafi_2015 [at] usk.ac.id Submitted: 26/12/2024 Accepted: 22/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
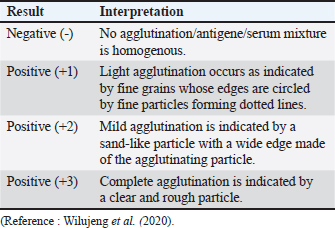
ABSTRACTBackground: Abortion refers to the premature expulsion of a fetus from a dam, typically following the death of the fetus in utero. If fetal death occurs within the first 1–2 months of gestation, it is often classified as “early embryonic death.” Such early losses generally proceed without noticeable clinical signs in the dam, and the termination of pregnancy may go undetected. In 2 months of gestation, fetal death usually results in evident clinical signs, culminating in the expulsion of the fetus and the associated fetal membranes. Aim: The objective of this research was to identify the causative agent of abortion in achenes cows using the rose bengal test (RBT), complement fixation test (CFT), ELISA, and card agglutination test (CATT). The identified causative agents were then mapped according to geographical distribution. Methods: Seventy-four blood serum samples were collected from cattle at the UPTD IBI Saree and 30 samples were collected from the UPTD Lembah Seulawah, Lhut/Jaya, Lhut/Teunom, and Reuleut/Ulim. The blood serum samples were then subjected to a series of serological tests: RBT and CFT, to detect the presence of Brucella (an agent thought to induce abortion). Detection of the infectious bovine rhinotracheitis (IBR) causative agent Bovine Herpesvirus-1 was performed using ELISA, whereas the Toxoplasma seropositive test was performed using CATT. Result: The abortion seropositive examination of cows by RBT and CFT methods achieved the same result 22.1%, with 15.3% in males and 28.8% in females. The Toxoplasma serologic test by CATT revealed a prevalence rate of 1.92% (2/140). The IBR examination with ELISA revealed that 85% of the samples were seropositive, 1.92% were suspected, and 16.35% were seronegative. From this series of examinations, we detected infections of Brucellosis, IBR, and toxins in the samples. Therefore, the infective causative agents of abortion in cows reared in the UPTD IBI Saree and other regions in Aceh are Brucella sp, Toxoplasma, and IBR. The geographical distribution of the data showing abortion causative agents in Acehnese cows are in the UPTD IBI Saree, Lembah Seulawah, Lhut/Jaya, Lhut/Teunom, and Reuleut/Ulim. Conclusion: The Acehnese cows examined by RBT, CFT, ELISA, and CATT were positive at different prevalences. The test results showed that the causative agent of abortion in cattle was not only caused by Brucella but could be caused by other agents or could occur in the same cattle caused by more than one agent simultaneously in one cattle. Keywords: Abortion, Brucella, IBR, Toxoplasma, ELISA, IBR, Cattle. IntroductionAbortion is the expulsion of a fetus before the gestation period is completed; therefore, although organ generation is complete, the fetus cannot survive. Abortion, mainly thought to be caused by infectious agents, is one of the most serious problems facing those who make a living from rearing cows (Givens, 2006; Ortega-Mora et al., 2007). Keshavarzi et al. (2020) added that the presence of health and reproduction problems, such as abortion, poses a threat to the farming industry. Several infectious agents have been reported to cause abortions in cows all over the world, including bacterial, viral, and parasitic agents. Abortion causative agents in cows during the middle to the end of gestation are Brucella spp., Chlamydia spp., Salmonella spp., Campylobacter spp., Listeria monocytogenes, and Coxiella burnetii (Tramuta et al., 2011; Yang et al., 2012; Boukary et al., 2013). Abortion in cows may also be caused by viral infectious agents such as Bovine Herpesvirus-1 (BHV-1), which causes infectious bovine rhinotracheitis (IBR) (Zachary et al., 2016; Sarangi et al., 2021). Meanwhile, parasitic agents like Trichomoniasis, Neosporosis, and Toxoplasmosis are also thought to cause abortion cases that are significantly related to economic loss (Barros et al., 2020). Abortifacient agents are essential to the livestock industry. These losses are caused by infertility, stillbirths, repeated breeding, decreased milk production, meat loss by aborted fetuses, additional treatment management costs, and veterinary services (Givens, 2006; Ortega Mora et al., 2007). Brucellosis is a pervasive zoonotic disease affecting various Brucella species. The disease mainly affects livestock and wildlife and poses significant public health threats, especially in regions with suboptimal hygiene, food safety, and veterinary care standards. Brucellosis infections have been reported in 32 provinces of Mongolia, with the highest prevalence being 40%. As a result, the transfer of infected livestock was limited, and animal trading was forbidden. The clinical symptoms of IBR include issues with the respiratory, nervous, and reproductive systems that may cause abortion and calves death (Foster et al., 2018). Generally, IBR morbidity ranges between 30% and 90%, and the mortality rate is less than 3% (Valas et al., 2019). Infection by Toxoplasma gondii generally occurs in hot-blooded animals and humans (Pudjiatmoko, 2014). Based on secondary data obtained by the UPTD Veterinary Laboratory Aceh Animal Husbandry Agency, the prevalence of Brucellosis using the rose bengal test (RBT) method was 6.49%, and that using the complement fixation test (CFT) method was 4.25%. The prevalence of toxins was 6.6% in 2021 and increased to 27.7% in 2022. Today, it is assumed that livestock declared positive for brucellosis still produce calves, leaving farmers in the dark about which infectious agent is responsible for abortion and whether it is possible for cows to be infected by more than one abortion-causing agent. The geographical distribution of abortion cases in livestock, including those in cattle, showed changes. When a new study focuses on a region declared to be free of cases, the infection is suddenly found in previously free areas. Therefore, a complete understanding of the epidemiology of animal diseases, especially regarding the geographical distribution of causative agents, is very important in implementing appropriate and efficient management measures for abortion from a “One Health” perspective (Dubey, 2022). The objective of this research was to identify the causative agents of abortion in Acehnese cows using the RBT, CFT, ELISA, and card agglutination test (CATT) Tests and to create geographic distribution data for the causative agents. Materials and MethodsSampling methodBlood serum samples were obtained from 104 adult Aceh cows with or without abortion symptoms through random sampling in several areas of Aceh Province, including Sukamakmur/Lembah Seulawa at an altitude of 1,800 above sea level, lowlands 8 m above sea level, Lhut/Jaya Lhut/Teunom, and Reuleut/Ulim. The cow blood was extracted from the jugular vein by a 3-ml syringe without anticoagulant, left to agglutinate, placed inside microtubes, and stored in an icebox before being transferred to a refrigerator. Brucella serologic identification using rapid radiographyThe rose bengal test uses serology to determine the presence of brucellosis by testing serum samples for agglutination against Brucella antigens. Serum samples, reagents, and antigens used were stored at room temperature (22°C + 4oC), afterward 25–30 μl of each serum were pipetted inside the RBT plate or the WHO hemoglobination plate. Brucella antigen was added to the same volume. The antigen and serum were mixed using a glass stirrer or microtip until a round or oval zone with a diameter of around 2 cm was formed, and the mixture was left to agitate for around 4 minutes at room temperature. The formation of globules is considered positive, whereas the absence of clear globules is considered negative (Lounes et al., 2021). After 4 minutes of agglutination, agglutination was observed and scored as follows: +1, +2, +3, or negative, with several criteria as follows:
Brucella serologic identification by complement fixation test methodSamples that were found to be positive in RBT were then submitted to CFT testing, a confirmation test for the presence of antibodies. The serologic test using CFT was modified to a standard procedure. The CFT test result was based on the highest serum dilution that showed a positive reaction, which indicated no lists. The result was determined based on the finding of 50% hemolysis at the highest serum dilution. Serums with CFT titer 1/4 or higher are categorized as positive (Mohan et al., 2016; Ghurafa et al., 2019). Each well of the microdish has a U-shaped bottom, as represented by the A row. In each well, as much as 0,05 ml of serum was padded, including the negative and positive control serum, and the samples were inactivated at 58°C for 30 seconds in a water bath. Every well of the plate except the A row was filled with Barbital Buffer Saline (BBS) dilution liquid 0,025 ml. The serum was diluted in BBS by transferring 0,025 ml of serum from A to B row, and so on up to row H so that the serum dilution obtained would be 1/2, 1/4, 1/8, 1/16, and so on. Each microplate well from C to H was filled with 0 025 ml of antigen. From B to H row, 0,025 ml of complementary liquid was added to each well. To all wells in row B, 0 025-ml dilution liquid was added as the control for anticomplementary activity. The plates were incubated at 37°C for 30 minutes. After incubation, 0 025 ml of erythrocytes sensitized with hemolysin were added to each well from B to H row. The plates were then incubated again at 37°C for 30 minutes while shaking with a shaker. The microplates were spun at 2,000 rpm for 5 minutes and then set aside at 4°C overnight. The subsequent reaction was evaluated using the following criteria:
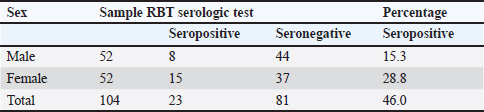
IBR Examination with ELISA MethodSerum samples stored in the refrigerator were taken out and placed at room temperature between 18°C and 26°C. The wash solution was prepared by diluting wash concentrate 10x under a 1:9 ratio or 30 ml wash concentrate: 270-ml distilled water. Samples were then homogenized using a vortex mixer to ensure that the serum did not gravitate at the bottom of the microtube. For all wells 50 μl of wash solution was deposited. In A1 and B1, 50 μl of negative control was added and 50 μl of positive control was added to C1 and D1. The test samples were vertically added as much as 50 μl into the well beginning from the E1 well. The samples were mixed multiple times to homogenize with the wash solution. After adding all samples to the wells, the plates were gently tapped for a while to ensure that none of the samples precipitated. The wells were then covered and incubated at 37°C for 2 hours. After 2 hours of incubation, the wells were removed from the incubator, emptied, and washed five times with 300 μl of a wash solution. Subsequently, 100 μl of the conjugate solution was added to each well. The wells were then incubated at room temperature (18°C–26°C) for 1 hour and uncovered. After 1 hour, the liquid was discarded, and the wells were washed again with 300 μl wash solution five times and then 100 μl of TBM substrate was added to all wells. The wells were incubated at room temperature (18°C–26°C) for 10 minutes and were placed away from direct light. After 10 minutes, 100 μl stop solution was added to each well. Finally, the results were read using an ELISA reader machine at wavelengths of 450 and 650 nm (Manual kit IDEXX). Toxoplasma serologic test by card agglutination testThe test was conducted by first preparing the positive and negative controls. This involved adding one drop of each control and evenly distributing them. Then, tests were performed on each sample. One drop (around 45 μl) of the antigen suspension was dripped on each circle of the plastic card. Test serum or plasma was added in as much as 25 μl to each circle and spread around 1 mm from the edge of the circle using a stirring stick” hich was cleaned using tissue between each circle. Cards for the agglutination test were kept between hands and tilted slowly in a circular motion for 5 minutes to prompt a rotating mixture reaction. The reaction was considered positive for toxins if the color of the sample was the same as that of the positive control. Data analysisThe prevalence examination data for each agent, such as Brucella sp, IBR, and Toxoplasma sp, were analyzed descriptively. ResultsBrucella serologic identification using the rose bengal testThe results of the examination of brucella antibody in 104 cows were collected from several locations in Aceh in June–September 2023 using the RBT method (Fig. 1). Serological analysis revealed several positive results with the discovery of antibodies in blood serum samples. The results of cow serum samples tested by the RBT showed that the Brucella seropositive percentage in Aceh cows was 46%, with details of 15.3% in male and 28.8% in female cows (Table 1).
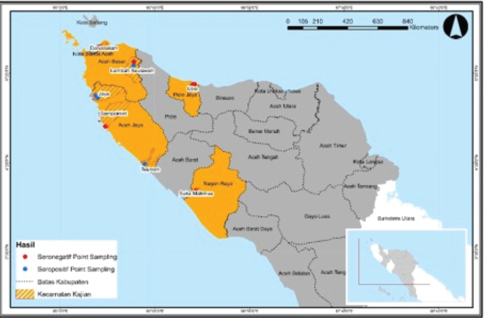
Fig. 1. Cow blood serum sampling was performed at several locations in Aceh. Table 1. The results of brucellosis examination of cows using the UPTD IBI saree rose bengal test.
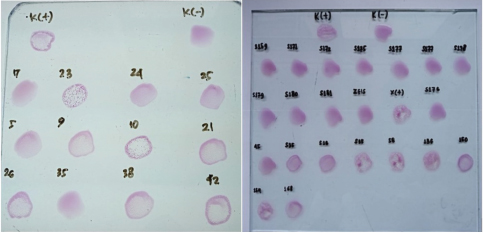
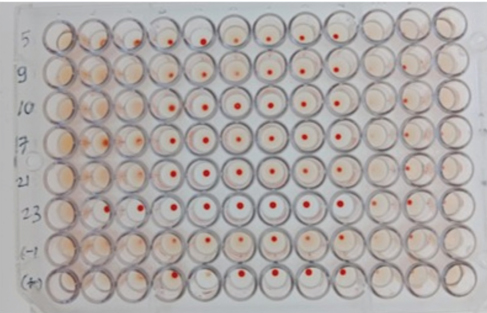
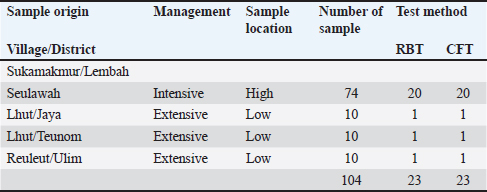
During discussions with farmers conducted as part of this study, many reported that their livestock had experienced multiple miscarriages. However, when attempts were made to conduct diagnostic tests to determine whether the miscarriages were caused by Brucella, Toxoplasma, IBR, Trichomonas, or other agents, testing could not be performed because the animals had already been sold. Livestock that has experienced miscarriages should ideally be reexamined because serological detection of brucellosis often produces false-negative results. Brucellosis typically does not cause clinical symptoms in young animals or nonpregnant females. However, infection in pregnant cows often leads to placentitis, which can result in miscarriages occurring between 5 and 9 months of gestation, corresponding to the second or third trimester (OIE, 2016). The results of blood serum sample examination to detect antibodies using the RBT test in this study revealed six samples with a triple-positive (+++) (Fig. 2), eleven samples with a double-positive (++), and two samples with a single-positive (+) result (Table 2). According to Wilujeng et al. (2020), triple-positive (+++) samples exhibited complete agglutination in a clear liquid that was easily visible, whereas double-positive (++) samples exhibited fine agglutination, resembling sand in a slightly clear liquid. Single-positive (+) samples exhibited light agglutination. The results were considered negative if no agglutination occurred and the serum color remained homogeneous, appearing reddish-purple. Some of the cow samples that were taken for examination included those from animals that had experienced miscarriage, as well as samples from others that had not miscarried but still tested positive for brucellosis. Aborted fetus data in cows is taken as calves that were already dead (Fig. 3). Complement fixation testOut of 104 serum samples initially tested using the RBT, 23 samples were found positive for Brucella antibodies. These samples were further tested using the CFT, which confirmed that all 23 samples were positive (23/81). The results of the examination of 104 cow blood samples by CFT are presented in Table 3 and Figure 4.
Fig. 2. The RBT method examination results for the aceh cow blood samples. Table 2. Rate of positive brucellosis.
Table 3. The number of positive samples from Acehnese cow blood serum samples using the CFT method.
Fig. 3. Cows suffering from abortions.
Fig. 4. CFT-positive result for the aceh cow serum sample. Table 4. The RBT and CFT results in the four Aceh villages and districts are presented below.
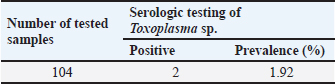
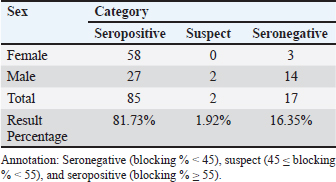
The serological detection results from both the RBT and CFT tests, which were conducted in parallel, were consistent, with 23 samples testing positive. As shown in Table 4, 22.1% of livestock were found to be positive for RBT and CFT was 22.1%. The complement fixation test results indicated a hemolysis rate of 25%, which was classified as positive (+++). This condition is characterized by partial lysis of erythrocytes, resulting in a slightly red liquid with clearly visible sedimentation of erythrocytes. The CFT titers observed in cattle with sample codes 5, 9, 10, 17, 21, and 23 were observed to have antibody titers labeled I/256, 1/256, 1/256, 2/256,/1/256, and 1/1026 (Fig. 3). Based on the results of this study, the elevation factor (highlands vs. lowlands) did not significant impact on positive brucellosis in cattle. In the highlands area (Sukamakmur Valley/Seulawah), 19.2% of cattle were declared positive for brucellosis by RBT and CFT tests, whereas in the lowlands, 71% of cattle were declared positive by RBT and CFT tests (Table 4). Serologic examination by card agglutination TestThe results of the seropositive examination of toxoplasmosis using CATT (Pastorex Toxo) on cow serum samples, the results obtained were 1.92% (Table 5). In comparison, (Elfahal et al., 2013) study who tested samples for T. gondii antibody using ELISA and found that the prevalence of T. gondii antibody in cows was 44.8% (13/29). Toxoplasma infection is highly influenced by the strain of each type of parasite (Halos et al., 2010; Samkange et al., 2023). IBR Examination by ELISASerological testing using ELISA on 20 serum samples collected from 20 cows at UPTD IBI Saree revealed 19 seropositive samples and 1 seronegative sample (Table 6). Based on the ELISA results shown in Table 6, 81.73% of the samples were seropositive, whereas 5% were seronegative. The high seropositivity rate for IBR observed in the samples is consistent with the reported morbidity rate of 30%–90% for IBR (Pudjiatmoko, 2014). According to Straub (1975), the mortality rate of IBR is relatively low, ranging from 0% to 15%, whereas morbidity can reach as high as 100%. In Australia, the prevalence of IBR in the native context ranges between 15% and 96% (AFFA, 2000). Table 5. A number of Acehnese cows serologically tested for toxoplasma.
Table 6. The serum sample results were tested by ELISA.
The interpretation was grouped into three categories: seronegative (blocking % < 45), suspect (45≤blocking % < 55), and seropositive (blocking % ≥ 55). The IBR detection in Aceh cows by ELISA resulted in 85% seropositive, 1.92% suspect, and 16.35% seronegative. The high seropositivity of IBR in the tested samples is directly proportional to the IBR morbidity value, which is 30%–90% (Pudjiatmoko, 2014). DiscussionAs shown in Table 1, 46.0% of samples were seropositive using the RBT method (Fig. 1) are 46.0%. The Brucella seropositive percentage was 15.3% in male and 28.8% in female Aceh cows (Table 2). The Brucella positivity result was based on the RBT test results, which showed clear agglutination (OIE, 2018). Female Acehnese cows positive for Brucella in this research is more than male Aceh cows. This is supported by Tagueha et al. (2020), who found that female cows produce higher amounts of erythritol in the uterus and fetal tissues, such as amniotic fluid, chorioallantois, and placenta. In addition, Dwi et al. (2018) explained that erythritol is a chemical naturally produced by certain microorganisms. Brucella bacteria are highly attracted to this substance, enabling their survival and reproduction. Erythritol is particularly abundant in the endometrium and the spaces between cotyledons. This result is higher when compared to research conducted by Aritayanthi et al. (2023) on cows in Polewali Regency, Mandar, West Sulawesi, which found the percentage of cows positive for brucellosis by RBT and CFT method to be 15.17% (22/145). The results of this study, when compared with the findings of Kustiningsih et al. (2023) in West Java, show a lower prevalence rate of 3.6%. The prevalence rate was 5.10% in the district and 15.77% in West Bandung Regency. Similarly, Winarsih (2018) reported a 3% incidence of brucellosis in Banyuwangi in 2017. The low prevalence observed in this study is likely attributable to the management practices applied to Aceh cattle at the UPTD IBI Saree, which employs a colony breeding system. This system enables the rapid isolation of positive cases from the rest of the livestock, thereby minimizing the spread of infection (Saymima, 2020). High rates of Brucella infection on farms can be influenced by several factors, including livestock population density within enclosures, inadequate supervision of livestock traffic control, cattle age, and management practices (Astari, 2016). According to Suputra et al. (2019), infected cows can spread disease faster because they can interact with healthy cows. The high density of animals in pens increases the risk of disease transmission through direct contact or exposure to infected body fluids, such as saliva, birth fluids, and urine. Poor husbandry practices, inadequate pen sanitation, and management systems that fail to separate sick and healthy animals can contribute to the rapid spread of infection. In addition, the lack of supervision over livestock movements between farms may facilitate disease transmission, especially when new animals are introduced without prior health screening. Adult livestock, particularly pregnant females, are more susceptible to Brucella infection, a common reproductive process. Farms without regular vaccination programs or effective disease control measures have a higher risk of contracting brucellosis. Furthermore, Brucella bacteria can survive in specific environments, such as soil and water, increasing the risk of livestock being exposed to contaminated areas (Astari 2016). In addition to the factors previously mentioned, abortion can also be caused by factors such as bacterial, viral, or parasitic infection, unsuitable living conditions, stress from environmental changes, hormonal disturbance, genetic factors, exposure to toxic substances such as dangerous chemicals, and physical trauma (Sarangi et al., 2021). Based on Table 2 and Figure 2, the percentage of brucellosis infection rate categorized as positive two (++) was higher than that categorized as positive one (+) and positive three (+++). This result differs from a study by Tagueha et al. (2020), which found that the rate of two positive (++) sample infections was 15.22%. The results of this study found six samples positive: three (+++), 11 samples positive two (++), and two samples positive one (+). The results of the RBT test depend on the stage of infection. For instance, low antibody titers in the early stage of infection can affect the accuracy of test results (Al-Gharadi et al., 2011; Priyadarshini et al., 2013). A positive RBT result is associated with the severity of the infection, as the test measures the antibody titer based on the level of agglutination between the antigen and antibody. RBT with a positive (+++) result indicates a high presence of Brucella antibody (Rohyati et al., (2018) therefore samples from cows in earlier stages of infection are more likely to be read as negative due to low antibody concentrations. Therefore, brucellosis is a challenging diagnosis. To date, serological tests, particularly the RBT, have been the preferred method in laboratories across Indonesia due to their relatively high sensitivity and specificity. These findings make RBT a reliable tool for the initial screening of brucellosis in livestock. Brucellosis diagnosis by RBT must be followed with the CFT method or confirmation test to further confirm the diagnosis and increase sensitivity (Kaltungo et al., 2014). According to Muslimin et al. (2017), the sensitivity and specificity of RBT are 88% while the specificity reaches 84%. It is higher than CFT, which only has 84% sensitivity. The complement fixation test is a widely used brucellosis confirmation test. However, this test is difficult to perform and requires a good laboratory facility and skilled staff; however, it is still chosen for the brucellosis test because of its very high specificity (OIE, 2018). Below is a table presenting the percentage of brucellosis infection-positive levels. A rapid and accurate diagnosis is crucial for the effective management and eradication of brucellosis (Gwida et al., 2020). The bacterial culture method is the gold standard for definitive brucellosis diagnosis (Jacques et al., 2020). However, culturing Brucella from field samples is challenging due to several factors: it is time-consuming, poses risks to personal health, and exhibits low sensitivity in most culture procedures. Consequently, serological testing has become the primary diagnostic method for detecting Brucella infections in livestock herds (Tsegay et al., 2017). Complement fixation testAs shown in Table 3, 22.1% of the livestock were found to be positive by RBT and CFT was 22.1%. This result is higher compared with the study conducted by Aritayanthi et al. (2023), whose RBT and CFT examination in cows found that 15.17% (22/145) of the serum was positive for brucellosis. The prevalence of brucellosis in cows in Polewali Regency, Mandar, West Sulawesi was 15.17% based on spot prevalence. The brucellosis disease was spread in six districts, namely Balanipa district with 18.75% prevalence; Campalagian with 7.5%; Luyo with 25.53%; Mapilli with 50%, West Mapilli with 100%, and Polewali with 25%, in line with the positive sample ratio against the total samples for every district used as sample sites. Testing using the CFT method was performed at the Aceh Animal Husbandry Agency. They used CFT to test the 11 samples found to be positive using the RBT test. CFT confirmed the findings of the RBT test, as all 11 samples were found to be positive by CFT. The CFT result showed 25% hemolysis (also called positive (+++ ). Figure 4 displays the geographical distribution of abortion causative agents, namely Brucella sp, IBR and Toxoplasma found in Acehnese cows. They are found in Lembah Seulawah, Lhut/Jaya, Lhut/Teunom, and Reuleut/Ulim. From Table 4, we can see that abortion occurs in cows that are either reared intensively (give a definition for this) or extensively (free-ranging). In the intensive rearing management system, the percentage of positive cases was 27.02%, whereas in the extensive management system, only a 10.0% positive rate was found via RBT and CFT. This result is slightly higher than that reported by Bagenda et al. (2023), who reported that extensive, intensive, and semi-intensive rearing systems resulted in 20%, 4%, and 52% positive results with CFT and 5%, 1%, and 18% negative results with CFT. The location of the herds (highland or lowland regions) influences the results. In the highlands, the positive results from the RBT and CFT tests were 27.02%, while in lowland livestock, it was 10.0%. This finding is supported by the research of Kong et al. (2016), which showed that the rates of Brucella infection were location-dependent. In their study, 5 out of 15 villages had 100% infection rates, with the following prevalence: Fundong (37.5%), Wum (25%), Nso (Jakiri) (12.5%), Metah (12.5%), and Fonta (12.5%). Serologic examination by card agglutination testThe result of the toxoplasma test by CATT was 1.92% (Table 5). In comparison, (Elfahal et al., 2013) study who tested samples for T. gondii antibody using ELISA and found that the prevalence of T. gondii antibody in cows was 44.8% (13/29). Toxoplasma infection is highly influenced by the strain of each type of parasite (Halos et al., 2010; Samkange et al., 2023). IBR examination by ELISAThe end results of the ELISA are presented in Table 6. The interpretation was grouped into three categories: seronegative (blocking % < 45), suspect (45≤blocking % < 55), and seropositive (blocking % ≥ 55). The IBR detection in Aceh cows by ELISA resulted in 85% seropositive, 1.92% suspect, and 16.35% seronegative. The high seropositivity of IBR in the tested samples is directly proportional to the IBR morbidity value, which is 30%–90% (Pudjiatmoko, 2014). High IBR prevalence may be caused by rapid and easy transmission. Virus transmission may occur through contaminated staff, equipment, semen (through natural mating or artificial insemination), embryo transfer, aerosol, or contact with secretion from the respiratory tract, eye, or reproduction tract of infected animals, which facilitates the easy transmission between livestock easy (Chase et al., 2017) IBR seropositive results in unvaccinated animals may be attributable to natural infection, even when there are clinically no respiratory or reproductive symptoms. Clinical IBR in Aceh has never been reported nor found by the Animal Husbandry Agency. IBR clinical symptoms varied from subclinical (latent) to acute, depending on the virus strain and the immunity of the infected cow. The symptoms may include respiratory, nerve, or reproductive problems that end in abortus or calf death (Valas et al., 2019). IBR cases are mostly subclinical (Kaashoek et al., 1996). Subclinical cases are difficult to observe by farmers, even more so for cows living in pastures, which are often left unsupervised by farmers. Cases can be easily observed in cows intensively reared in pens; thus, sick animals can be noticed based on clinical symptoms, pathological changes, and the effect of the disease that lowers weight gain or milk production. IBR disease in Indonesia is one of the 11 diseases that livestock in seed-producing units must be free of. There are several points that must be immediately addressed by the government to manage IBR in Indonesia. These involved (1) vaccine and vaccination, (2) diagnostic tools, (3) IBR-infected semen, (4) biosecurity, (5) revision on the importing requirements of cows from Australia regarding IBR, (6) regulation and IBR control guides, and (7) practical research on IBR. ConclusionThe research results showed that the aceh cows tested by RBT, CFT, ELISA, and CATT produced several positive results. The seropositive results in cows using RBT and CFT were the same (22.1%, that can be detailed for males (15.3%) and females (28.8%. The serologic examination of Toxoplasma by CATT revealed a prevalence rate of 1.92%. IBR examination by ELISA revealed 85% seropositivity, with the suspect values being 1.92% and 16.35%, respectively. This indicates that abortions occurring on livestock may not only be caused by Brucella, but may also be caused by other agents or an event in which livestock was infected by more than one infectious agent. AcknowledgmentsThe authors would acknowledge the UPTD Veterinary Laboratory Aceh Animal Husbandry Service for kind assistance during abortion detection using RBT and CFT tests to detect the presence of Brucella. Detection of the IBR causative agent BHV-1 was performed by ELISA, whereas the Toxoplasma seropositive test was performed by CATT in the Parasitology Laboratory, Universitas Syiah Kuala. Conflicts of interestThe authors declare no conflict of interest. FundingThis research was supported by the Ministry of Research Technology and Higher Education of the Republic of Indonesia through Grant No. 36/UN11.2.1/PT.01.03/PNBP/2023 Date 3 Mei 2023. Authors’ ContributionsMHF and AST: designed the study and wrote the manuscript. TS: Communicated with owners of cow farms, performed CATT Pastorex Toxo tests, and supervised the study. RHF: CFT and RBT methods. HML: ELISA preparation and results. CNS and NDW: Sample collection. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript, and no additional data sources are required. ReferencesAFFA. 2000. Discussion paper on Bovine Herpesvirus 1. Canberra, Australia: Agriculture, Fisheries and Forestry. Al-Gharadi, M.A., Khairani-Bejo, S., Zunita, Z. and Omar, A.R. 2011. Detection of Brucella melitensis in blood samples collected from goats. J. Anim. Vet. Adv. 10(11), 1437–1444. Aritayanthi, P.V., Mahatmi, H. and Dharmawan, N.S. 2023. Seroprevalence and risk factors of brucellosis in cattle of Polewali Mandar regency, west Sulawesi. Vet. Bul. Udayana. 15(5), 991–1001. Astari, D.P. 2016. Faktor-faktor yang mempengaruhi kejadian brucellosis pada ternak sapi di Indonesia. J. Vet. 17(1), 42–48. Bagenda, I., Malaka, R., Muflihanah, Waqiah, South North and Yousof, S.M. 2023. Impact and risk factors of Brucellosis (Brucella abortus) in beef cattle. J. Anim. Sci. 5(2), 66–83. Boukary, A.R., Saegerman, C., Abatih, D. and Alambe dji Bada, R. 2013. Seroprevalence and potential risk factors of Brucella spp. Infection in traditional cattle, sheep, and goats reared in urban, periurban, and rural areas of niger. PLoS One 8(12), e83175. Barros, L.D., Garcia, J.L., Bresciani, K.D.S., Cardim, S.T., Storte, V.S. and Headley, S.A. 2020. A review of toxoplasmosis and neosporosis in water buffalo (Bubalus bubalis). Front. Vet. Sci. 7, 455. Chase, C.C.L., Fulton, R.W., O. Toole, D., Gillette, B., Daly, R. F., Perry, G. and Clement, T. 2017. Bovine herpesvirus-1-modified live virus vaccines for cattle reproduction: Balancing protection with undesired effects. Vet. Microbiol. 206, 69–77. Dubey, J.P. 2022. Clinical toxoplasmosis in zoo animals and its management. Emerg. Ani. Species 2, 1–6. Dwi, W.K., Tyasningsih, W., Praja, R.N., Hamid, I.S., Sarudji, S.O. and Purnama, M.T.E. 2018. Brucella antibody detection on dairy cows in Purwoharjo sub district Banyuwangi regency implemented rose bengal test (RBT) method. Medic. Vet. Jl. 1(3), 142–147. Elfahal, A.M., Elhassan, A.M., Hussien, M.O., Enan, K.A., Musa, A.B. and El Hussein, A.M. 2013. Seroprevalence of Toxoplasma gondii in dairy cattle with reproductive problems in Sudan. ISRN Vet. Sci. 2013, 895165; doi: 10.1155/2013/895165 Givens, M.D. 2006. A clinical, evidence-based approach for identifying infectious causes of infertility in beef cattle. Theriogenology 66, 648–654. Ghurafa, R., Lukman, D. West and Latif, H. 2019. Indirect enzyme-linked immunosorbent assay as a method for tracking brucellosis in dairy cattle. J. Vet. 20(1), 30–37. Priyadarshini, A., Sarangi, L.N. and Palai, T.K. 2013. Brucellosis in cattle and occupationally exposed human beings: a serosurveys in Odisha, India. J. Pure Appl. Microbiol. 7, 3255–3260. Foster, J.T., Walker, F.M., Rannals, B.D., Hussain, M.H., Drees, K.P., Tiller, R.V., Saqib, M. 2018. African lineage Brucella melitensis isolates from Omani livestock. Front. microb. 8, 2702–2716. C., Geers, R., Alliot, A., Escotte-Binet, S., Ajzenberg, D., Dardé, M.L., Durand, B., Boireau, P. and Villena, I., 2010. An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int. J. Parasitol. 40, 193–200. Jacques, I., Lopez-Goni, I., Garcia-Yoldi, D., Marin, C.M., de Miguel, M.J., Munoz, P.M., Blasco, J.M., Grayon, M., Cloeckaert, A., Ferreira, A.C. 2020. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J. Clin. Microbiol. 46, 3484–3487. Kaashoek, M.J., Straver, P.H., Van, R.E., Quak, J. and Oirschot, J.T.V. 1996. Virulence, immunogenicity and reactivation of seven bovine herpesvirus 1.1 strains: clinical and virological aspects. Vet. Record 139, 416–421. Kaltungo, B.Y., Saidu, S.N.A., Sacey, A.K.B. dan Kazeem, H.M.A. 2014. Review on diagnostic techniques for brucellosis Afr. J. Biotechnol. 13(1), 1–10. Keshavarzi, H., Sefidmazgi, A. South, Ghorbani, G.R., Kowsar, R., Razmkabir, M. and Amer, P. 2020. The effects of abortion on the milk production, health, and reproductive performance of Holstein dairy cattle. Anim. Repro. Sci. 217, 106458. Kong, A.T., Nsongka M.V., South M. Itoe, S.M., Hako, A.T. and Leinyuy, I. 2016. Seroprevalence of Brucella abortus in the Bamenda Municipal Abattoir of the Western Highlands, of Cameroon. Greener J. Agri. Sci. 6(8), 245–251. Kustiningsih, H., Sudarnika, E., Saleh, A., Basri, C. and Sudarwanto, M. 2023. Role of dairy farmers in surveillance programs and brucellosis control in Bogor regency. J. Vet. Sci. 41(1), 51–62. Lounes, N., Melzer, F., Sayour, A.E., Maamar, H.T., Rahal, K., Benamrouche, N., Lazri, M., Bouyoucef, A., Hendam, A., Neubauer, H. and El-Adawy, H. 2021. Identification, geographic distribution and risk factors of Brucella abortus and Brucella melitensis infection in cattle in Algeria. Vet. Microbiol. 254, 109004. Muslimin, L., Bangsawan, A.T.danUtami, S. 2017. Brucellosis identification on farmers in Pinrang district. Nusa. Med. Sci. J. 1(2), 33–37. Mohan, A., Saxena, H.M. and Malhotra, P. 2016. A comparison of titers of anti-Brucella antibodies in naturally infected cattle and healthy vaccinated cattle by standard tube agglutination test, microtiter plate agglutination test, indirect hemagglutination assay, and indirect enzyme-linked immunosorbent assay. Vet. World 9, 717–722. OIE. (2018). Brucellosis (Brucella abortus, B. melintensis and B. suis). (Infection with Brucella abortus and B. suis). Paris, France: OIE, 355–398. Ortega-Mora, L.M., Gottstein, B., Conraths, F.J. and Buxton, D. 2007. Ruminants: guidelines for diagnosis and control in farm protozoal abortion. Wallingford, UK: CAB International. Priyadarshini, A., Sarangi, L.N. and Palai, T.K. 2013. Brucellosis in cattle and occupationally exposed human beings: a serosurveys in Odisha, India. J. Pure Appl. Microbiol. 7, 3255–3260. Pudjiatmoko. 2014. The manual of mammalian diseases, sub-directorate for animal disease observation, directorate of animal health, directorate general of animal husbandry and animal health. Jakarta, Indonesia: Ministry of Agriculture. Rohyati, E., Toelle, N.N. and Hau, E.R. 2018. Brucellosis screening test on cattle at the Oeba RPH, Kupang City using the RBT test. Partner J. 23(2), 705–709. Samkange, A., Chitanga, S., Tjipura-Zaire, G.N., Mutjavikua, V.G. Smith, J.W. Neves, L. and Matjila, T. 2023. Seroprevalence and associated risk factors for Toxoplasma gondii infection of goats and sheep in the Khomas region of Namibia. J. South Afr. Vet. Assoc. 94, 123–129; doi: 10.36303/JSAVA.548 Saymima, A. 2020. Analisis epidemiologi brucellosis di Asia Tenggara. J. Epidemiol. Trop. 15(2), 123–130 Suputra. G. West K., I P. Sampurna, T. and Agustina, K.K. 2019. Clustering of Bali Cattle Herd Management in Simantri, Badung Regency, Bali. Udayana Vet. Bul. 11(2), 128–135. Sarangi, L.N., Tharani, N., Polapally, S., Rana, S.K., Thodangala, N., Bahekar, V.S., Prasad, A., Reddy, R.V.C., Surendra, K.S.N.L.,Gonuguntla,H.N.,Ponnanna, N.M.dan Sharma,G.K. 2021. Infectious bovine abortions: observations from an organized dairy herd. Braz J Micro. 52(1), 439–448. Tagueha, A.D., Souhoka, D.F. and Leklioy, B.B. 2020. Prevalence of brucellosis reactor in a cattle population in Letti District, Southwest Maluku Regency. Filiia Cendikia Sci. J. 5(2), 54–60. Tramuta, C., Lacerenza, D., Zoppi, S., Goria, M., Dondo, A., Ferroglio, E. and Rosati, S. 2011. Development of a set of multiplex standard polymerase chain reaction assays for identifying infectious agents in aborted bovine clinical samples. J. Vet. Diagn. Investig. 23(4), 657–664. Tsegay, S.M., Kansale, C. and Goll, South P. 2017. An analysis of early childhood education policy in China. Asia-Pacific J. Res. Early Child Educ. 11(1), 69–84. Valas, S., Brémaud, I., Stourm, S., Croisé, B., Mémeteau, S., Mbot, D.N., dan Tabouret, M. 2019. Improvement of the eradication programme for infectious bovine rhinotracheitis in France based on the serological monitoring of singleton reactors in certified BoHV1-free herds. Preve. Vet. Med. 171(1), 104743. Wilujeng, E., Suarno, R.N., Hamid, I.S., Yunita, M.N. and Wibawati, P.A. 2020. Sero detection of brucellosis using the rose bengal and complement fixation test methods in dairy cows in Banyuwangi. Medic. Vet. J. 3(2), 188–195. Winarsih, W.H. 2018. Livestock diseases need to be monitored in relation to food safety. Cakrawala J. 12(2), 208–221. Yang N., Cui X., Qian W., Yu S. and Liu Q. 2012. Survey of nine abortifacient infectious agents in aborted bovine fetuses from dairy farms in Beijing, China, by PCR. Acta. Vet. Hung. 60, 83–92. Zachary, J.F. 2016. Pathologic basis of veterinary disease disease expert consult - E-BOOK. Philadelphia, PA: Elsevier Health Sciences. | ||
| How to Cite this Article |
| Pubmed Style Hanafiah M, Sutriana A, Helmi TZ, Fajriah R, Mulyana H, Sabila CN, Dewantara N. Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Vet. J.. 2025; 15(4): 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 Web Style Hanafiah M, Sutriana A, Helmi TZ, Fajriah R, Mulyana H, Sabila CN, Dewantara N. Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. https://www.openveterinaryjournal.com/?mno=234746 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i4.22 AMA (American Medical Association) Style Hanafiah M, Sutriana A, Helmi TZ, Fajriah R, Mulyana H, Sabila CN, Dewantara N. Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Vet. J.. 2025; 15(4): 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 Vancouver/ICMJE Style Hanafiah M, Sutriana A, Helmi TZ, Fajriah R, Mulyana H, Sabila CN, Dewantara N. Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Vet. J.. (2025), [cited January 24, 2026]; 15(4): 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 Harvard Style Hanafiah, M., Sutriana, . A., Helmi, . T. Z., Fajriah, . R., Mulyana, . H., Sabila, . C. N. & Dewantara, . N. (2025) Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Vet. J., 15 (4), 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 Turabian Style Hanafiah, Muhammad, Amalia Sutriana, Teuku Zahrial Helmi, Ruhul Fajriah, Happy Mulyana, Cut Nanda Sabila, and Nadhira Dewantara. 2025. Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Veterinary Journal, 15 (4), 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 Chicago Style Hanafiah, Muhammad, Amalia Sutriana, Teuku Zahrial Helmi, Ruhul Fajriah, Happy Mulyana, Cut Nanda Sabila, and Nadhira Dewantara. "Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia." Open Veterinary Journal 15 (2025), 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 MLA (The Modern Language Association) Style Hanafiah, Muhammad, Amalia Sutriana, Teuku Zahrial Helmi, Ruhul Fajriah, Happy Mulyana, Cut Nanda Sabila, and Nadhira Dewantara. "Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia." Open Veterinary Journal 15.4 (2025), 1702-1712. Print. doi:10.5455/OVJ.2025.v15.i4.22 APA (American Psychological Association) Style Hanafiah, M., Sutriana, . A., Helmi, . T. Z., Fajriah, . R., Mulyana, . H., Sabila, . C. N. & Dewantara, . N. (2025) Epidemiological study of the serology and geographic distribution of abortion causative agents in aceh cows in Indonesia. Open Veterinary Journal, 15 (4), 1702-1712. doi:10.5455/OVJ.2025.v15.i4.22 |