| Research Article | ||
Open Vet. J.. 2025; 15(4): 1695-1701 Open Veterinary Journal, (2025), Vol. 15(4): 1695-1701 Research Article Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approachDhirgo Adji1*, Bambang Sutrisno2, Artina Prastiwi1, Dito Anggoro1 and Hastari Wuryastuti31Department of Surgery and Radiology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 2Department of Pathology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 3Department of Internal Medicine, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia *Corresponding Author: Dhirgo Adji. Department of Surgery and Radiology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia. Email:dhirgo.aji [at] ugm.ac.id Submitted: 24/12/2024 Accepted: 22/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
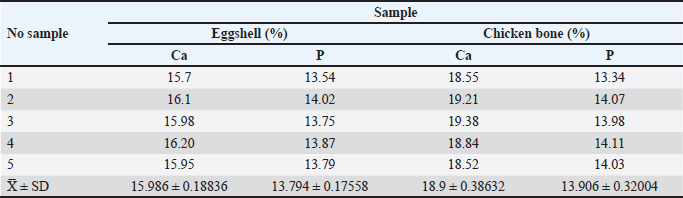
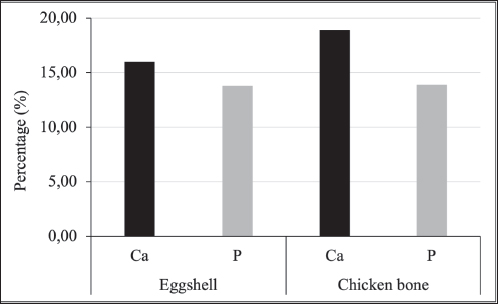
AbstractBackground: Chicken eggshells and bone waste are waste materials rich in calcium (Ca) and phosphorus (P), and they are often discarded despite their potential value. These materials are promising precursors for hydroxyapatite (HA), a biocompatible material structurally similar to natural bone that is widely used in biomedical applications. Aim: To evaluate the Ca and P contents of broiler chicken eggshells and bone waste and to synthesize HA from these waste materials. The results highlight the potential of broiler chicken bones as a sustainable source of HA, particularly for veterinary bone graft applications. Methods: Eggshell and chicken bone waste samples were collected from restaurants. The samples were washed, cleaned, dried, and heated at 800°C to produce calcite. The resulting calcite suspension was synthesized by heating it at 70°C and gradually mixing it with an ammonium phosphate ((NH4)2HPO4) suspension over 30 minutes. During the mixing process, the solution pH was maintained above 9. The HA synthesized from eggshells was then analyzed using titration to determine Ca content and spectrophotometry to measure P content. Results: The analysis revealed that eggshells contained 15.986 ± 0.188% Ca and 13.794 ± 0.176% P, whereas chicken bones had higher Ca and P levels, with 18.9 ± 0.386% Ca and 13.906 ± 0.320% P. The average Ca:P ratio was 1:1.158 for eggshells and 1:1.359 for chicken bones. Conclusion: This study demonstrates the feasibility of converting poultry industry waste into valuable biomaterials to promote sustainable practices. The higher Ca and P levels observed in chicken bones than in eggshells may offer superior potential for treating pet fractures. Keywords: Calcium, Chicken bone waste, Eggshell, Hydroxyapatite, Phosphorus. IntroductionSmall animal fractures are prevalent in Indonesia, posing a significant health concern for domestic dogs and cats. These injuries, often resulting from trauma, can lead to complex fractures with severe comminution, making the anatomical alignment of bone fragments challenging (Scott and McLaughlin, 2007). Bone defect healing is a complex process involving the reconstruction of bone tissue, and it typically involves multiple procedures within an overlapping timeline (Blumenfeld, 2002). While most bone defects can heal spontaneously under favorable physiological and environmental conditions because of the inherent regenerative ability of bone, the healing process is often slow. This is primarily due to reduced blood flow to the fracture site and insufficient Ca and P levels to strengthen and mineralize the new bone (Li et al., 2010). Hydroxyapatite (HA), a biocompatible ceramic material with the chemical formula Ca10(PO4)6(OH)2, has been widely used in medical applications as a bone filler and scaffold, aiding in bone formation and promoting healing (Peon et al., 2004; Amaral et al., 2023). HA has been proven to be particularly useful in treating pet fractures in veterinary medicine. The ability of HA to promote bone healing and its biocompatibility with animal tissue make it an attractive option for grafting materials in orthopedic procedures (Fitzpatrick and Guthrie, 2018). The application of HA as a bone substitute can significantly reduce healing time and improve outcomes, especially in complex or severe fracture cases. Given these properties, HA is widely regarded as one of the most effective materials for bone grafting, offering a solution to many of the challenges faced in bone repair and regeneration (Oki et al., 2001; Munoz et al., 2009; Pratama et al., 2023). Although HA has been synthesized from various calcium- and phosphorus-rich sources, including animal bones, crab shells, and chicken eggshells (Rocha et al., 2005), few studies have explored broiler chicken bones and eggshells as sustainable raw materials for HA production. The stoichiometric Ca/P ratio of HA is 1.67, which is chemically identical to the mineral component of bone, making it highly suitable for bone repair (Othsuki et al., 2009; Liu et al., 2012; Santos et al., 2019). Its osteoconductive properties facilitate bone integration and healing, making it valuable for veterinary orthopedic procedures (Fakoor et al., 2013). Despite its recognized potential, there remains a need to identify the most efficient precursors for HA synthesis between broiler chicken bones and eggshells, particularly in veterinary applications. The primary objective of this study was to investigate the synthesis of HA from broiler chicken eggshells and chicken bone waste sourced from the Yogyakarta Special Territory, using a calcination process at 800°C. We hypothesized that chicken bones, a more direct source of mineralized tissue, will exhibit higher Ca and P levels compared with eggshell samples, making them more efficient for HA synthesis. By comparing these two waste materials, the author aims to determine which offers the most promising source of HA for veterinary bone repair while promoting sustainable resource utilization. Materials and MethodsCollection and preparation of materialsEggshells and chicken bone materials were collected from five restaurants in the Special Region of Yogyakarta, Indonesia. A total of 1 kg of chicken eggshells and broiler chicken bone waste was gathered from each stall (Fig. 1). To ensure decontamination and uniformity, visible debris was removed, and the samples were washed with soapy water, rinsed with distilled water, and soaked in 70% ethanol for 10 minutes. After a final rinse with sterile distilled water, the samples were air-dried for 3 days. The cleaned eggshells and bones were separately coded (1–5) and placed in five containers. The eggshells were blended to form coarse particle fragments, whereas the chicken bones were cut into small pieces (0.5–1 cm) using bone-cutting scissors. Both eggshell and bone fragments were ground, weighed, and dried at 110°C for 2 hours, followed by 12 hours at room temperature. Calcination and precursor preparationThe dried eggshells and bones were then calcined in a furnace (Heraeus Instrument UG/FN/004016/New Model) at 800°C for 3 hours, with a subsequent cooling period of 12 hours. This temperature was selected based on literature and preliminary studies indicating its effectiveness in removing organic matter while preserving the crystalline integrity of HA (Wibisono et al., 2018). No additional trials at other temperatures were conducted. The next step involved preparing Ca and phosphate precursor solutions. The Ca precursor solution was prepared by weighing the calcined shell and bone powders, calculating the required stoichiometric amounts, and dissolving the calcium oxide in 10 ml of 65% HNO3 solution (Thermo Fisher Scientific, Waltham, MA, USA). The solution was then diluted with distilled water (Waterone, OneMed, Indonesia) to 100 ml and homogenized. Ammonium hydroxide (NH4OH) (Sigma-Aldrich, Merck, Germany) was added until the pH reached 10, followed by a buffer. The phosphate precursor solution was prepared by dissolving (NH4)2HPO4 crystals in 10 ml of distilled water, homogenizing the mixture, and diluting it with additional distilled water to reach a total volume of 10 ml. Synthesis of HAHA synthesis was performed by slowly adding phosphate into the Ca solution at 70°C and stirring at 300 RPM using a magnetic stirrer on a hot plate (IKA Works, Staufen, Germany). The stirring was continued for 30 minutes until the phosphate solution was completely reacted. The gradual addition of phosphate was essential to prevent rapid precipitation, which could lead to non-uniform particle size and phase impurities (Ismail et al., 2021). It is crucial to sustain a pH above 9, implemented with the addition of NH4OH, to promote the development of stoichiometric HA and reduce secondary phases such as calcium phosphate (Ca3(PO4)2) or calcium oxide (CaO) (Ortiz et al., 2020). The mixed solution was then allowed to cool and age for 24 hours, allowing precipitates to form. The precipitate was filtered using Whatman 42 filter paper (Cytiva, Marlborough, MA, USA), washed with distilled water to remove residual ammonium nitrate, and dried at 110°C for 2 hours. The resulting material was then calcined in a furnace at 800°C for 3 hours and sieved to obtain uniformly sized particles (Haryati et al., 2021). Characterization of HAThe processed material was then analyzed to determine its HA characteristics by measuring the calcium-to-phosphorus (Ca:P) ratio. A 2.5-g sample was dissolved in 25 ml of a 7:3 mixture of HNO3 and H2O2, heated in boiling water, and then cooled at room temperature. The phosphorus-binding (PB) reagent was prepared before phosphorus (P) measurement by dissolving 3.8 g of ammonium molybdate in 300 ml of distilled water and 50 g of boric acid in 300 ml of hot distilled water. Subsequently, 75 ml of concentrated hydrochloric acid was added, and the solution was brought to a final volume of 1 l using distilled water in a 1,000 ml volumetric flask. The phosphorus-reactive color (PC) reagent was prepared by dissolving 0.1 g of vitamin C (ascorbic acid) in 100 ml of distilled water. For P content measurement, a 5 ml aliquot was pipetted, and 5 ml of PB reagent and PC reagent were added, following the method of Boltz and Howel (1978). The absorbance was measured using a UV-2600 spectrophotometer (Shimadzu Corporation, Japan) at 650 nm. A standard curve was generated using KH2PO4 solutions (0–50 mg/L), and all samples were measured in triplicate to ensure accuracy. For calcium (Ca) content measurement, 1 g of HA was dissolved in 10 ml of concentrated HCl and analyzed using atomic absorption spectroscopy (PinAAcle 900T, PerkinElmer, Waltham, MA, USA) at 422.7 nm (Melgar et al., 2021). Standard solutions of Ca(NO3)2 (0–100 mg/l) were calibrated, and each sample was analyzed in triplicate. The relative SD was maintained below 5% to ensure precision. Statistical analysisCa and P levels from eggshell and chicken bone samples were analyzed using t-tests in SPSS 27 (IBM SPSS Statistics, version 27, SPSS Inc, Chicago, IL, USA). Data normality was assessed using the Shapiro-Wilk test, and homogeneity of variance was verified using Levene’s test. If assumptions were violated, then the Mann–Whitney U test was used as a nonparametric alternative. Ethical approvalNot needed for this study. ResultsThe Ca and P contents in broiler chicken eggshells and chicken bone waste were analyzed to evaluate their potential as raw materials for HA synthesis. The compositional assessment focused on determining the levels of Ca and P in both materials and calculating their calcium-to-phosphorus (Ca:P) ratios, as these are critical indicators of suitability for HA production. The findings highlight the distinct compositional characteristics of the two sources, providing valuable insights into their comparative chemical profiles and potential applicability in biomedical material synthesis. Calcium and phosphorus levelsThe Ca and P levels in broiler chicken eggshells and chicken bone samples were measured and are presented in Table 1. The eggshell samples contained an average Ca content of 15.986 ± 0.188%, with P levels averaging 13.794 ± 0.176%. In comparison, the chicken bone samples had higher Ca levels, averaging 18.9 ± 0.386%, and P levels averaging 13.906 ± 0.320%. Statistical analysis using a t-test showed significant differences in both Ca and P levels between eggshells and chicken bones (p < 0.05) (Fig. 2), with higher concentrations observed in chicken bones. Table 1. Comparison of calcium (Ca) and phosphorus (P) levels in eggshells and chicken bones.
Fig . 1. Laboratory procedures for synthesizing HA from eggshells and chicken bones. A–D illustrates the stepwise processing of eggshells, from raw material preparation (A) to grinding (B), calcination (C), and the final HA product (D). E–H depicts the same stages for chicken bones, highlighting differences in raw material handling and processing.
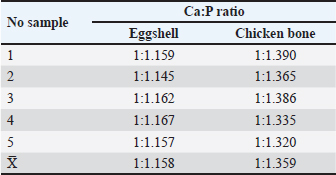
Fig. 2. Bar graph comparing Ca and P levels in synthesized HA from eggshells and chicken bones. Ca:P ratioThe Ca:P ratios of the eggshell and chicken bone samples were calculated and summarized in Table 2. The average Ca:P ratio of the eggshell samples was 1:1.158. 1:1.158, with individual ratios ranging from 1:1.145 to 1:1.167, indicating a relatively uniform distribution. The Ca:P ratio was higher in chicken bone samples, with an average of 1:1.359, with individual values ranging from 1:1.320 to 1:1.390. The higher Ca:P ratio in chicken bones suggests a higher Ca content relative to P than in eggshells. These differences in Ca:P ratios reflect the compositional variations between the two biological sources and may influence their performance in HA synthesis. DiscussionThe synthesis of HA from broiler chicken eggshells and chicken bone waste via calcination at 800°C demonstrated the feasibility of using these biomaterials as sustainable sources for HA production. Notably, chicken bone-derived HA had higher Ca and P content than eggshell-derived HA, which is consistent with our hypothesis. These findings underscore the potential of chicken bones as a more efficient raw material for HA synthesis, with promising applications in bone repair and regeneration in veterinary medicine. Furthermore, the comparison highlights the importance of selecting optimal raw materials to maximize HA yield and quality. The observed differences in Ca and P levels between eggshells and bones can be attributed to their distinct biological functions. Eggshells primarily serve as protective barriers and are rich in calcite, a crystalline form of calcium carbonate, whereas bones are composed predominantly of HA and other calcium phosphate-based minerals (Betancourt and Cree, 2017; Hahn et al., 2017). The compositional disparity is evident in the slightly higher Ca levels in bones, which are necessary for mechanical strength and structural integrity (Opris et al., 2020). Such levels are expected because 98% of Ca is stored in bones, whereas Ca and P are distributed to the eggshell only during production (Pelicia et al., 2009). In contrast, eggshells’ relatively lower Ca content may be due to their thinner and less mineralized structure than bone tissue (Kattimani and Lingamaneni, 2019). Compared with other studies, broiler chicken bone’s Ca and P levels are consistent with previously reported values for poultry bone-derived HA, reinforcing their suitability as raw materials (Kim et al., 2017; Li et al., 2020). However, the Ca levels in eggshells were slightly lower than those reported in other studies on calcium carbonate-rich materials (Brun et al., 2013; Bartter et al., 2018). This discrepancy may be due to regional variations in diet, genetics, or environmental factors influencing mineral deposition in broiler chickens from the Yogyakarta Special Territory. Table 2. Comparison of Ca and P ratio.
The Ca:P ratio is a critical determinant of the synthesis of HA. This study calculated the Ca:P ratios for broiler chicken eggshells and bones as 1:1.158 and 1:1.359, respectively, which were lower than the ideal value (1.67). In contrast, a notable study reported an average bone Ca:P ratio of 1.64 ± 0.14, closely aligning with the theoretical stoichiometric value (Mahamid et al., 2008). These deviations suggest potential compositional differences that influence the quality of the synthesized HA. A Ca:P ratio lower than 1.67 can affect the synthesized material’s crystallinity, mechanical properties, and biocompatibility. Specifically, a lower ratio may lead to calcium-deficient HA (CDHA) formation, which has higher solubility and faster degradation rates than stoichiometric HA (Wu et al., 2021). While this property might benefit applications requiring rapid resorption, such as bone grafts, it could disadvantage load-bearing implants where durability and strength are critical. Interestingly, the observed Ca:P ratios also align with natural variations reported in other studies on poultry-derived materials. The diet, environment, and genetic background of broiler chickens can influence the mineral composition of their bones and eggshells (Han et al., 2016; Li et al., 2020; Lee et al., 2023). For example, diets deficient in P or with an imbalanced Ca:P ratio can lower P deposition in skeletal tissues and eggshells, potentially contributing to the observed ratios (Li et al., 2020). Calcination at 800°C is crucial for removing organic materials while preserving the mineral phase required for HA synthesis. At 800°C, significant weight loss was observed in the eggshells and bone samples due to the elimination of volatile organic components, resulting in whiter, more brittle materials, indicative of successful organic residue removal (Chraibi et al. 2016). A study on tilapia fish bones indicated that calcination at 800°C eliminated collagen and organic molecules, resulting in a composition primarily of HA with sharper and more significant X-ray diffraction peaks, signifying enhanced mineral crystallinity (Fara et al., 2015). Other investigations also found that calcination at 700°C–900°C alters the microstructure of HA in cattle bone, significantly affecting particle shape and porosity, which are crucial for enhancing bioactivity (Aissa and Othmani, 2023). In contrast, excessively high temperatures (>1,000°C) can decompose HA into undesired phases, such as tricalcium phosphate or calcium oxide, compromising its structural integrity and biological properties (Lima et al., 2006; Hossain et al., 2021; Razak, 2023). The calcination process also significantly influences the Ca:P ratio, which is a crucial parameter for HA synthesis (Fara et al., 2015). While raw materials exhibited Ca:P ratios below the stoichiometric value of 1.67, calcination helped refine this ratio by reducing interference from non-phosphatic organic compounds. However, minor deviations from the ideal ratio were observed, which can be attributed to precursor selection, reaction conditions, and thermal treatment. These variations may affect the physicochemical properties and bioactivity of the final product. Additional processing steps, such as ionic doping, can enhance the stability and bioactivity of the resulting material, whereas further calcination under controlled conditions may improve the phase purity and crystallinity. Moreover, calcination at 800°C is energy-efficient, cost-effective, and feasible when compared with conventional furnaces, making it scalable for industrial applications. Upcycling broiler chicken eggshells and bone waste supports the sustainable recycling of agricultural by-products for biomedical and industrial use. The current study has several limitations that should be addressed. First, the Ca:P ratios in eggshells and bones were lower than the ideal stoichiometric value, which could affect the crystallinity, mechanical properties, and biocompatibility of the synthesized HA. Further optimization of the calcination process or additional chemical treatments may be necessary to achieve the desired Ca:P ratio and enhance the material properties. Additionally, regional variations in the diet, genetics, and environment of broiler chickens from the Yogyakarta Special Territory may influence the mineral composition, limiting the generalizability of the results to other regions. ConclusionChicken bone-derived HA exhibited higher Ca and P content than eggshell-derived HA, suggesting that chicken bones are a more efficient raw material for HA synthesis. In veterinary practice, synthesized HA holds potential applications in bone grafts, dental implants, and orthopedic coatings, offering a cost-effective alternative to commercial HA. However, scalability challenges remain, particularly in terms of ensuring consistent material quality and optimizing production costs. Future research should focus on refining synthesis methods, assessing in vivo biocompatibility, and exploring the mechanical performance of HA-based implants to facilitate clinical adoption in veterinary orthopedics. AcknowledgmentsThe authors thank Sarah Alvania Joseph, DVM, M.Sc., from Nusa Cendana University, Kupang, East Nusa Tenggara, for her invaluable assistance in sample collection and laboratory work. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe Faculty of Veterinary Medicine, Gadjah Mada University, Indonesia, funded the study through the Postgraduate Collaboration Research Program 2022 (Grant No: 1705/UN.1/FKH/HK4/2022). Authors’ contributionsDhirgo Adji conceptualized the study, designed the experimental approach, and contributed to manuscript preparation. Bambang Sutrisno and Dito Anggoro conducted the calcination experiments, collected the data, and analyzed the data. Artina Prastiwi interpreted the data, and Hastari Wuryastuty contributed to the statistical analysis and assisted with manuscript preparation. All authors have approved the final version of the manuscript. Data availabilityAll data supporting the findings of this study are presented in the manuscript. ReferencesAissa, A. and Othmani, M. 2023. Heavy metal removal using nano-hydroxyapatite extracted from cattle bones. Chem. Select 8(13), e202300165. Amaral, M.B.D., Viana, R.B., Viana, K.B., Diagone, C.A., Denis, A.B. and Plepis, A.M.D.G. 2023. Bone regeneration in critica-sizede calvarial defects using synthetic hydroxyapatite and mineralized bovine tendon base materials: a microcomputed tomography analysis. Acta Sci. Technol. 46(1), e63001. Bartter, J., Diffey, H., Yeung, Y.H., O’Leary, F., Hasler, B., Maulaga, W. and Alders, R. 2018. Use of chicken eggshell to improve dietary calcium intake in rural sub-saharan Africa. Matern. Child. Nutr. 14(Suppl 3), e12649. Betancourt, N. and Cree, D. 2017. Mechanical properties of poly (lactic acid) composites reinforced with caco3 eggshell based fillers. MRS Adv. 2(47), 2545–2550. Blumenfeld, I., Srouji, S., Lanir, Y., Laufer, D. and Livne, E. 2002. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp. Gerontol. 37(4), 553–565. Boltz, D.F. and Howel, J.A. 1978. Colorimetric determination of nonmetals. New York, NY: John Wiley & Sons; pp: 120–125. Brun, L., Lupo, M., Delorenzi, D., Loreto, V.D. and Rigalli, A. 2013. Chicken eggshell as a suitable calcium source at home. Int. J. Food Sci. Nutr. 64(6), 740–743. Chraibi, S., Moussout, H., Boukhlifi, F., Ahlafi, H. and Alami, M. 2016. Utilization of calcined eggshell waste as an adsorbent for the removal of phenol from aqueous solution. J. Encap. Adsorp. Sci. 6(4), 132–146. Fakoor, M., Sarrafan, N. and Morteza, F. 2013. Assessment of prophylactic bone grafting effect on the union of open tibial fracture. Pak. J. Med. Sci. 29(1), 112–114. Fara, A.N.K.A., Pragash, G. and Abdullah, H.Z. 2015. Effect of calcination on the properties of hydroxyapatite from tilapia fish bones. Adv. Mater. Res. 1125, 474–478. Fitzpatrick, N. and Guthrie, J. 2018. Hemipelvic and proximal femoral limb salvage endoprosthesis with tendon ongrowth in a dog. Vet. Surg. 47(7), 963–969. Hahn, E.N., Sherman, V., Pissarenko, A., Rohrbach, S.D., Fernandes, D.J. and Meyers, M.A. 2017. Nature’s technical ceramic: the avian eggshell. J. R. Soc. Interface. 14(126), 20160804. Han, J., Wang, J., Chen, G., Qu, H., Zhang, J., Shi, C., Yan, Y. and Cheng, Y. 2016. Effects of calcium to nonphytate phosphorus ratio and different sources of vitamin d on growth performance and bone mineralization in broiler chickens. R. Bras. Zootec. 45(1), 1–7. Haryati, E., Dahlan, K., Togibasa, O. and Dahlan, K. 2021. The influence of calcination temperature on calcium content in mangrove crab shells (Scylla serrata) from merak. J. Phys. Conf. Ser. 1940, 012024. Hossain, M.S., Mahmud, M., Sultana, S., Bin Mobarak, M., Islam, M.S. and Ahmed, S. 2021. The combined effect of particle size of the source materials and calcination temperature on the direct synthesis of hydroxyapatite. Open Sci. 8(9), 210684. Ismail, R., Laroybafih, M.B., Fitriyana, D.F., Nugroho, S., Santoso, Y.I., Hakim, A.J., Mulqi, M.S. and Bayuseno, A.P. 2021. The effect of hydrothermal holding time on the characterization of hydroxyapatite synthesized from green mussel shells. J. Adv. Res. Fluid Mech. Therm. Sci. 80(1), 84–93. Kattimani, V. and Lingamaneni, K. 2019. Natural bioceramics: our experience with changing perspectives in the reconstruction of maxillofacial skeleton. J. Korean Assoc. Oral Maxillofac. Surg. 45(1), 34–42. Kim, J.H., Jung, H., Pitargue, F.M., Han, G.P., Choi, H.S. and Kil, D.Y. 2017. Effects of dietary calcium concentrations in low nonphytate phosphorus diets containing phytase on growth performance, bone mineralization, litter quality, and footpad dermatitis incidence in growing broiler chickens. Asian-Australas. J. Anim. Sci. 30(7), 979–984. Lee, J., Tompkins, Y., Kim, D., Kim, W. and Lee, K. 2023. Increased sizes and improved qualities of tibial bones caused by myostatin mutation in Japanese quail. Front. Physiol. 13:1085935. Li, Y., Li, Q., Zhu, S., Luo, E., Li, J., Feng, G., Liao, Y. and Hu, J. 2010. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 31(34), 9006–9014. Li, T., Xing, G., Shao, Y., Zhang, J., Li, S., Lu, L., Liu, Z., Liao, X. and Luo, X. 2020. Dietary calcium or phosphorous deficiency impairs bone development by regulating related calcium or phosphorous metabolic utilization parameters in broilers. Poult. Sci. 99(6), 3207–3214. Lima, I., Costa, A.M., Bastos, I.N., Granjeiro, J.M. and Soares, G.D., 2006. Development and characterization of 5% mol zn bioceramics in granular form. Mater. Res. 9(4), 399–403. Liu, M., Zhou, G., Song, W., Li, P., Liu, H. and Niu, X. 2012. Effect of nano-hydroxyapatite on the axonal guidance growth of rat cortical neurons. Nanoscale 4, 3201–3207. Mahamid, J., Sharir, A., Addadi, L. and Weiner, S. 2008. Amorphous calcium phosphate is a significant component of the zebrafish fin bones: indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA. 105(35), 12748–12753. Melgar, A.E., Fagundes, A.P., Macuvele, D.L.P., Padoin, N., Soares, C. and Riella, H.G., 2021. Green synthesis of hydroxyapatite nanoparticles for biomedical application: a brief review. Int. J. Adv. Med. Biotechnol. 4(1), 54–60. Munoz, L.F.S., Ramírez, S. and Silva, R., 2019. Comparison of subcutaneous inflammatory response to commercial and engineered zinc hydroxyapatite implants in rabbits. Arq. Bras. Med. Vet. Zootec. 71(6), 1873–1879. Oki i, N., Nishimura, S., Kurisu, K., Takeshima, Y. and Uozumi, T. 2001. In vivo histological changes occurring in hydroxyapatite cranial reconstruction. Neurol. Med. Chir. 41(2), 100–104. Opris, H., Dinu, C., Băciuţ, M., Băciuț, G., Miter, I., Crișan, B., Armencea, G., Prodan, D.A. and Bran, S. 2020. The influence of eggshells on bone regeneration in preclinical in vivo studies. Biology 9(12), 476. Ortiz, S.L., Mendoza-Anaya, D., Sãnchez-Campos, D., Fernãndez-García, M.E., Salinas-Rodríguez, E., Reyes-Valderrama, M.I. and Rodríguez-Lugo, V. 2020. The pH effect on the growth of hexagonal and monoclinic hydroxyapatite synthesized by the hydrothermal method. J. Nanomaterials. 2, 1–10. Othsuki, M. 2009. Bone-grafting materials, their uses advantages, and disadvantages. J. Am. Dent. Assoc. 133(8), 1125–1126. Pelicia, K., Garcia, E.A., Faitarone, A., Silva, A., Berto, D.A., Molino, A. and Vercese, F. 2009. Calcium and available phosphorus levels for laying hens in second production cycle. Rev. Bras. Cienc. Avic. 11(1), 39–49. Peon, E., Fuentes, G., Delgado, J.A., Morejon, L, Almirall, A. and Garcia, R. 2004. Preparation and characterization of porous blocks of synthetics hydroxyapatite. Lat. Am. Appl. Res. 34(4), 225–228. Pratama, A.M., Abadi, R.D., Ratrisono, R.B., Budhi, S. and Kristanto, D. 2023. Effect of administration of a bovine xenograft on accelerated healing of femoral fracture in domestic dogs. Vet. Biomed. Clin. J. 5(2), 70–77. Razak, A. 2023. Effect of calcination temperature on the properties of eggshell waste (EW) powder for biomedical application. Joint J. Novel Carbon Resource Sci. Green Asia Strategy 10(2), 782–791. Rocha, J.H.G., Lemons, A.F., Kannan, S., Agathopoulos, S., Ferreira, J.M.F., Valeiro, P., Oktar, F.N., 2005. Scaffolds for bone restoration from cuttlefish. Bone. 37(6), 850–857. Santos, M.H., Oliveira, M., Souza, L.P.F., Mansur, H.S., Vasconcelos, W.L. 2004. Synthesis control and characterization of hydroxyapatite were prepared using a wet precipitation process. Mater. Res. 7(4), 625–630. Scott, H.W. and McLaughlin, R. 2007. Introduction to feline orthopedic surgery. In Feline Orthopedics. Eds., Scott, H.W., McLauglin, R. Manson Publishing, pp: 9–16. Wibisono, Y., Dwijaksara, N.L.B., Widayatno, W.B., Wismogroho, A.S., Amal, M.I., Rochman, N.T., Nishimura, T. and Noviyanto, A., 2018. Synthesis and sinterability of hydroxyapatite from fishery by-products. J. Korean Ceram. Soc. 55(6), 570–575. Wu, N., Liu, J., Ma, W., Dong, X., Feng, W., Yang, D. and Xu, Y. 2021. Degradable calcium-deficient hydroxyapatite/poly (lactic-glycolic acid copolymer) bilayer scaffold through integral molding 3d printing for bone defect repair. Biofabrication 13(2), 025005. | ||
| How to Cite this Article |
| Pubmed Style Adji D, Sutrisno B, Prastiwi A, Anggoro D, Wuryastuti H. Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Vet. J.. 2025; 15(4): 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 Web Style Adji D, Sutrisno B, Prastiwi A, Anggoro D, Wuryastuti H. Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. https://www.openveterinaryjournal.com/?mno=234419 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.21 AMA (American Medical Association) Style Adji D, Sutrisno B, Prastiwi A, Anggoro D, Wuryastuti H. Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Vet. J.. 2025; 15(4): 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 Vancouver/ICMJE Style Adji D, Sutrisno B, Prastiwi A, Anggoro D, Wuryastuti H. Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 Harvard Style Adji, D., Sutrisno, . B., Prastiwi, . A., Anggoro, . D. & Wuryastuti, . H. (2025) Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Vet. J., 15 (4), 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 Turabian Style Adji, Dhirgo, Bambang Sutrisno, Artina Prastiwi, Dito Anggoro, and Hastari Wuryastuti. 2025. Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Veterinary Journal, 15 (4), 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 Chicago Style Adji, Dhirgo, Bambang Sutrisno, Artina Prastiwi, Dito Anggoro, and Hastari Wuryastuti. "Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach." Open Veterinary Journal 15 (2025), 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 MLA (The Modern Language Association) Style Adji, Dhirgo, Bambang Sutrisno, Artina Prastiwi, Dito Anggoro, and Hastari Wuryastuti. "Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach." Open Veterinary Journal 15.4 (2025), 1695-1701. Print. doi:10.5455/OVJ.2025.v15.i4.21 APA (American Psychological Association) Style Adji, D., Sutrisno, . B., Prastiwi, . A., Anggoro, . D. & Wuryastuti, . H. (2025) Sustainable synthesis of hydroxyapatite from poultry waste for veterinary applications: A calcination approach. Open Veterinary Journal, 15 (4), 1695-1701. doi:10.5455/OVJ.2025.v15.i4.21 |