| Review Article | ||
Open Vet. J.. 2025; 15(4): 1505-1519 Open Veterinary Journal, (2025), Vol. 15(4): 1505-1519 Review Article The alarming spread of Japanese encephalitis: A growing public health concernAnindita Riesti Retno Arimurti1*, Aswin Rafif Khairullah2, Dita Artanti1, Vella Rohmayani3, Tara Puri Ducha Rahmani4, Arifin Budiman Nugraha5, Abdul Hadi Furqoni6, Muhammad Khaliim Jati Kusala2, Bantari Wisynu Kusuma Wardhani7, Fitrine Ekawasti2, Ikechukwu Benjamin Moses8, Wasito Wasito2, Syahputra Wibowo9, Julaeha Julaeha10, Riza Zainuddin Ahmad2, Kartika Afrida Fauzia10,11, Ima Fauziah2 and Dea Anita Ariani Kurniasih121Diploma Medical Laboratory Technology, Faculty of Health Science, University of Muhammadiyah Surabaya, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Bachelor Medical Laboratory Technology, Faculty of Health Science, University of Muhammadiyah Surabaya, Surabaya, Indonesia 4Department of Biology, Faculty of Science and Technology, Universitas Islam Negeri Walisongo Semarang, Semarang, Indonesia 5Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia 6Center for Biomedical Research, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 8Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 9Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 11Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan 12Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Anindita Riesti Retno Arimurti. Diploma Medical Laboratory Technology, Faculty of Health Science, University of Muhammadiyah Surabaya, Surabaya, Indonesia. Email: aninditariesti [at] um-surabaya.ac.id Submitted: 18/12/2024 Accepted: 22/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
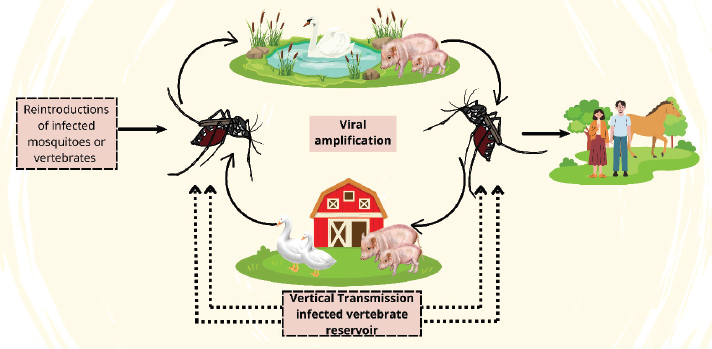
AbstractJapanese encephalitis (JE) is a zoonotic infectious disease transmitted by mosquitoes. JE is caused by the Japanese encephalitis virus (JEV), a member of the Orthoflavivirus genus. In 1871, the first JEV-related encephalitis outbreak was documented in Japan. JEV is the primary cause of viral encephalitis in the Asian subcontinent. In addition, isolated outbreaks have been noted in northern Australia and the Western Pacific. JEV infection usually results in a strong physiological immune response in humans, including cellular and adaptive humoral immunity. The neurological system is the primary site of alterations. JE mostly affects the cerebral cortex, cerebellum, anterior horn cells of the spinal cord, and thalamus. The disease’s initial symptoms show up six to fourteen days after incubation. Typically, it begins with a temperature above 38°C, chills, headaches similar to meningitis, muscle aches, and vomiting. The established method for diagnosing JE is the enzyme-linked immunosorbent assay (ELISA) test. JEV can be spread by a variety of Culex mosquito species. Risk factors for JE include travel, outdoor activities, accommodation, proximity to animals, gender, and age. The World Health Organization estimates that 15,000 people die of JE each year, with about 50,000 cases occurring globally. This illness has no known cure. The only available treatment is supportive. A large number of approved vaccines are used to prevent JEV infection. Strategies to avoid human illness and interfere with the virus’s enzootic cycle are employed in controlling JE. Keywords: Culex, JE, JEV, Public Health, Virus. IntroductionThe zoonotic infectious disease known as Japanese encephalitis (JE) is commonly reported in temperate and subtropical regions and is spread by mosquitoes (Yun and Lee, 2014). JE is caused by the Japanese encephalitis virus (JEV), a member of the Orthoflavivirus genus that is linked to the tick-borne encephalitis virus (TBEV), West Nile virus (WNV), dengue virus (DENV), and Zika virus (ZIKV) (Maeki et al., 2019). JEV was initially isolated from the brain of a deceased JE patient in 1935, while JE was originally documented in Japan in 1871 (Kuno, 2022). Positive-sense single-stranded RNA makes up the genome of JEV, an enclosed virus with a virion size of about 50 nm and minimal genotype-to-genotype variation in length (Kumar et al., 2022). Numerous mammalian and avian species, such as pigs, sheep, horses, cattle, goats, donkeys, dogs, cats, herons, egrets, and humans, are susceptible to contracting JEV (Nemeth et al., 2012; Mansfield et al., 2017). JE is distributed over the Western Pacific Islands (Choe et al., 2020), Australia (McGuinness et al., 2023), and Asia (Suresh et al., 2022). JE is one of the leading causes of viral encephalitis globally, accounting for an estimated 50,000 cases and 15,000 fatalities per year, despite being viewed by many Westerners as an uncommon and exotic sickness (Zheng et al., 2012). In endemic locations, children between the ages of three and six have the highest attack rate by age. Approximately half of survivors have significant neuropsychiatric disorders, and approximately one-third of patients die (Amicizia et al., 2018). The virus, which is spreading alarmingly, has infected a large portion of China, Southeast Asia, and the Indian subcontinent. The severity of the illness is demonstrated in these regions by overcrowded hospital wards with children and young adults afflicted with JE (Srivastava et al., 2023). The potential for an epidemic and its mortality rate makes JE a public health issue. Most JEV infections do not cause symptoms, but when they do, they can lead to serious morbidity and death (Sunwoo et al., 2017). In contrast to most endemic locations with long-term JEV transmission, where the illness burden is in children, cases in adults are frequently found when JEV enters new areas (Hills et al., 2023). Compared with adults, newborns and children under the age of 15 are more vulnerable to JE and have a higher chance of developing neurological problems (Srivastava et al., 2023). Nearly 2 billion people in endemic nations are constantly at risk of contracting JE, and growing mosquito populations raise the possibility that the disease will spread to other regions (Amicizia et al., 2018). The JE case has significantly affected public health, animal productivity, trade, and health (Dixon et al., 2024). The World Health Organization (WHO) has advised that the JE vaccine be included in national immunization programs in all regions where JE is acknowledged as a public health priority to lessen the disease’s burden. A review of the JE can help with public health decision-making on disease control measures. The purpose of this review article is to explain the etiology, history, epidemiology, pathogenesis, immune response, pathology, clinical symptoms, diagnosis, transmission, risk factors, public health importance, economic impact, treatment, vaccination, and control of JE. EtiologyThe JEV is an enveloped, single-stranded, positive-sense virus that exhibits cubic spheroidal symmetry, with a diameter ranging from 40 to 50 nm in diameter (Srivastava et al., 2023). The approximately 11-kb viral RNA genome contains genes for seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) that have a methylated 5’ cap but no poly-A tail at the 3’ end, as well as three structural proteins: the capsid (C), precursor membrane (prM), and envelope (E) (Guo et al., 2024). These 10n proteins (7 nonstructural and 3 structural) were translated from a single open reading frame of genomic RNA, resulting in a chain of 3,432 amino acids. After replication, the virus genome is encapsidated by capsid protein (C) and then transported to the endoplasmic reticulum (ER), where a lipid bilayer surrounded by E proteins and membrane proteins (PrM) inculcates the nucleocapsid. The immature virions consisting of capsid proteins, genomic RNA, and PrM-E heterodimers then travel through the trans-Golgi network for maturation through furin cleavage (Kumar et al., 2022). The capsid structural protein (C) of JEV dimerizes in an antiparallel manner from head to tail. Viral genomic RNA is encased within a spherical nucleocapsid, which is often composed of many copies of the capsid dimer (Luca et al., 2012). A recently discovered secondary structure of α-helical regions 1–4 in the crystal structure of protein C shows significant similarity to the capsid proteins of DENV, WNV, and ZIKV (Poonsiri et al., 2019). A short loop connects the four helices that make up each JEV capsid protein monomer: α1 (amino acids 29–38), α2 (amino acids 44–57), α3 (amino acids 63–70), and the longest, α4 (amino acids 74–96) (Zhang et al., 2021). The variable, closed-and-open conformation formed by the amino terminus of α helix-1 makes it a desirable target for antiviral agents. Nonetheless, the 4–4 α-helical arrangement’s carboxyl-terminal pairing results in a structure resembling a coil, which could facilitate nucleic acid bonds (Poonsiri et al., 2019). Following co-translational cleavage by the signal peptide, the newly produced polyprotein gives rise to the viral prM, also known as the pre-membrane (prM), which then starts to assemble into vesicles containing the viral genomic RNA in the ER (Mi et al., 2024). The vesicles leave the ER shortly after they are assembled and arrive at the Golgi apparatus network, where the enzyme furin cleaves prM to produce the M protein and mature virus particles, which are then expelled from the host cell (Xiong et al., 2022). It remains unclear exactly what role prM/M plays in JEV. However, it has been extensively studied in related viruses of the same family, such as DENV and YFV, where it assists in the assembly of mature viruses and provides a site for proper conformational modulation of the E protein. Moreover, the binding of the E protein domain to host receptors during infection is significantly influenced by viral membrane proteins (Guo et al., 2024). Membrane proteins facilitate the fusion of viral vesicles to endosomes following virion internalization. JEV’s envelope protein (E), which envelops the virion, is a crucial protein that fuses with the host plasma membrane and binds to host cell receptors (Zhu et al., 2023a). The primary protein identified by virus-neutralizing antibodies is the E protein of JEV. Envelope domain I (EDI), envelope domain II (EDII), and envelope domain III (EDIII) are the three domains that comprise the E proteins of other flaviviruses (Zhang et al., 2017). The E protein’s crystal structure has shown that, like other flaviviruses, it dimerizes antiparallel in a head-to-tail fashion with a comparatively small interface (Luca et al., 2012). Between the globular EDIII and expanded EDII domains lies a nine-stranded β-barrel that forms the core of EDI. Fundamentally, EDII is composed of two long loops that extend from EDI. The peptide is stabilized by binding with three disulfide bonds, which maintains its fusion peptide at the top. At the carboxyl terminus of the ectodomain, EDIII, like other flaviviruses, maintains an immunoglobulin-like structure that is probably responsible for binding host receptors and facilitating the internalization of its virions (Wu et al., 2003). Given these characteristics, the EDIII domain could be a target for antiviral drug development aimed at reducing JEV infection. The first step in JEV productive infection and pathogenesis is the initiation process of virus-host interactions. The three major steps involved in the entry of JEV into host cells to cause diseases are: recognition of host cell surface receptors by JEV and JEV-receptor binding, endocytosis, and membrane fusion and uncoating (Zhu et al., 2023a). These processes are followed by diffusion or directed migration on the cell membrane. The JEV then internalizes into the endocytic vesicle via clathrin-mediated endocytosis (CME) or clathrin-independent endocytosis (CIE). The JEV envelope then fuses with the membrane and uncoats within acidic endosomes before the final release of the viral RNA materials into the cytoplasm; thus, leading to productive infection (Lopez et al., 2015). Some important characteristics of JE include viral infection of the central nervous system (CNS), reactive gliosis, uncontrolled inflammatory response, and neuronal cell death (Zhu et al., 2023a). HistoryIn 1871, the first JEV-related encephalitis outbreak was reported in Japan (Kuno, 2022). Over 6,000 cases were recorded in a severe outbreak in Japan in 1924, and major epidemics have been reported roughly every 10 years (Erlanger et al., 2009). The first Nakayama strain was identified from an encephalitis patient’s brain in 1935. Since then, the virus has been categorized as a group B arbovirus in the Togaviridae family, along with other flaviviruses. To differentiate these summer outbreaks from von Economo’s lethargy/sleeping sickness, often referred to as type A encephalitis, which manifests in the winter with a distinct clinical appearance, the name “type B” encephalitis was first employed (Mohsin et al., 2022). Later, the “type B” label was dropped, and JEV was classified as a member of the genus OrthoFlavivirus and placed in the distinct family Flaviviridae in 1985 (Solomon et al., 2003). The genus Orthoflavivirus comprises seventy tiny, enclosed viruses with positive-sense single-stranded RNA and is named after the prototype yellow fever virus (from the Latin word flavi) (Laureti et al., 2018). JEV has only one serotype, but it can be classified into five genotypes according to the full-length genome sequence or E gene sequence, namely genotypes I, II, III, IV, and V (GI, GII, GIII, GIV, and GV) (Xia et al., 2023). Between 1935 and the 1990s, type GIII was reported to be the most widespread genotype in Asia, particularly China, and has historically been reported as the source of many JEV strains (Zheng et al., 2012; Xia et al., 2023). The GI JEV strain was first reported in Cambodia and China in 1967 and 1979, respectively. GII, GIII, GIV, and GEV JEV have also been reported widely in Australia, Malaysia, Indonesia, India, Vietnam, Japan, South Korea, Thailand, and China (Xia et al., 2023). More than one JEV genotype may be simultaneously transmitted, especially in JEV-endemic countries, and the predominant genotype may change. However, studies suggest that JEV originated from ancestral viruses in the Indonesia–Malaysia region and evolved there into different genotypes that subsequently spread throughout Asia. EpidemiologyJEV is the primary cause of viral encephalitis in Asia (Srivastava et al., 2023). It encompasses a large region, primarily tropical Asia, which includes all of Southeast Asia, as well as China, Taiwan, South Korea, Japan, the Philippines, and India. India, Pakistan, Myanmar, Japan, Laos, Malaysia, Singapore, Sri Lanka, the Philippines, China, Indonesia, maritime Siberia, Nepal, Vietnam, and South Korea are among the nations that have documented JE epidemics (Suresh et al., 2022). Furthermore, isolated outbreaks have also been noted in northern Australia and the Western Pacific (Choe et al., 2020; McGuinness et al., 2023). Although there have been outbreaks like JE in Japan in the late 1800s, the first definite case of JE was reported in Japan in 1924. South Korea (1933), China (1940), the Philippines (1950), India (1955), and numerous other Asian nations followed (Erlanger et al., 2009). In recent decades, South Asian nations such as Bangladesh, India, and Pakistan have become the primary focus of JE transmission, replacing East Asian nations like Japan, South Korea, and Taiwan (Mulvey et al., 2021). The Indian subcontinent has seen a rise in JEV activity since the early 1970s. The virus then spread to nearby regions of southern Pakistan and the Kathmandu Valley in Nepal in the late 1990s (Zimmerman et al., 1997). Serological investigations yielded the first indication of JE in India in 1952, and the country’s first case of JEV was reported in 1955. Subsequently, outbreaks were regularly recorded in every region of India (Kulkarni et al., 2018). In 1973, a significant epidemic occurred with a 42.6% fatality rate in the West Bengal district of Bankura (Tiwari et al., 2012). In 2005, the Gorakhpur district of Uttar Pradesh experienced the longest JE outbreak, with over 5500 recorded cases of viral encephalitis and over 23% fatality (Patel et al., 2021). Cases suggestive of extensive transmission have been documented, particularly in urban places like New Delhi, and some reports indicate that the virus has expanded to new non-endemic areas, such as the north and northeast of the Indian subcontinent (Kumari et al., 2013; Singh et al., 2023). The threat of JEV infection has recently increased, as evidenced by a notable epizootic epidemic in Australia in 2022 (van den Hurk et al., 2022). The virus was dormant for the previous 20 years in Australia, where the first JE cases were discovered in 1995 (Furuya-Kanamori et al., 2022). The virus resurfaced in early 2021 and was identified in a northern Queensland resident who died as a result. The recent epidemic in 2022 in the southern Australian state seems to have been a sentinel occurrence. Later, JEV was found in newborn piglets, mummified fetuses, and stillborn piglets from a number of commercial pig farms, mostly in Australia’s four southern states (Queen, South Australia, Victoria, and New South Wales) (Hick et al., 2024). In Australia, there have been 45 documented human cases of JEV, 35 of which have been confirmed with strong clinical evidence and seven of which have resulted in death (Waller et al., 2022). The Culex sitiens subgroup, particularly Culex annulirostris, is the primary cause of JE transmission (Chiou et al., 2021). Although further mosquito studies and surveillance are required to determine their regional range and abundance in Australia, it is believed that other Culex species, specifically Culex quinquefasciatus, Culex gelidus, and Culex tritaeniorhynchus, are responsible for disease transmission (van den Hurk et al., 2022). However, neighboring territorial jurisdictions like Western Australia and the Northern Territory, are seriously at risk from the present JEV outbreaks in the state of South Australia (Furlong et al., 2023). PathogenesisThe incubation time for JEV ranges from 6 to 16 days. The factors that determine whether infections cause disease are unknown, although they might include host features like age, genetic composition, physical health, and pre-existing immunity, as well as viral factors like method of entry, titer, and neurovirulence of the inoculum (Mohsin et al., 2022). Following a mosquito bite, the virus replicates in the epidermis before moving on to nearby lymph nodes (Sharma et al., 2021). It has also been demonstrated that Langerhans dendritic cells in the skin facilitate the spread of viruses, including West Nile fever and dengue fever, which are illnesses caused by flaviviruses (Dobrzyńska et al., 2023). After transient viremia occurs in the peripheral nervous system, it spreads to the CNS. Early viremia involves the seeding of additional neuronal networks with viral particles. Some of the most crucial locations for extra neural replication include tissues like connective and lymphocytic tissues, muscles like skeletal and smooth muscles, organs like the heart, and glands like endocrine and exocrine (Yadav et al., 2022). The virus enters the bloodstream and travels to the CNS. Clinical symptoms can be used to diagnose a number of diseases by identifying the mechanisms by which the virus enters CNS cells (Li et al., 2015). Although JEV infection is caused by the pathogen in question in the nerve tissue; symptoms may be minimal or nonexistent if the infection is limited to the tissues (extra-neural) (Ashraf et al., 2021). Therefore, understanding the pathophysiology of viral illnesses requires an understanding of how viruses enter the CNS. The mechanism by which JEVs can pass through the blood-brain barrier (BBB) is unknown. However, immunohistochemical labeling of human postmortem material showed a broad infection across the brain, indicating a hematogenous invasion mechanism (Ludlow et al., 2016). Nevertheless, experimental evidence indicates that some flaviviruses may be able to pass across the BBB primarily by reproduction within endothelial cells; passive transmission through endothelial cells seems to be a more likely mechanism for JEV (de Vries and Harding, 2023). The likelihood of neuroinvasion has also been linked to other factors related to the BBB integrity. Numerous studies have found that neurocysticercosis was present in disproportionate numbers of deceased patients at autopsy (Handique et al., 2008). Immune responseJEV infection usually results in a strong physiological immune response in humans, including cellular and adaptive humoral immunity (Filgueira and Lannes, 2019). The production of neutralizing antibodies against the envelope (E) protein is necessary for a protective humoral immune response because the E protein is in charge of binding and subsequent entry into target cells (Fan et al., 2024). Thus, as demonstrated by animal studies, neutralizing antibodies against JEV E protein can be effectively employed for passive vaccination in the early treatment of JEV-infected people (Cao et al., 2016). Five genotypes have been identified so far, each having 1%–5% variants in the E protein and matching differences in the epitopes and antibodies generated (Lee et al., 2024). However, antibodies that cross-react with all JEV genotypes are typically formed as a result of infection or immunization. Because of this, vaccines against JEV genotype III have mostly been created using formalin-fixed live attenuated or inactivated viruses (like IXARIO®), which produce long-lasting immunity and are well tolerated by both young people and the elderly (Hegde and Gore, 2017). It should be mentioned that vaccination and JEV infection can produce antibodies that react with DENV and other flaviviruses (Pushpakumara et al., 2020). Cross-reacting antibodies produced by JEV infection or immunization may have the ability to enhance DENV infection, which could lead to DENV infection deaths (Saito et al., 2016). It has also been shown that immunization increases JEV infection in pigs in an antibody-dependent manner (García-Nicolás et al., 2017). Adoptive T cell-mediated immunity is necessary to boost B cell growth, including somatic mutation processes to maximize antigen-binding capabilities and antibody class switching, to create specific humoral immunity to JEV (Amanna and Slifka, 2011). As a result, particular T cell responses are crucial during JEV infection, and different T cell subpopulations may have different consequences (Zhang et al., 2024). Through cytotoxic mechanisms, JEV-specific CD8+ cytotoxic αβ T cells and γδ T cells target and kill JEV-infected cells, thereby preventing brain infection and subsequent encephalitis. This helps to restrict JEV production and dissemination (Jain et al., 2017). The immunogenic peptides of NS proteins, which are shown by major histocompatibility complex (MHC) class I, have been discovered by cytotoxic T cells (Sayeed et al., 2018). B cell assistance in germinal centers is largely provided by JEV-specific CD4+ helper T cells that are able to identify the JEV E protein (Rathore and St John, 2020). In contrast, CD4+ cells that identify NS protein epitopes may aid in specific cytotoxic immune responses by generating antiviral cytokines like interferon-γ, which in turn regulate and ultimately resolve the cellular immune response while also supplying T cell memory (Turtle et al., 2016). T-cell activation requires dendritic cells. MHC class I and class II cells process and display JEV antigens, and they associate acquired immunity with innate immunity (Wieczorek et al., 2017). However, JEV can also target dendritic cells, which may lead to a fatal infection (Lannes et al., 2017). Therefore, it has been demonstrated that dendritic cells can be infected by weakened viruses. In this sense, it is still unknown whether JEV infection of dendritic cells is crucial for developing permanent immunity or whether JEV infection of immune cells aids in impairing the immune response or even results in encephalitis. First, the protective immune response and the identification of JEV infection are significantly aided by innate immune cells and processes. The important pattern recognition receptors that detect JEV components and alert immune cells are toll-like receptors (TLRs), such as TLR7 and TLR8 (Nazmi et al., 2014). The first line of cytotoxic defense is provided by natural killer (NK) and natural killer T (NKT) cells until acquired immunity takes over (Sooryanarain et al., 2012). The immunological response to JEV involves mast cells, which are also a component of innate immunity. Mast cells, however, are more likely to exacerbate JEV invasion and inflammation in the brain than to aid in its resolution (Hsieh et al., 2019). Overall, JEV infection triggers every immunological mechanism and process that is known to exist, which typically leads to enduring immunity. To better understand the connections between JEV and immune cells, particularly in cases resulting in encephalitis, further research is necessary. PathologyThere are several known pathological abnormalities in JE. The neurological system is the primary site of alterations. It is possible to artificially cause nonsuppurative encephalitis in piglets inoculated with JEV in animal models (Yamada et al., 2004). JE mostly affects the cerebral cortex, cerebellum, anterior horn cells of the spinal cord, and thalamus (Patel et al., 2015). The brain exhibits herniation, edema, and congestion during the acute phase of the illness (Mehta et al., 2021). Microglial proliferation resulting in glial nodules, perivascular lymphocytic infiltration, neuronal degeneration and neuronophagy, and meningeal inflammation are examples of microscopic lesions (Mohapatra et al., 2023). These alterations mostly affect the diencephalic, mesencephalic, and brainstem regions and typically occur in the gray matter. A clear topographic distribution of JEVs in the brain has been demonstrated by immunohistochemical investigations in fatal human cases (Hills et al., 2009). JE virus antigen is immunohistochemically detectable in the gray matter of the thalamus and midbrain, as well as in the cytoplasm of nerve cells in the frontal and temporal lobe cortex (Han et al., 2021). Autopsy findings of JE reveal an inflammatory response to widespread neuronal infection caused by the virus (Upadhyay, 2013). The leptomeninges may appear normal or blurred. Focal petechiae or gray matter hemorrhages clog the brain parenchyma (Money et al., 2023). Extending survival beyond 7 days results in necrotic zones in the spots. The white matter often has a normal appearance. Some patients experience poliomyelitis-like discoloration and coalescence of the spinal cord’s gray matter (Solomon et al., 1998). Diffusion-weighted imaging shows the typical bilateral thalamic involvement in humans (Prakash et al., 2004). Additionally, lesions in the cerebellum, cerebral cortex, pons, midbrain, subcortical white matter, and basal ganglia can be found using magnetic resonance imaging (Phukan et al., 2021). These individuals typically present clinically with classic Parkinson’s symptoms following recovery from acute encephalitis (Tadokoro et al., 2018). As previously indicated, anterior horn cell involvement has been reported in addition to brain lesions. The distribution of cell types was identical in both fatal and nonfatal cases, did not change between the first and last days of hospitalization, and was unaffected by the use of steroids (Monath, 2023). Clinical symptomsMost JE infections are often mild, with either no symptoms or subclinical fever. However, the case fatality rate might be as high as 30% in patients who develop severe clinical disease (encephalitis) (Srivastava et al., 2023). Approximately 30%–50% of individuals who survive severe infections nevertheless have neurological or behavioral problems, such as paralysis, recurrent seizures, and speech impairment (Sunwoo et al., 2017). Clinical indications appear in only 1 out of every 300 cases (Solomon et al., 2003). The disease’s initial symptoms show up six to fourteen days after incubation. Typically, it begins with a temperature above 38°C, chills, headaches similar to meningitis, muscle aches, and vomiting (Tiwari et al., 2012). The initial symptoms in children, such as nausea, vomiting, and stomach discomfort, are similar to those observed in cases of acute gastrointestinal sickness (Kamath et al., 2023). Possible symptoms include paradoxes, paralysis, changes in postural patterns, Parkinson’s rhythm, seizures, and coma (Tadokoro et al., 2018). The acute flaccid paralysis that a small percentage of patients with JE can be mistaken for poliomyelitis, which typically manifests as extensive convulsions (Grewe et al., 2022). In 20%–30% of cases, signs of acute cerebral or pulmonary edema causing severe respiratory distress result in death (Mohsin et al., 2022). Children with significant behavioral and neurological effects, such as persistent extrapyramidal syndromes, sensorimotor seizures, epileptic seizures, and severe mental illnesses, are frequently neglected by recovery (Sunwoo et al., 2017). Recurrent seizures, peduncular injuries, or intracranial hypertension are linked to the length of a person’s coma stay and may indicate poor mortality (Solomon et al., 2002). The development of a disease occurs in four stages. It is possible to identify the stage as prodromal by looking for symptoms such as (high) temperature and headache, as well as less specific ones like nausea, vomiting, anorexia, and malaise (Mohsin et al., 2022). The acute stage, often referred to as the second stage, encompasses the range of consciousness that includes moderate cloudiness, fainting, semi-coma, and coma (Mehta et al., 2021). Both localized and widespread seizures are frequent, as are stiff necks and limb pain (Michael and Solomon, 2012). Fatal cases advance quickly and pass away at this point. In straightforward cases, the third stage is distinguished by improved neurological outcomes and a decrease in body temperature (Srivastava et al., 2023). The last stage is the residual symptom phase, which involves the improvement of neurological abnormalities in severe cases and full recovery in moderate cases (Mayxay et al., 2020). DiagnosisLow virus titers and the rapid generation of neutralizing antibodies are likely the main reasons why attempts to isolate JEV from clinical material typically fail. Isolates can occasionally be extracted from brain tissue (either at necropsy or postmortem needle biopsy) or cerebrospinal fluid (CSF) (in which case it is linked to lack of antibody generation and increased mortality) (Desai et al., 1995). Polyclonal antibodies against JEV may show positive immunohistochemical staining of CSF cells or necropsy tissue (Bharucha et al., 2020). Nonetheless, JE is typically identified through serology. The hemagglutination inhibition test was in use for a long time, but it had a number of practical drawbacks, including the inability to make an early diagnosis because it required paired sera (Upadhyay, 2013). The established method for diagnosing JE is the enzyme-linked immunosorbent assay (ELISA) test, which was created in the 1980s and captures both IgM and IgG (Ravi et al., 2006). The sensitivity and specificity of anti-JEV IgM in the CSF for viral CNS infection are >95% after the initial days of sickness (false negatives may occur before this) (Robinson et al., 2010). However, ELISA is mostly used in a small number of academic or referral institutes rather than in the rural areas where JE occurs because it requires sophisticated equipment. Recently, IgM ELISA was altered to use a straightforward nitrocellulose membrane-based format, which produces a color shift that is apparent to the unaided eye (Ravi et al., 2009). This rapid, easy-to-use test, which does not require special equipment, should prove useful for the diagnosis of the disease in rural hospitals. Reverse transcriptase polymerase chain reaction (PCR) has been used to identify JEV RNA in human CSF samples; however, its applicability as a standard diagnostic procedure has not been proven (Swami et al., 2008). A promising diagnostic technique which employs a CRISPR-Cas12a-based molecular diagnostic method combined with isothermal nucleic acid amplification in a DNA endonuclease-targeted CRISPR trans reporter is emerging. This emerging technology utilizes reverse transcription-recombinase polymerase amplification (RT-RPA) for the diagnosis of JEV. Interestingly, this technique has been used for the successful detection of RNA copies for JEV genotypes I (GI), GIII, and GV (Kwak et al., 2023). The lateral flow assay (LFA) was also developed for the detection of JEV antibodies in serum. This diagnostic innovation involves the conjugation of the recombinant NS1 protein with serum antibodies for the detection of JEV. This LFA technique was demonstrated to be suitable for screening swine serum samples during the monsoon and post-monsoon periods (Dhanze et al., 2020). TransmissionVertebrates contract JEV from mosquitoes. The transmission of mosquitoes began in the early 1930s. The research conducted in Japan by Buescher et al. (1959) is the source of the JEV ecology, which has been reviewed numerous times. JEV can be spread by a variety of Culex mosquito species. C. tritaeniorhynchus is the primary JE vector for South, East, and Southeast Asia (Tong et al., 2023). C. annulirostris is the primary vector for Northern Australia (Klein et al., 2024). However, a number of additional secondary vectors may be significant. Numerous secondary vectors, such as C. whitmorei, C. pseudovishnui, C. epidesmus, C. gelidus, Anopheles subpictus, A. peditaeniatus, Mansonia indiana, and M. uniform, have been found in studies conducted in India in particular (Tiwari et al., 2012). In Asia, Culex mosquitoes and water birds participate in the natural JEV cycle (Mulvey et al., 2021). In contrast to many other diseases spread by mosquitoes, the epidemiology of human JE depends on reproducing hosts. Pigs are regarded as the most significant breeding hosts in Asia, serving as a conduit to people because of their close proximity to houses (Damayanti et al., 2017). In addition to pigs and mosquitoes, JEV hosts other animals, including bats, birds, and a number of other wild animals. The virus life cycle is illustrated in Figure 1. There are two epidemiological patterns of transmission: an epidemic pattern in more temperate regions with a noticeable summer season and an endemic type in tropical regions where the virus circulates throughout the year but has large seasonal peaks, most likely as a result of irrigation techniques (Hills et al., 2010). Risk factorsRisk factors for JE include travel, outdoor activities, accommodation, proximity to animals, gender, and age (Liu et al., 2010). Long-term visitors have been observed to have the highest prevalence of disease (Moore, 2021). Long-term travel increases the chance of coming into contact with infected mosquitoes, but there is no set length of time that puts tourists at risk for JE (Shlim and Solomon, 2002). Chronic stays in JE-endemic regions are included in long-term travel; this includes regular visitors and city dwellers who frequently visit high-risk rural regions (Hills et al., 2019). Transmission of the JE virus occurs year-round in certain places and seasonally in others (Xia et al., 2023).
Fig. 1. The transmission cycle of the Japanese Encephalitis Virus (JEV): key interactions between mosquito vectors, amplifying hosts, and incidental hosts. Culex mosquitoes are vectors of reinfection and viral amplification in hosts such as pigs and waterfowl. Humans and horses are incidental dead-ends because they cannot be perpetuated by the virus. The level of persistence is enhanced when the reintroduction of both infected vectors and vertebrates occurs consistently and via vertical viral transmission within the amplification hosts. This cycle presents some important targets for impeding JEV transmission in addition to minimizing its public health vector. In rural or agricultural settings, exposure to mosquitoes poses the greatest risk (Kulkarni et al., 2018). The breeding grounds for JEV mosquitoes are usually marshes, flooded rice fields, and other areas with stagnant water (Keiser et al., 1995). Travelers visiting coastal regions or resorts in or close to rural or rice-growing regions have been the subjects of multiple occurrences (Connor and Bunn, 2017). JE can occur in large, localized outbreaks, indicating widespread transmission of the active JE virus in the area. Living close to a farm also increases the risk of JEV transmission, particularly if the farm is close to rice fields (Kumar et al., 2018). The majority of JEV-transmitting mosquitoes eat outside, particularly between sunset and daybreak (Van den Eynde et al., 2022). Activities that raise risk include outdoor recreation, such as camping, hiking, trekking, cycling, rafting, hunting, fishing, gardening, and spending a lot of time outside, particularly at night or in the evening (Hills et al., 2019). The risk of mosquito exposure is higher in accommodations without air conditioning, screens, or mosquito nets. Males are more likely than to be infected with JEV than females, the older adults and infants are more prone to severe disease (Monath, 2023). Public health importanceJE is one of the most important causes of viral encephalitis in Asia. This geographically defined focal disease is considered a public health issue due to its high case fatality rate and notable long-term neurological effects among survivors (Cheng et al., 2022). The WHO recommends including the JE vaccine in national vaccination programs in every region where the illness is a top public health concern. The WHO estimates that 15,000 people die of JE each year, with about 50,000 cases occurring globally (Erlanger et al., 2009). The first recorded case of JE occurred in Japan in 1871 (Kuno, 2022). The yearly incidence of JE varies between 10 and 100 per 100,000 people in endemic locations (Singh and Agarwal, 2005). Taiwan, Vietnam, Thailand, South Korea, North Korea, Japan, and the People’s Republic of China routinely vaccinate children against JE (Vannice et al., 2021). Insufficient laboratory assistance, reporting issues, and challenges in clinically recognizing the disease have prevented countries from producing sufficient JE surveillance data. Progress toward JE prevention and control will be sustained with the support of increased JE surveillance, sustained dedication, and sufficient funding for JE immunization (Asawapaithulsert et al., 2023). Children under the age of 15 years account for 75% of JE cases (Park, 2022). JEV transmission is endemic in 24 countries in the WHO South-East Asia and Western Pacific area, placing over 3 billion people at risk of contracting the virus (Sahu et al., 2022). This illness has no known cure. The only type of treatment is supportive. There is a vaccination against JE that is both safe and effective. In any region where JE disease is acknowledged as a public health issue, the WHO recommends that JE vaccination be included in the national immunization schedule (Heffelfinger et al., 2016). Economic impactThe effects of JEV on the economy are not well understood. From 2013 to 2018, the combined direct (U.S. $8.32 million) and indirect (U.S. $3.69 million) expenses of JEV in Zhejiang Province, China, were projected to be U.S. $12.01 million (Deng et al., 2021). The estimated economic impact was based on 149 verified JEV cases reported in China’s case-based JEV surveillance system and National Notifiable Diseases Registration System. The authors estimated the actual number of JEV cases using an expansion factor (EF) of three because misdiagnosis of JEV is widespread and JEV cases are underreported worldwide. According to the 2006–2009 Acute Meningitis and Encephalitis Syndrome Surveillance study, which found that JEV cases were underreported in China by two to three times, an EF of three was utilized (Deng et al., 2021). Both medical and non-medical expenses related to treating a JEV infection are considered direct costs (e.g., nutritional supplements, transportation to doctor’s appointments, and employing caregivers) (Nguyen et al., 2023). Time missed working for patients and their caregivers are examples of indirect expenses. The average cost per JEV case was U.S. $26,871, and it was determined that men’s mean costs were higher (U.S. $30,018) than women’s (U.S. $21,435) (Nguyen et al., 2024). The economic costs of JEV were further categorized by occupation, age, insurance coverage, place of residence (rural vs. urban), and whether the patient had received a JEV vaccination. About 60% of Australia’s commercial pig industry was impacted by the 2022 JEV genotype IV outbreak, with an estimated U.S. $250,000 loss per 1,000 sows. It is estimated that impacted farms will experience yearly production losses of 3%–6% (Harrison et al., 2024). The economic impact of an outbreak on domestic swine herds is unknown if JEVs expand in the US. On March 6, 2024, the Swine Health Information Center published an economic assessment of a hypothetical JEV outbreak in the United States. According to estimates of the number of commercial pigs kept in naturally aired versus mechanically ventilated pens in the United States and comparable production losses in Australia, the outbreak is expected to cost between U.S. $306 million and U.S. $612 million (Hoad et al., 2022). TreatmentSupportive treatment for JE includes seizure management and lowering elevated intracranial pressure when they happen. Corticosteroids have been used for many years; however, dexamethasone did not show any effect in double-blind, placebo-controlled randomized trials (Ajibowo et al., 2021). To lower the risk of contractures, malnourishment, and bedsores, careful nursing care and physical therapy are required (Kunjarkar et al., 2022). Patients with a diminished gag reflex are frequently at risk of aspiration pneumonia. As of this writing, JE has no known cure. In animal models, monoclonal antibodies appear to work well, whereas isoquinolone molecules work well in vitro (Calvert et al., 2019). At present, the most promising possible treatment is interferon-α. Antiviral properties in vitro and are naturally generated in the CSF in response to JEV infection. Recombinant interferon-α is presently undergoing evaluation in double-blind, placebo-controlled trials after being administered to a small number of patients in open trials with positive outcomes (Sahu et al., 2022). Other potential therapeutic strategies are being explored, including the use of ribosome inhibitors such as N-nonyl-deoxynojirimycin (N-N-DNJ) to stop the progression of JEV and synthetic oligonucleotide dnase targeting the 3”′-untranslated region of JEV to inhibit viral replication. An example is nitazoxanide (Su et al., 2024). VaccinationCurrently, a large number of approved vaccines are used to prevent JEV; nevertheless, safety and specificity metrics are obtained through thorough testing in animal models. In the Asia–Pacific, three JE vaccines, namely JE live-attenuated vaccine (JEV-L), JE inactivated vaccine (Vero cell) (JEV-I(Vero)), and JE inactivated vaccine (primary hamster kidney cell) (JEV-I(PHK)), are commonly used to protect children from JE infection, and their safety and effectiveness have been the subject of meta-analyses (Wang et al., 2015). Three JEV vaccinations that are commonly used in the Asia–Pacific region to protect newborns and children against JE were the subjects of a previously published meta-analysis on their safety and effectiveness. The use of JEV-I (PHK) and JEV-L (PHK) is questioned logically by immunologists because of the presence of diverse cellular matrix proteins in vaccines made by PHK cells (Satchidanandam, 2020). The host may become more sensitive to vaccinations as a result of the expression of these cellular matrix proteins. However, the JEV (Vero) vaccine exhibited very low immunogenicity despite demonstrating high safety. The positive therapeutic effects of IgM, TLR7, and type I IFN on JE pathogenesis have also been demonstrated in a number of mouse models (Upadhyay, 2013). Similar to this, substances such as ribavirin, arctigenin, curcumin, minocycline, and glucosidase inhibitors aid in controlling the generation of proinflammatory mediators, microglial activation and differentiation, caspase-3 activation, and cellular death in JEV infection (Zhu et al., 2023b). ControlIn general, strategies to prevent human illness and interfere with the virus’s enzootic cycle are used to control JE. Attempts to prevent Culex mosquitoes from reproducing, such as by applying insecticides and using larvicides in rice fields, have not worked (Sahu et al., 2022). Pigs are vaccinated against the virus using attenuated and inactivated vaccines; however, most regions do not allow for extensive immunization (Srivastava et al., 2023). To reduce the frequency of Culex mosquito bites, residents, and visitors to endemic areas should wear personal protective equipment. These precautions include sleeping under a mosquito net, wearing clothing that exposes as little skin as possible, applying insect repellent with at least 30% DEET (N, N-diethyl-3 methylbenzamide), and limiting time spent outside at nightfall and dawn (Ghali and Albers, 2024). These precautions are mainly unfeasible for residents of endemic areas, although they might be possible for temporary visitors. According to high-level experts, two rounds of indoor residual spray (IRS) with benzene hexachloride (B.H.C.) 50% water dispersible powder (wdp) at 60-day intervals each, outdoor room spray with malathion technical (5.0%), and indoor room spray with pyrethrum (0.1%), along with personal protective measures, were advised because the occurrence of seizures and encephalopathy was thought to be caused by JE and necessitated anti-vector measures (Chandra et al., 2021). Following the program experts’ recommendations, B.H.C. 50% gdp (active ingredient dose 0.2 g/m2) was sprayed on the entire population of the severely affected areas in two rounds of 60 days each (Chandra et al., 2021). Additionally, motorized sprayers or indoor spraying equipment are available in these regions, as well as portable and vehicle-mounted thermal fogging machines for outdoor spraying. Resources were also provided for IRS and outdoor spraying in sole-impacted regions, although not everywhere. ConclusionJE is a serious zoonotic infectious disease caused by the JEV, a member of the Orthoflavivirus genus. JEV is primarily transmitted by mosquitoes of the Culex species. The disease poses a serious threat to public health, particularly in endemic areas of Asia and the Western Pacific. The first JEV-related encephalitis outbreak was documented in Japan and was recognized as the main cause of viral encephalitis in the Asian subcontinent. JE outbreaks have also been reported in northern Australia. JE mostly affects the cerebral cortex, cerebellum, anterior horn cells of the spinal cord, and thalamus. The disease’s initial symptoms show up six to fourteen days after the incubation period and typically begin with a temperature above 38°C, chills, headaches similar to meningitis, muscle aches, and vomiting. Important risk factors include travel to JE-endemic regions, proximity to animals, and age. According to the WHO, an estimated 15,000 people die due to JE each year, with about 50,000 cases occurring globally. Unfortunately, there is no known cure for JE, as the only available treatment is supportive. Intriguingly, there are some well-known vaccines that help in the prevention of JEV infection. A large number of approved vaccines are used to prevent JEV infection. The high morbidity and mortality rates associated with the disease, particularly among children, underscore the urgent need for effective vaccination and public health strategies to reduce its impact. AcknowledgmentsThe authors are grateful to Universitas Muhammadiyah Surabaya and Badan Riset dan Inovasi Nasional (BRIN). Author’s contributionsARRA, ARK, KAF, and AHF drafted the manuscript. IBM, SW, DAAK, and JJ revised and edited the manuscripts. FE, TPDR, WW, RZA, and IF participated in the preparation and critical checking of the manuscript. DA, ABN, MKJK, BWKW, and VR edited the references. All authors have read and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThis research was funded by the Faculty of Health Science, Universitas Muhammadiyah Surabaya, Surabaya, Indonesia. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAjibowo, A.O., Ortiz, J.F., Alli, A., Halan, T. and Kolawole, O.A. 2021. Management of Japanese encephalitis: a current update. Cureus 13(4), e14579. Amanna, I.J. and Slifka, M.K. 2011. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 411(2), 206–215. Amicizia, D., Zangrillo, F., Lai, P.L., Iovine, M. and Panatto, D. 2018. Overview of Japanese encephalitis disease and its prevention. Focus on IC51 vaccine (IXIARO®). J. Prev. Med. Hyg. 59(1), E99–E107. Asawapaithulsert, P., Ngamprasertchai, T. and Kitro, A. 2023. Japanese encephalitis vaccine acceptance and strategies for travelers: insights from a scoping review and practitioners in endemic countries. Vaccines 11(11), 1683. Ashraf, U., Ding, Z., Deng, S., Ye, J., Cao, S. and Chen, Z. 2021. Pathogenicity and virulence of Japanese encephalitis virus: neuroinflammation and neuronal cell damage. Virulence 12(1), 968–980. Bharucha, T., Shearer, F.M., Vongsouvath, M., Mayxay, M., de Lamballerie, X., Newton, P.N., Zitzmann, N., Gould, E. and Dubot-Pérès, A. 2020. A need to raise the bar - A systematic review of temporal trends in diagnostics for Japanese encephalitis virus infection, and perspectives for future research. Int. J. Infect. Dis. 95(1), 444–456. Buescher, E.L., Scherer, W.F., Rosenberg, M.Z., Gresser, I., Hardy, J.L. and Bullock, H.R. 1959. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am. J. Trop. Med. Hyg. 8(1), 651–664. Calvert, A.E., Bennett, S.L., Dixon, K.L., Blair, C.D. and Roehrig, J.T. 2019. A monoclonal antibody specific for Japanese encephalitis virus with high neutralizing capability for inclusion as a positive control in diagnostic neutralization tests. Am. J. Trop. Med. Hyg. 101(1), 233–236. Cao, L., Fu, S., Gao, X., Li, M., Cui, S., Li, X., Cao, Y., Lei, W., Lu, Z., He, Y., Wang, H., Yan, J., Gao, G.F. and Liang, G. 2016. Low protective efficacy of the current japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PLoS Negl. Trop. Dis. 10(5), e0004686. Chandra, R., Singh, N.P., Sharma, R.S., Kamal, S., Aggrawal, H.K. and Sharma, S.N. 2021. A review of the current status of Japanese encephalitis in Uttar Pradesh, India. J. Commun. Dis. 53(4), 119–134. Cheng, Y., Minh, N.T., Tran Minh, Q.T., Khandelwal, S. and Clapham, H.E. 2022. Estimates of Japanese Encephalitis mortality and morbidity: a systematic review and modeling analysis. PLoS Negl. Trop. Dis. 16(5), e0010361. Chiou, S.S., Chen, J.M., Chen, Y.Y., Chia, M.Y. and Fan, Y.C. 2021. The feasibility of field collected pig oronasal secretions as specimens for the virologic surveillance of Japanese encephalitis virus. PLoS Negl. Trop. Dis. 15(12), e0009977. Choe, Y.J., Jee, Y., Takashima, Y. and Lee, J.K. 2020. Japanese encephalitis in the Western Pacific Region: Implication from the Republic of Korea. Vaccine 38(13), 2760–2763. Connor, B. and Bunn, W.B. 2017. The changing epidemiology of Japanese encephalitis and new data: the implications for New recommendations for Japanese encephalitis vaccine. Trop. Dis. Travel Med. Vaccines 3(1), 14. Damayanti, P.A.A., Adi, A.A.A.M., Astawa, I.N.M., Sudarmaja, I.M., Kardena, I.M. and Swastika, I.K. 2017. Incidence of japanese encephalitis among children is associated with the presence of Pigs in Bali, Indonesia. Biomed. Pharmacol. J. 10(3), 1333–1338. de Vries, L. and Harding, A.T. 2023. Mechanisms of neuroinvasion and neuropathogenesis by pathologic flaviviruses. Viruses 15(2), 261. Deng, X., Yan, R., Li, Z.Q., Tang, X.W., Zhou, Y. and He, H. 2021. Economic and disease burden of Japanese encephalitis in Zhejiang Province, 2013-2018. PLoS Negl. Trop. Dis. 15(6), e0009505. Desai, A., Shankar, S.K., Ravi, V., Chandramuki, A. and Gourie-Devi, M. 1995. Japanese encephalitis virus antigen in the human brain and its topographic distribution. Acta Neuropathol. 89(4), 368–373. Dhanze, H., Bhilegaonkar, K.N., Kumar, C., Kumar, M.S., Singh, P. and Kumar, A. 2020. Development and evaluation of lateral flow assay for sero-diagnosis of Japanese encephalitis in swine. Anim. Biotechnol. 31(4), 350–356. Dixon, A.L., Oliveira, A.R.S., Cohnstaedt, L.W., Mitzel, D., Mire, C. and Cernicchiaro, N. 2024. Revisiting the risk of introduction of Japanese encephalitis virus (JEV) into the United States?an updated semi-quantitative risk assessment. One Health 19(1), 100879. Dobrzyńska, M., Moniuszko-Malinowska, A. and Skrzydlewska, E. 2023. Metabolic response to CNS infection with flaviviruses. J. Neuroinflammation. 20(1), 218. Erlanger, T.E., Weiss, S., Keiser, J., Utzinger, J. and Wiedenmayer, K. 2009. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 15(1), 1–7. Fan, Y.-C., Chen, J.-M., Chen, Y.-Y., Ke, Y.-D., Chang, G.-J.J. and Chiou, S.-S. 2024. Epitope(s) involving amino acids of the fusion loop of Japanese encephalitis virus envelope protein is(are) important to elicit protective immunity. J. Virol. 98(4), e0177323. Filgueira, L. and Lannes, N. 2019. Review of emerging japanese encephalitis virus: new aspects and concepts about entry into the brain and inter-cellular spreading. Pathogens 8(3), 111. Furlong, M., Adamu, A.M., Hoskins, A., Russell, T.L., Gummow, B., Golchin, M., Hickson, R.I. and Horwood, P.F. 2023. Japanese encephalitis enzootic and epidemic risks across Australia. Viruses 15(2), 450. Furuya-Kanamori, L., Gyawali, N., Mills, D.J., Hugo, L.E., Devine, G.J. and Lau, C.L. 2022. The emergence of Japanese encephalitis in australia and the implications for a vaccination strategy. Trop. Med. Infect. Dis. 7(6), 85. García-Nicolás, O., Ricklin, M.E., Liniger, M., Vielle, N.J., Python, S., Souque, P., Charneau, P. and Summerfield, A. 2017. A Japanese encephalitis virus vaccine inducing antibodies strongly enhancing in vitro infection is protective in pigs. Viruses 9(5), 124. Ghali, H. and Albers, S.E. 2024. An updated review on the safety of N, N-diethyl-meta-toluamide insect repellent use in children and the efficacy of natural alternatives. Pediatr. Dermatol. 41(3), 403–409. Grewe, S., Gliem, M., Abrar, D.B., Feldt, T., Wojtecki, L., Tan, V., Lifea, Afzal, S., Meuth, S.G., Luedde, T. and Orth, H.M. 2022. Myelitis with flaccid paralysis due to Japanese encephalitis: case report and review of the literature. Infection 50(6), 1597–1603. Guo, J., Mi, Y., Guo, Y., Bai, Y., Wang, M., Wang, W. and Wang, Y. 2024. Current advances in Japanese encephalitis virus drug development. Viruses 16(2), 202. Han, W., Gao, M., Xie, C., Zhang, J., Zhao, Z., Hu, X., Zhang, W., Liu, X., Cao, S., Cheng, G. and Gu, C. 2021. Precise localization and dynamic distribution of Japanese encephalitis virus in the rain nuclei of infected mice. PLoS Negl. Trop. Dis. 15(6), e0008442. Handique, S.K., Das, R.R., Saharia, B., Das, P., Buragohain, R. and Saikia, P. 2008. Coinfection of Japanese encephalitis with neurocysticercosis: an imaging study. AJNR Am. J. Neuroradiol. 29(1), 170–175. Harrison, J.J., Nguyen, W., Morgan, M.S., Tang, B., Habarugira, G., de Malmanche, H., Freney, M.E., Modhiran, N., Watterson, D., Cox, A.L., Yan, K., Yuen, N.K.Y., Bowman, D.H., Kirkland, P.D., Bielefeldt-Ohmann, H., Suhrbier, A., Hall, R.A., Rawle, D.J. and Hobson-Peters, J. 2024. A chimeric vaccine derived from Australian genotype IV Japanese encephalitis virus protects mice from lethal challenge. NPJ Vaccines 9(1), 134. Heffelfinger, J.D., Li, X., Batmunkh, N., Grabovac, V., Diorditsa, S., Liyanage, J.B., Pattamadilok, S., Bahl, S., Vannice, K.S., Hyde, T.B., Chu, S.Y., Fox, K.K., Hills, S.L. and Marfin, A.A. 2016. Japanese encephalitis surveillance and immunization?Asia and Western Pacific regions, 2016. MMWR Morb. Mortal. Wkly. Rep. 66(22), 579–583. Hegde, N.R. and Gore, M.M. 2017. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin. Immunother. 13(6), 1–18. Hick, P.M., Finlaison, D.S., Parrish, K., Gu, X., Hayton, P., O’Connor, T., Read, A., Zhang, J., Spiers, Z.B., Pinczowski, P., Ngo, A.L. and Kirkland, P.D. 2024. Experimental infections of pigs with Japanese encephalitis virus genotype 4. Microorganisms 12(11), 2163. Hills, S., Dabbagh, A., Jacobson, J., Marfin, A., Featherstone, D., Hombach, J., Namgyal, P., Rani, M., Solomon, T. and Japanese Encephalitis Core Working Group. 2009. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BMC Infect. Dis. 9(1), 214. Hills, S.L., Griggs, A.C. and Fischer, M. 2010. Japanese encephalitis in travelers from non-endemic countries, 1973-2008. Am. J. Trop. Med. Hyg. 82(5), 930–936. Hills, S.L., Netravathi, M. and Solomon, T. 2023. Japanese encephalitis among adults: a review. Am. J. Trop. Med. Hyg. 108(5), 860–864. Hills, S.L., Walter, E.B., Atmar, R.L., Fischer, M. and ACIP Japanese Encephalitis Vaccine Work Group. 2019. Japanese encephalitis vaccine: recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 68(2), 1–33. Hoad, V.C., Kiely, P., Seed, C.R., Viennet, E. and Gosbell, I.B. 2022. An outbreak of Japanese encephalitis virus in Australia; what is the risk to blood safety? Viruses 14(9), 1935. Hsieh, J.T., Rathore, A.P.S., Soundarajan, G. and St John, A.L. 2019. Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat. Commun. 10(1), 706. Jain, N., Oswal, N., Chawla, A.S., Agrawal, T., Biswas, M., Vrati, S., Rath, S., George, A., Bal, V. and Medigeshi, G.R. 2017. CD8 T cells protect adult naive mice from JEV-induced morbidity via lytic function. PLoS Negl. Trop. Dis. 11(2), e0005329. Kamath, S.D., Jha, B., Ahmed, T. and Sarkar, N. 2023. A profile study of Japanese encephalitis in an industrial hospital in Eastern India. Cureus 15(5), e38455. Keiser, J., Maltese, M.F., Erlanger, T.E., Bos, R., Tanner, M., Singer, B.H. and Utzinger, J. 2005. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 95(1), 40–57. Klein, M.J., Jackson, S.A., Suen, W.W., Payne, J., Beveridge, D., Hargreaves, M., Gillies, D., Wang, J., Blasdell, K.R., Dunn, M., López-Denman, A.J., Durr, P.A., Williams, D.T. and Paradkar, P.N. 2024. Australian Culex annulirostris mosquitoes are competent vectors for Japanese encephalitis virus genotype IV. Emerg. Microbes Infect. 13(1), 2429628. Kulkarni, R., Sapkal, G.N., Kaushal, H. and Mourya, D.T. 2018. Japanese encephalitis: a brief review on Indian perspectives. Open Virol. J. 12(1), 121–130. Kumar, K., Arshad, S.S., Selvarajah, G.T., Abu, J., Toung, O.P., Abba, Y., Bande, F., Yasmin, A.R., Sharma, R., Ong, B.L., Rasid, A.A., Hashim, N., Peli, A., Heshini, E.P. and Shah, A.K.M.K. 2018. Prevalence and risk factors of Japanese encephalitis virus (JEV) in livestock and companion animal in high-risk areas in Malaysia. Trop. Anim. Health Prod. 50(4), 741–752. Kumar, S., Verma, A., Yadav, P., Dubey, S.K., Azhar, E.I., Maitra, S.S. and Dwivedi VD. 2022. Molecular pathogenesis of Japanese encephalitis and possible therapeutic strategies. Arch. Virol. 167(9), 1739–1762. Kumari, R., Kumar, K., Rawat, A., Singh, G., Yadav, N.K. and Chauhan, L.S. 2013. First indigenous transmission of Japanese Encephalitis in urban areas of National Capital Territory of Delhi, India. Trop. Med. Int. Health 18(6), 743–749. Kunjarkar, K., Harjpal, P. and Samal, S. 2022. Rehabilitative approach toward a japanese encephalitis patient via therapy ball: a case report. Cureus 14(10), e30883. Kuno, G. 2022. Contrasting the practices of virus isolation and characterization between the early period in history and modern times: the case of Japanese encephalitis virus. Viruses 14(12), 2640. Kwak, N., Park, B.J., Song, Y.J. (2023). A CRISPR-Cas12a-based diagnostic method for Japanese encephalitis virus genotypes I, III, and V. Biosensors.13(8): 769. Lannes, N., Summerfield, A. and Filgueira, L. 2017. Regulation of inflammation in Japanese encephalitis. J. Neuroinflammation. 14(1), 158. Laureti, M., Narayanan, D., Rodriguez-Andres, J., Fazakerley, J.K. and Kedzierski, L. 2018. Flavivirus receptors: diversity, identity, and cell entry. Front. Immunol. 9(1), 2180. Lee, A.R., Kim, S.H., Hong, S.Y., Lee, S.H., Oh, J.S., Lee, K.Y., Kim, S.J., Ishikawa, T., Shim, S.M., Lee, H.I. and Seo, S.U. 2024. Characterization of genotype V Japanese encephalitis virus isolates from Republic of Korea. Emerg. Microbes Infect. 13(1), 2362392. Li, F., Wang, Y., Yu, L., Cao, S., Wang, K., Yuan, J., Wang, C., Wang, K., Cui, M. and Fu, Z.F. 2015. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J. Virol. 89(10), 5602–5614. Liu, W., Gibbons, R.V., Kari, K., Clemens, J.D., Nisalak, A., Marks, F. and Xu, Z.Y. 2010. Risk factors for Japanese encephalitis: a case-control study. Epidemiol. Infect. 138(9), 1292–1297. Lopez, A.L., Aldaba, J.G., Roque, V.G. Jr., Tandoc, A.O., Sy, A.K., Espino, F.E., DeQuiroz-Castro, M., Jee, Y., Ducusin, M.J., Fox, K.K. 2015. Epidemiology of Japanese encephalitis in the Philippines: a systematic review. PLoS Negl. Trop. Dis. 9(3): e0003630. Luca, V.C., AbiMansour, J., Nelson, C.A. and Fremont, D.H. 2012. Crystal structure of the Japanese encephalitis virus envelope protein. J. Virol. 86(4), 2337–2346. Ludlow, M., Kortekaas, J., Herden, C., Hoffmann, B., Tappe, D., Trebst, C., Griffin, D.E., Brindle, H.E., Solomon, T., Brown, A.S., van Riel, D., Wolthers, K.C., Pajkrt, D., Wohlsein, P., Martina, B.E.E., Baumgärtner, W., Verjans, G.M. and Osterhaus, A.D.M.E. 2016. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 131(2), 159–184. Maeki, T., Tajima, S., Ikeda, M., Kato, F., Taniguchi, S., Nakayama, E., Takasaki, T., Lim, C.K. and Saijo, M. 2019. Analysis of cross-reactivity between flaviviruses with sera of patients with Japanese encephalitis showed the importance of neutralization tests for the diagnosis of Japanese encephalitis. J. Infect. Chemother. 25(10), 786–790. Mansfield, K.L., Hernández-Triana, L.M., Banyard, A.C., Fooks, A.R. and Johnson, N. 2017. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 201(1), 85–92. Mayxay, M., Douangdala, P., Vilayhong, C., Phommasone, K., Chansamouth, V., Vongsouvath, M., Rattanavong, S., Chang, K., Sengvilaipaseuth, O., Chanthongthip, A., Thongpaseuth, S., Newton, P.N. and Dubot-Pérès, A. 2020. Outcome of Japanese Encephalitis Virus (JEV) infection in pediatric and adult patients at Mahosot Hospital, Vientiane, Lao PDR. Am. J. Trop. Med. Hyg. 104(2), 567–575. McGuinness, S.L., Lau, C.L. and Leder, K. 2023. The evolving Japanese encephalitis situation in Australia and implications for travel medicine. J. Travel. Med. 30(2), taad029. Mehta, A., Singh, R., Mani, V.E. and Poddar, B. 2021. Japanese B Encephalitis. Indian J. Crit. Care Med. 25(Suppl 2), S171–S174. Mi, Y., Guo, Y., Luo, X., Bai, Y., Chen, H., Wang, M., Wang, Y. and Guo, J. 2024. Natural products and derivatives as Japanese encephalitis virus antivirals. Pathog. Dis. 82(1), ftae022. Michael, B.D. and Solomon, T. 2012. Seizures and encephalitis: clinical features, management, and potential pathophysiologic mechanisms. Epilepsia 53(Suppl 4), 63–71. Mohapatra, S., Chakraborty, T. and Basu, A. 2023. Japanese Encephalitis virus infection in astrocytes modulate microglial function: correlation with inflammation and oxidative stress. Cytokine 170(1), 156328. Mohsin, F., Suleman, S., Anzar, N., Narang, J. and Wadhwa, S. 2022. A review on Japanese Encephalitis virus emergence, pathogenesis and detection: from conventional diagnostics to emerging rapid detection techniques. Int. J. Biol. Macromol. 217(1), 435–448. Monath, T.P. 2023. Japanese encephalitis: risk of emergence in the United States and the resulting impact. Viruses 16(1), 54. Money, K.M., Chauhan, L., Piquet, A.L., Tyler, K.L. and Pastula, D.M. 2023. An overview of Japanese encephalitis. Neurohospitalist. 13(3), 328–330. Moore, S.M. 2021. The current burden of Japanese encephalitis and the estimated impacts of vaccination: Combining estimates of the spatial distribution and transmission intensity of a zoonotic pathogen. PLoS Negl. Trop. Dis. 15(10), e0009385. Mulvey, P., Duong, V., Boyer, S., Burgess, G., Williams, D.T., Dussart, P. and Horwood, P.F. 2021. The ecology and evolution of Japanese encephalitis virus. Pathogens 10(12), 1534. Nazmi, A., Mukherjee, S., Kundu, K., Dutta, K., Mahadevan, A., Shankar, S.K. and Basu, A. 2014. TLR7 is a key regulator of innate immunity against Japanese encephalitis virus infection. Neurobiol. Dis. 69(1), 235–247. Nemeth, N., Bosco-Lauth, A., Oesterle, P., Kohler, D. and Bowen, R. 2012. North American birds as potential amplifying hosts of Japanese encephalitis virus. Am. J. Trop. Med. Hyg., 87(4), 760–767. Nguyen, A., Sultana, R., Vodicka, E., Tasnim, Z., Mehedi, K., Islam, M.M., Al Murad, S.M.A., Ullah, M.R., Sultana, S., Shirin, T. and Pecenka, C. 2024. Cost-effectiveness analysis of Japanese encephalitis vaccination for children <15 years of age, Bangladesh. Emerg. Infect. Dis. 30(12), 2593–2603. Nguyen, A.L.T., Slavkovsky, R., Phan, H.T., Nguyen, H.T.T., Vannachone, S., Le, D.H., Dubot-Pérès, A., Vongsouvath, M., Dinh, S.T., Marfin, A.A., Letson, G.W., Vu, H.M., Tham, D.C., Mayxay, M., Ashley, E.A., Pham, T.Q. and Pecenka, C. 2023. Estimating the cost of illness of acute Japanese encephalitis and sequelae care in Vietnam and Laos: a cross-sectional study. PLOS Glob. Public Health 3(6), e0001873. Park, S.E. 2022. Importance of maintaining a high childhood vaccination rate and surveillance program against Japanese encephalitis in Korea. Clin. Exp. Pediatr. 65(3), 127–128. Patel, B., Bhatt, G.C., Kushwaha, K.P. and Gore, M.M. 2015. Japanese encephalitis presenting without cerebrospinal fluid pleocytosis. Pediatr. Infect. Dis. J. 34(12), 1416. Patel, J.P., Verma, K. and Singh, V. 2021. Japanese encephalitis (JE): a curse for people living in Uttar Pradesh, India. J. Vaccines Immunol. 7(1), 36–40. Phukan, P., Sarma, K., Sharma, B.K., Boruah, D.K., Gogoi, B.B. and Chuunthang, D. 2021. MRI spectrum of Japanese encephalitis in Northeast India: a cross-sectional study. J. Neurosci. Rural Pract. 12(2), 281–289. Poonsiri, T., Wright, G.S.A., Solomon, T. and Antonyuk, S.V. 2019. Crystal structure of the Japanese encephalitis virus capsid protein. Viruses 11(7), 623. Prakash, M., Kumar, S. and Gupta, R.K. 2004. Diffusion-weighted MR imaging in Japanese encephalitis. J. Comput. Assist. Tomogr. 28(6), 756–761. Pushpakumara, P.D., Jeewandara, C., Gomes, L., Perera, Y., Wijewickrama, A., Malavige, G.N. and Goonesekara, C. 2020. Development and validation of an assay for detection of Japanese encephalitis virus specific antibody responses. PLoS One 15(10), e0238609. Rathore, A.P.S. and St John, A.L. 2020. Cross-reactive immunity among flaviviruses. Front. Immunol. 11(1), 334. Ravi, V., Desai, A., Balaji, M., Apte, M.P., Lakshman, L., Subbakrishna, D.K., Sridharan, G., Dhole, T.N. and Ravikumar, B.V. 2006. Development and evaluation of a rapid IgM capture ELISA (JEV-Chex) for the diagnosis of Japanese encephalitis. J. Clin. Virol. 35(4), 429–434. Ravi, V., Robinson, J.S., Russell, B.J., Desai, A., Ramamurty, N., Featherstone, D. and Johnson, B.W. 2009. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am. J. Trop. Med. Hyg. 81(6), 1144–1150. Robinson, J.S., Featherstone, D., Vasanthapuram, R., Biggerstaff, B.J., Desai, A., Ramamurty, N., Chowdhury, A.H., Sandhu, H.S., Cavallaro, K.F. and Johnson, B.W. 2010. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am. J. Trop. Med. Hyg. 83(5), 1146–1155. Sahu, R.C., Suthar, T., Pathak, A. and Jain, K. 2022. Interventions for the prevention and treatment of Japanese encephalitis. Curr. Infect. Dis. Rep. 24(11), 189–204. Saito, Y., Moi, M.L., Takeshita, N., Lim, C.K., Shiba, H., Hosono, K., Saijo, M., Kurane, I. and Takasaki, T. 2016. Japanese encephalitis vaccine-facilitated dengue virus infection-enhancement antibody in adults. BMC Infect. Dis. 16(1), 578. Satchidanandam, V. 2020. Japanese encephalitis vaccines. Curr. Treat Options Infect. Dis. 12(4), 375–386. Sayeed, U., Wadhwa, G., Jamal, Q.M.S., Kamal, M.A., Akhtar, S., Siddiqui, M.H. and Khan, M.S. 2018. MHC binding peptides for designing of vaccines against Japanese encephalitis virus: a computational approach. Saudi J. Biol. Sci. 25(8), 1546–1551. Sharma, K.B., Vrati, S. and Kalia, M. 2021. Pathobiology of Japanese encephalitis virus infection. Mol. Aspects Med. 81(1), 100994. Shlim, D.R. and Solomon, T. 2002. Japanese encephalitis vaccine for travelers: exploring the limits of risk. Clin. Infect. Dis. 35(2), 183–188. Singh, U., Padhi, B.K., Suresh, V., Jindal, H. and Sah, R. 2023. Emergence of Japanese encephalitis in nonendemic regions of India: a public health concern? Ann. Med. Surg. 85(5), 2250–2252. Singh, Z. and Agarwal, V.K. 2005. Japanese encephalitis: is routine immunization required? Med. J. Armed. Forces India 61(4), 357–359. Solomon, T., Dung, N.M., Kneen, R., Thao, L.T.T., Gainsborough, M., Nisalak, A., Day, N.P., Kirkham, F.J., Vaughn, D.W., Smith, S. and White, N.J. 2002. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 125(Pt 5), 1084–1093. Solomon, T., Kneen, R., Dung, N.M., Khanh, V.C., Thuy, T.T., Ha, D.Q., Day, N.P., Nisalak, A., Vaughn, D.W. and White, N.J. 1998. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 351(9109), 1094–1097. Solomon, T., Ni, H., Beasley, D.W., Ekkelenkamp, M., Cardosa, M.J. and Barrett, A.D. 2003. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 77(5), 3091–3098. Sooryanarain, H., Ayachit, V. and Gore, M. 2012. Activated CD56(+) lymphocytes (NK+NKT) mediate immunomodulatory and anti-viral effects during Japanese encephalitis virus infection of dendritic cells in-vitro. Virology 432(2), 250–260. Srivastava, K.S., Jeswani, V., Pal, N., Bohra, B., Vishwakarma, V., Bapat, A.A., Patnaik, Y.P., Khanna, N. and Shukla, R. 2023. Japanese encephalitis virus: an update on the potential antivirals and vaccines. Vaccines 11(4), 742. Su, Y., Wang, Y., Xiong, C., Wang, X., Wang, C., Zhou, W., Zhou, D., Zhang, K. 2024. The modulation of proteomics and antioxidant stress is involved in the effect of nitazoxanide against Japanese encephalitis virus in vitro. Vet Microbiol. 298, 110289. Sunwoo, J.S., Lee, S.T., Jung, K.H., Park, K.I., Moon, J., Jung, K.Y., Kim, M., Lee, S.K. and Chu, K. 2017. Clinical characteristics of severe Japanese encephalitis: a case series from South Korea. Am. J. Trop. Med. Hyg. 97(2), 369–375. Suresh, K.P., Nayak, A., Dhanze, H., Bhavya, A.P., Shivamallu, C., Achar, R.R., Silina, E., Stupin, V., Barman, N.N., Kumar, S.K., Syed, A., Kollur, S.P., Shreevatsa, B. and Patil, S.S. 2022. Prevalence of Japanese encephalitis (JE) virus in mosquitoes and animals of the Asian continent: a systematic review and meta-analysis. J. Infect. Public Health 15(9), 942–949. Swami, R., Ratho, R.K., Mishra, B. and Singh, M.P. 2008. Usefulness of RT-PCR for the diagnosis of Japanese encephalitis in clinical samples. Scand. J. Infect. Dis. 40(10), 815–820. Tadokoro, K., Ohta, Y., Sato, K., Maeki, T., Sasaki, R., Takahashi, Y., Shang, J., Takemoto, M., Hishikawa, N., Yamashita, T., Lim, C.K., Tajima, S. and Abe, K. 2018. A Japanese encephalitis patient presenting with parkinsonism with corresponding laterality of magnetic resonance and dopamine transporter imaging findings. Intern. Med. 57(15), 2243–2246. Tiwari, S., Singh, R.K., Tiwari, R. and Dhole, T.N. 2012. Japanese encephalitis: a review of the Indian perspective. Braz. J. Infect. Dis. 16(6), 564–573. Tong, Y., Jiang, H., Xu, N., Wang, Z., Xiong, Y., Yin, J., Huang, J., Chen, Y., Jiang, Q. and Zhou, Y. 2023. Global Distribution of Culex tritaeniorhynchus and Impact Factors. Int. J. Environ. Res. Public Health 20(6), 4701. Turtle, L., Bali, T., Buxton, G., Chib, S., Chan, S., Soni, M., Hussain, M., Isenman, H., Fadnis, P., Venkataswamy, M.M., Satishkumar, V., Lewthwaite, P., Kurioka, A., Krishna, S., Shankar, M.V., Ahmed, R., Begum, A., Ravi, V., Desai, A., Yoksan, S., Fernandez, S., Willberg, C.B., Kloverpris, H.N., Conlon, C., Klenerman, P., Satchidanandam, V. and Solomon, T. 2016. Human T cell responses to Japanese encephalitis virus in health and disease. J. Exp. Med. 213(7), 1331–1352. Upadhyay, R.K. 2013. Biomarkers in Japanese encephalitis: a review. Biomed. Res. Int. 2013(1), 591290. Van den Eynde, C., Sohier, C., Matthijs, S. and De Regge, N. 2022. Japanese encephalitis virus interaction with mosquitoes: a review of vector competence, vector capacity and mosquito immunity. Pathogens 11(3), 317. van den Hurk, A.F., Skinner, E., Ritchie, S.A. and Mackenzie, J.S. 2022. The emergence of Japanese encephalitis virus in Australia in 2022: existing knowledge of mosquito vectors. Viruses 14(6), 1208. Vannice, K.S., Hills, S.L., Schwartz, L.M., Barrett, A.D., Heffelfinger, J., Hombach, J., Letson, G.W., Solomon, T., Marfin, A.A. and Japanese encephalitis vaccination experts panel. 2021. The future of Japanese encephalitis vaccination: expert recommendations for achieving and maintaining optimal JE control. NPJ Vaccines 6(1), 82. Waller, C., Tiemensma, M., Currie, B.J., Williams, D.T, Baird, R.W. and Krause, V.L. 2022. Japanese encephalitis in Australia?a sentinel case. N. Engl. J. Med. 387(7), 661–662. Wang, S.Y., Cheng, X.H., Li, J.X., Li, X.Y., Zhu, F.C. and Liu, P. 2015. Comparing the immunogenicity and safety of 3 Japanese encephalitis vaccines in Asia–Pacific area: a systematic review and meta-analysis. Hum. Vaccin. Immunother. 11(6), 1418–1425. Wieczorek, M., Abualrous, E.T., Sticht, J., Álvaro-Benito, M., Stolzenberg, S., Noé, F. and Freund, C. 2017. Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front. Immunol. 8(1), 292. Wu, S.C., Yu, C.H., Lin, C.W. and Chu, I.M. 2003. The domain III fragment of Japanese encephalitis virus envelope protein: mouse immunogenicity and liposome adjuvanticity. Vaccine 21(19–20), 2516–2522. Xia, Q., Yang, Y., Zhang, Y., Zhou, L., Ma, X., Xiao, C., Zhang, J., Li, Z., Liu, K., Li, B., Shao, D., Qiu, Y., Wei, J. and Ma, Z. 2023. Shift in dominant genotypes of Japanese encephalitis virus and its impact on current vaccination strategies. Front. Microbiol. 14(1), 1302101. Xiong, J., Yan, M., Zhu, S., Zheng, B., Wei, N., Yang, L., Si, Y., Cao, S. and Ye, J. 2022. Increased cleavage of Japanese encephalitis virus prM protein promotes viral replication but attenuates virulence. Microbiol. Spectr. 10(3), e0141722. Yadav, P., Chakraborty, P., Jha, N.K., Dewanjee, S., Jha, A.K., Panda, S.P., Mishra, P.C., Dey, A. and Jha, S.K. 2022. Molecular mechanism and role of Japanese encephalitis virus infection in central nervous system-mediated diseases. Viruses 14(12), 2686. Yamada, M., Nakamura, K., Yoshii, M. and Kaku, Y. 2004. Nonsuppurative encephalitis in piglets after experimental inoculation of Japanese encephalitis flavivirus isolated from pigs. Vet. Pathol. 41(1), 62–67. Yun, S.I. and Lee, Y.M. 2014. Japanese encephalitis: the virus and vaccines. Hum. Vaccin. Immunother. 10(2), 263–279. Zhang, W., Yu, Q., Gao, X., Chen, H., Su, J., Chen, Y., Li, Y., Zhang, N., Fu, Z. and Cui, M. 2024. Myeloid-derived suppressor cells induce exhaustion-like CD8+ T cells during JEV infection. Int. J. Biol. Sci. 20(15), 5959–5978. Zhang, X., Jia, R., Shen, H., Wang, M., Yin, Z. and Cheng, A. 2017. Structures and functions of the envelope glycoprotein in flavivirus infections. Viruses 9(11), 338. Zhang, X., Zhang, Y., Jia, R., Wang, M., Yin, Z. and Cheng, A. 2021. Structure and function of capsid protein in flavivirus infection and its applications in the development of vaccines and therapeutics. Vet. Res. 52(1), 98. Zheng, Y., Li, M., Wang, H. and Liang, G. 2012. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev. Med. Virol. 22(5), 301–322. Zhu, Y., Chen, S., Lurong, Q. and Qi, Z. 2023b. Recent advances in antivirals for Japanese encephalitis virus. Viruses 15(5), 1033. Zhu, Y., He, Z. and Qi, Z. 2023a. Virus-host interactions in early Japanese encephalitis virus infection. Virus Res. 331(1), 199120. Zimmerman, M.D., Scott, R.M., Vaughn, D.W., Rajbhandari, S., Nisalak, A. and Shrestha, M.P. 1997. Short report: an outbreak of Japanese encephalitis in Kathmandu, Nepal. Am. J. Trop. Med. Hyg. 57(3), 283–284. | ||
| How to Cite this Article |
| Pubmed Style Arimurti ARR, Khairullah AR, Artanti D, Rohmayani V, Rahmani TPD, Nugraha AB, Furqoni AH, Kusala MKJ, Wardhani BWK, Ekawasti F, Moses IB, Wasito W, Wibowo S, Julaeha J, Ahmad RZ, Fauzia KA, Fauziah I, Kurniasih DAA. The alarming spread of Japanese encephalitis: A growing public health concern. Open Vet. J.. 2025; 15(4): 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 Web Style Arimurti ARR, Khairullah AR, Artanti D, Rohmayani V, Rahmani TPD, Nugraha AB, Furqoni AH, Kusala MKJ, Wardhani BWK, Ekawasti F, Moses IB, Wasito W, Wibowo S, Julaeha J, Ahmad RZ, Fauzia KA, Fauziah I, Kurniasih DAA. The alarming spread of Japanese encephalitis: A growing public health concern. https://www.openveterinaryjournal.com/?mno=233527 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.1 AMA (American Medical Association) Style Arimurti ARR, Khairullah AR, Artanti D, Rohmayani V, Rahmani TPD, Nugraha AB, Furqoni AH, Kusala MKJ, Wardhani BWK, Ekawasti F, Moses IB, Wasito W, Wibowo S, Julaeha J, Ahmad RZ, Fauzia KA, Fauziah I, Kurniasih DAA. The alarming spread of Japanese encephalitis: A growing public health concern. Open Vet. J.. 2025; 15(4): 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 Vancouver/ICMJE Style Arimurti ARR, Khairullah AR, Artanti D, Rohmayani V, Rahmani TPD, Nugraha AB, Furqoni AH, Kusala MKJ, Wardhani BWK, Ekawasti F, Moses IB, Wasito W, Wibowo S, Julaeha J, Ahmad RZ, Fauzia KA, Fauziah I, Kurniasih DAA. The alarming spread of Japanese encephalitis: A growing public health concern. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 Harvard Style Arimurti, A. R. R., Khairullah, . A. R., Artanti, . D., Rohmayani, . V., Rahmani, . T. P. D., Nugraha, . A. B., Furqoni, . A. H., Kusala, . M. K. J., Wardhani, . B. W. K., Ekawasti, . F., Moses, . I. B., Wasito, . W., Wibowo, . S., Julaeha, . J., Ahmad, . R. Z., Fauzia, . K. A., Fauziah, . I. & Kurniasih, . D. A. A. (2025) The alarming spread of Japanese encephalitis: A growing public health concern. Open Vet. J., 15 (4), 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 Turabian Style Arimurti, Anindita Riesti Retno, Aswin Rafif Khairullah, Dita Artanti, Vella Rohmayani, Tara Puri Ducha Rahmani, Arifin Budiman Nugraha, Abdul Hadi Furqoni, Muhammad Khaliim Jati Kusala, Bantari Wisynu Kusuma Wardhani, Fitrine Ekawasti, Ikechukwu Benjamin Moses, Wasito Wasito, Syahputra Wibowo, Julaeha Julaeha, Riza Zainuddin Ahmad, Kartika Afrida Fauzia, Ima Fauziah, and Dea Anita Ariani Kurniasih. 2025. The alarming spread of Japanese encephalitis: A growing public health concern. Open Veterinary Journal, 15 (4), 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 Chicago Style Arimurti, Anindita Riesti Retno, Aswin Rafif Khairullah, Dita Artanti, Vella Rohmayani, Tara Puri Ducha Rahmani, Arifin Budiman Nugraha, Abdul Hadi Furqoni, Muhammad Khaliim Jati Kusala, Bantari Wisynu Kusuma Wardhani, Fitrine Ekawasti, Ikechukwu Benjamin Moses, Wasito Wasito, Syahputra Wibowo, Julaeha Julaeha, Riza Zainuddin Ahmad, Kartika Afrida Fauzia, Ima Fauziah, and Dea Anita Ariani Kurniasih. "The alarming spread of Japanese encephalitis: A growing public health concern." Open Veterinary Journal 15 (2025), 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 MLA (The Modern Language Association) Style Arimurti, Anindita Riesti Retno, Aswin Rafif Khairullah, Dita Artanti, Vella Rohmayani, Tara Puri Ducha Rahmani, Arifin Budiman Nugraha, Abdul Hadi Furqoni, Muhammad Khaliim Jati Kusala, Bantari Wisynu Kusuma Wardhani, Fitrine Ekawasti, Ikechukwu Benjamin Moses, Wasito Wasito, Syahputra Wibowo, Julaeha Julaeha, Riza Zainuddin Ahmad, Kartika Afrida Fauzia, Ima Fauziah, and Dea Anita Ariani Kurniasih. "The alarming spread of Japanese encephalitis: A growing public health concern." Open Veterinary Journal 15.4 (2025), 1505-1519. Print. doi:10.5455/OVJ.2025.v15.i4.1 APA (American Psychological Association) Style Arimurti, A. R. R., Khairullah, . A. R., Artanti, . D., Rohmayani, . V., Rahmani, . T. P. D., Nugraha, . A. B., Furqoni, . A. H., Kusala, . M. K. J., Wardhani, . B. W. K., Ekawasti, . F., Moses, . I. B., Wasito, . W., Wibowo, . S., Julaeha, . J., Ahmad, . R. Z., Fauzia, . K. A., Fauziah, . I. & Kurniasih, . D. A. A. (2025) The alarming spread of Japanese encephalitis: A growing public health concern. Open Veterinary Journal, 15 (4), 1505-1519. doi:10.5455/OVJ.2025.v15.i4.1 |