| Research Article | ||
Open Vet. J.. 2025; 15(4): 1685-1694 Open Veterinary Journal, (2025), Vol. 15(4): 1685-1694 Research Article The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practicesKaula A. Saad1*, Hanan A. Ageehal2, Ahmed S. Elgrari2 and Saleh A. Ammar21Libyan Authority for Scientific Research, Tripoli, Libya 2Administration of Zoonotic Disease Control, National Center of Diseases Control, Tripoli, Libya *Corresponding Author: Kaula. A. Saad. Libyan Authority for Scientific Research, Tripoli, Libya. Email: saadkaula [at] gmail.com Submitted: 17/12/2024 Accepted: 03/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
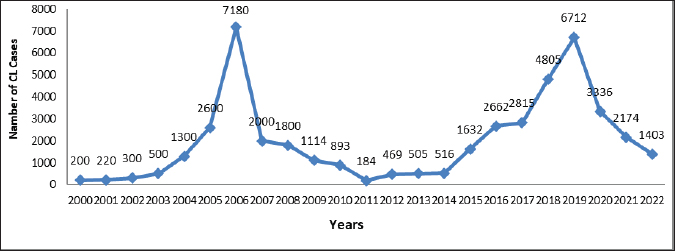
AbstractBackground: Cutaneous leishmaniasis (CL) has been a significant public health concern in Libya for five decades, with transmission dynamics that are both zoonotic and anthroponotic. The disease was first reported in 1930, and since then, it has become widespread, particularly in the north-western region. Aim: This study aimed to illustrate changes in the epidemiological characteristics and treatment practices of CL in Libya during the period 2019–2022. Methods: Data for this study were obtained from the Administration of Zoonotic Disease Control at the Libyan National Center for Disease Control NCDCL in Tripoli. The dataset included information from 40 endemic sites across 10 municipalities. A chi-square test was performed to analyze the relationships among 19 key variables related to the incidence, demographic distribution, and treatment patterns of CL. Results: Between 2019 and 2022, an estimated 13,625 CL cases were reported in Libyan hospitals, with fluctuating annual incidence rates. The highest number of cases was recorded in 2019, followed by a gradual decline over subsequent years. The age range of affected individuals ranged from 1 month to 95 years, with males accounting for 57.60% of cases and females accounting for 42.40%. Clinical diagnosis relied primarily on lesion features and epidemiological data, while laboratory confirmation was achieved using Giemsa staining. Cryotherapy has emerged as the predominant first-line treatment in regions with high disease prevalence. Conclusion: CL continues to pose a substantial public health challenge in Libya, intensified by the ongoing socio-political instability affecting disease surveillance and management systems. Comprehensive strategies integrating medical interventions and community-based initiatives are essential for mitigating the adverse medical and social impacts of CL in the country. Keywords: Cutaneous leishmaniasis, Epidemiology, Demographic criteria, Treatment practices. IntroductionLeishmaniasis, caused by various Leishmania parasite species (Sadeghi-Nejad and Saki, 2014), ranks as the fourth leading cause of morbidity among tropical diseases globally (Heidari-Kharaji et al., 2016). This condition predominantly affects impoverished individuals, particularly those living in poor housing and environmental conditions. According to the World Health Organization (WHO), Leishmaniasis is endemic in 98 countries, including Libya, where varying clinical forms cause the disease in >12 million people worldwide (Sadeghi-Nejad and Saki, 2014). This is an infectious disease transmitted by the bite of an infected female phlebotomine sand fly (Sadeghi-Nejad and Saki, 2014). The disease manifestations are cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis, and visceral leishmaniasis (VL) (PAHO/WHO, 2019). Libya is endemic for both VL and CL (Mehabresh, 1994; Postigo, 2010). CL: there are two forms Zoonotic:veterinary reservoirs/wild and domestic mammals as Zoonosis (ZCL), and anthroponotic (ACL): humans as the main reservoir (Postigo, 2010). Leishmania major in Libya may cause epidemiological CL in the rural areas of Jabal al Gharbi and Jafara districts (Ashford et al., 1976; AI-Buni et al., 2000; Amro et al., 2012). On the other hand, Leishmania tropica-induced ACL spread up to Misrata for the first time in 2006 (Aoun et al., 2006) and later on in Al Jabal Al Gharbi, Murqub (Amro et al., 2012), Tripoli, Zantan and Gharyan later on (El-Badry et al., 2017). A major concern is that Libya does not have a reliably estimated burden of CL-level data due to suboptimal and incomplete surveillance and underreporting (Amro et al., 2012). As shown in Figure 1, the prevalence of the disease is high, as evidenced by the presence of 7,180 cases in different areas of rural governorates in 2006 (WHO/EMRO, 2009). The national annual data obtained from the hospitals for the treatment of CL in Libya by the National Center for Disease Control (NCDC) reported a total of 19,396 cases between 1971 and 2011. The vector control national leishmaniasis program control implemented in Libya seems to have had a positive response as the number of reported cases has been decreasing. After program initiation, the number of cases was significantly reduced, with only 1,800 cases reported by 2008 (Fig. 1) (WHO/EMRO, 2009). The WHO recommends a stepwise approach for CL treatment, commencing with local therapies and subsequently providing systemic treatment when appropriate. According to the WHO guidelines, first-line treatment consists of local approaches and simple wound care. These treatments include cryotherapy, intralesional antimonials, and topical treatments (Morizot et al., 2013; Aronson and Joya, 2019). If local therapy fails or is impractical, systemic treatment is recommended. The most widely used systemic treatments include sodium stibogluconate and meglumine antimoniate, which are pentavalent antimonials. Although there are doubts about its toxicity and the need for regular assessment, they are still the most popular systemic therapies for New World CL (Mitropoulos et al., 2010; Brito et al., 2017). Other treatment options such as fluconazole, itraconazole, and paromomycin have been found to have differing degrees of success against Old World CL (Gonzãlez et al., 2017; De Souza et al., 2021). The major methods for treating CL in Libya include cryotherapy and intralesional antimony, as well as systemic treatment using sodium stibogluconate or meglumine antimoniate (Alrashah, 2023). Other forms of systemic therapy, including oral rifampicin and isoniazid, have been effective in CL cases in Libya where systemic antimony is contraindicated or failed to provide satisfactory results. This combination therapy for 8 weeks was effective in managing CL in a Libyan patient with HIV (Al-Dwibe et al., 2014). In Libya, an armed civil struggle has persisted since 2011, which has seen people leave their homes forcibly and caused the deterioration of the health care system and the disruption of national health programs. Such risk factors contributed to the spread of CL, leading to a significant increase in the total number of registered cases, which exceeded 27,029 between 2012 and 2022 (Fig. 1). Against this backdrop, the present study aimed to evaluate the status of CL in Libya from 2019 to 2022, with a particular focus on prevalence rates, demographic factors, and treatment methods. The findings of this study have substantial implications for understanding the epidemiology and treatment of CL in Libya, which is critical for developing targeted public health interventions. By analyzing changes in epidemiological characteristics and treatment practices over this 4-year period, this study provides essential data for designing effective control strategies, optimizing resource allocation, and improving patient outcomes. Furthermore, the use of a comprehensive dataset from 40 endemic sites across 10 municipalities, combined with robust statistical analyses (chi-square tests), enhances the reliability and generalizability of the findings. These insights could inform future biomedical research, such as the development of new diagnostic tools, treatments, and vaccines, and contribute to global efforts to combat neglected tropical diseases.
Fig. 1. Cutaneous leishmaniasis (CL) disease annual incidence in Libya between 2000 and 2022.
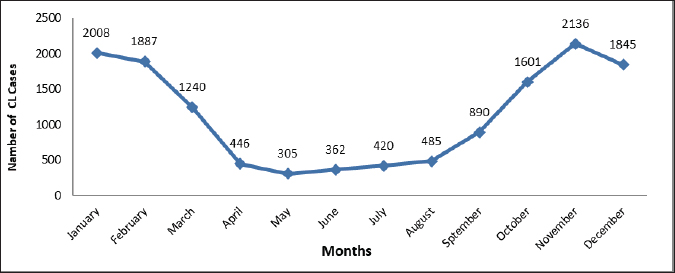
Fig. 2. Cutaneous leishmaniasis (CL) disease annual incidence rate in Libya from 2019 to 2022.
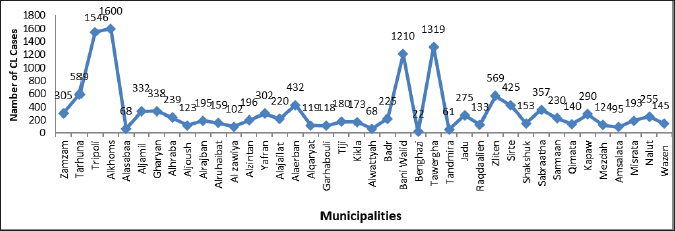
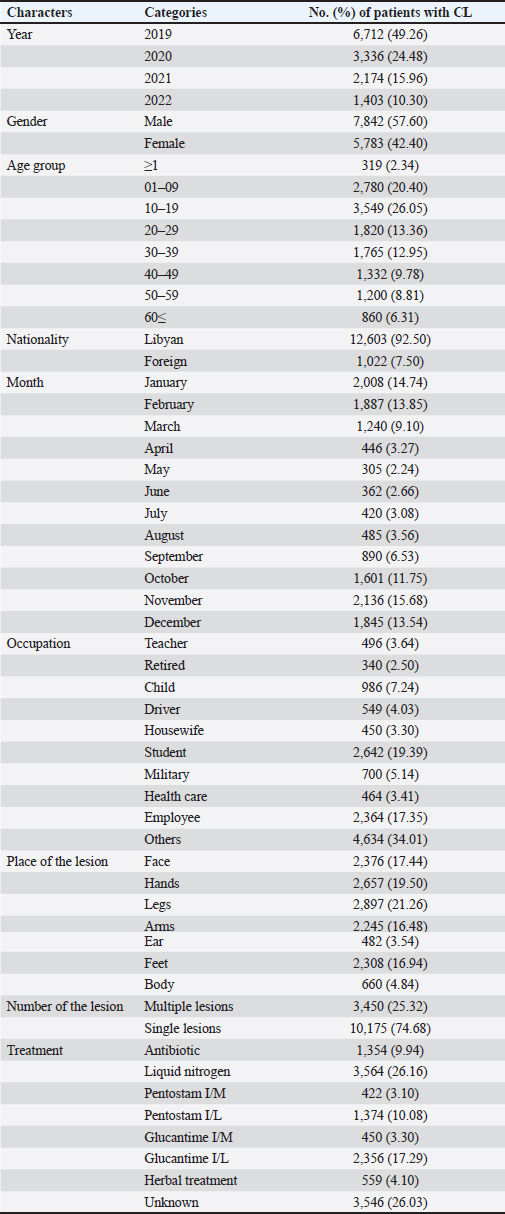
Fig. 3. Prevalence of cutaneous leishmaniasis (CL) in Libya during 2019–2022 by municipalities. Materials and MethodsThis was a retrospective descriptive study and did not require ethical approval or informed consent. The data made use of in these studies were obtained from the Administration of Zoonotic Disease Control-National Center for Disease Control, Libya (NCDCL) in Tripoli, which collected data from 40 endemic areas that take place in 10 municipalities shown in Figure 3. Individuals were diagnosed with CL in Libyan hospitals from January 2019 to December 2022 during the implementation of a national leishmaniasis-control program. Clinical diagnosis depends on the characteristics of the lesions and epidemiological data, while laboratory diagnosis is made using Giemsa staining, in which samples are taken from the patient’s lesions and then examined under a light microscope for signs of the leishmania parasite. Epidemiologic data included patient demographic characteristics such as age, sex, occupation, location or number of acute lesion(s), date of onset of lesion(s), previous travel to an area affected by the disease, place of residence, results of clinical examination and laboratory tests, type of treatment, and nationality of patients Excel program for Windows was used to perform a chi-square test for the relationships between the variables. Student’s t test was used for mean values comparison. p value of < 0.05 is regarded as a significant level. ResultsBetween January 2019 and December 2022, a cumulative total of 13,625 CL cases were reported from Libyan hospitals. In 2019, the number of cases was maximum, with 6,712 cases; the infection rate was 111 per 100,000 people. In 2020, there were 3,336 cases of CL, with an infection rate of 51 cases per 100,000 people. This trend was followed by 2,174 in 2021 and 1,403 in 2022, with infection rates of 20 cases per 100,000 population. The results indicated that the monthly prevalence of the disease differed. The highest numbers of cases were recorded in January (14.74%) and February (13.85%), followed by November (15.68%) and December (13.54%). The prevalence of CL varies by month, according to Figure 2. The demographic characteristics of the patients are presented in Table 1. We collected 13,625 cases, of which 42.4 % were female and 57.6 % were male. The results of the chi-square test in our analysis showed that there was no significant association between sex and disease (p < 0.05). However, the main disparities between age groups (p < 0.05) were observed. The patients aged 10–19 years accounted for the majority of patients with CL. Less than 6% of patients were 60 years old or older; only 2.3 years old or less. Of our study population, the youngest individual was 1-month-old and the oldest had reached 95 years of age. Table 1. Distribution of characteristics of cutaneous leishmaniasis (CL) in 13,625 patients in Libya from January 2019 to December 2022 (p value of < 0.05).
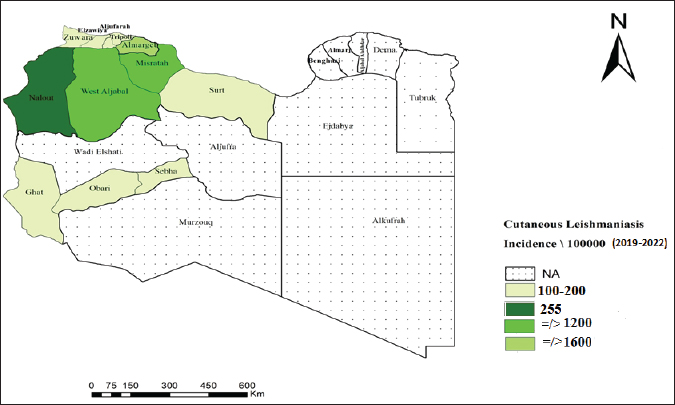
Lesions were found in various parts of the body, starting with the legs (21.26 %), followed by the hands (19.50%), face (17.44%), and other areas of the body. Approximately one-quarter (25.32 %) of patients had multiple lesions spread across their bodies. In addition, most patients (74.68 %) had only one lesion. The majority of patients (92.50 %) were Libyan nationals (p=0.001), and 1,022 (7.50%) were foreigners. The students had the highest lesion rate (19.39 %) among all occupations studied. No significant association was observed between occupation and disease (p < 0.05). The distribution of CL cases among Libyan municipalities, as depicted in Figure 3, indicates that cases were reported in 40 municipalities, with no reports from the eastern region. Notably, cases in Benghazi originated from residents displaced from Tawergha due to conflict. Figure 4 provides a geographical representation of the distribution of CL cases in Libya, expressed as incidence rates per 100,000 population, across various health areas from 2019 to 2022. The map highlights the spatial heterogeneity of CL cases, revealing areas with higher and lower disease burdens. The majority of cases were concentrated in the western region, particularly in the Al Jabal Al-Gharbi region and Tawergha (1,319 cases). Alkhoms had the highest number of infections (1,600), followed by Bani Walid (1,210), Tarhuna (589), Zliten (569 cases), Alaerban (432 cases), and others. Tripoli hospitals also documented 1,546 cases, including individuals with travel histories to endemic areas. The results of this study showed that 26.16 % of infected cases were treated with Liquid nitrogen, 17.29% were treated with Glucantime I/L, and 10.08% were treated with Pentostam I/L. Additionally, 3.10% and 3.30% were treated with Pentostam I/M and Glucantime I/M, respectively. Furthermore, 4.10% of the patients received herbal treatment, whereas 26.03% did not receive any recorded treatment (Table 1). DiscussionCL has been a persistent health concern in Libya for over four decades. The disease has posed significant challenges to public health in the region (Ashford et al., 1976; Ashford et al., 1977; Arshah, 2023). The distribution of CL cases among Libyan municipalities shows that cases were reported in 40 municipalities. The occurrence of CL cases in Benghazi linked to displaced residents from Tawergha due to conflict highlights the impact of social disruptions on disease transmission dynamics. This situation underscores the importance of considering population movements and living conditions to understand the epidemiology of infectious diseases, particularly in conflict-affected regions. The majority of cases were concentrated in the western region, with Alkhoms having the highest number of infections followed by Tawergha, Bani Walid, Zliten, Alaerban, and others. Tripoli hospitals also documented 1,546 cases, including individuals with travel histories to endemic areas. Various studies and reports have highlighted the prevalence and impact of CL in northern Libya (El-Buni et al., 2000, Ashour et al., 2022). These include the regions of Tawerga, Beni Walid, al Khams, Zawiya, and Jafara (Aoun et al., 2006), Yafran, Nalut, Al-Jabal Al-Gharbi (El-Buni et al., 2000; Belal et al., 2012; Abdellatif et al., 2013; Ashour et al., 2022), Sirte (Fathy et al., 2009; Abdel-Magied et al., 2016), Zantan, and Gharyan (El-Badry et al., 2015). In 2006, the Libyan National Center for Disease Control (NCDC) reported 7,180 cases of CL in humans in several districts (WHO/EMRO, 2009). Most CL cases were reported in the Tawerga villages, which are considered the highest endemic foci in Libya. High infection rates have given rise to public health warnings. After the initiation of the leishmaniasis control programme in 2008 in endemic areas under the auspices of the National Center for Disease Control (NCDC), aimed at reducing the number of vectors and reservoirs involved in the transmission of the infection, a substantial decrease in the number of cases was observed, with only 1,800 cases reported compared with 7,180 in 2006. This highlights the effectiveness of targeted control measures in mitigating the burden of CL in Libya (WHO/EMRO, 2009). The armed conflict in Libya since 2011 has had severe repercussions, including the disruption of the health care system and disease control programs, leading to a surge in CL cases. The escalation of risk factors due to the conflict has significantly impacted the proliferation of CL, with the number of registered cases increasing by more than 27,029 from 2012 to 2022, based on national annual reported cases in hospitals for the treatment of CL lesions. This study documented approximately 13,625 cases during the period 2019–2022, reflecting the substantial rise in CL cases amidst the challenging circumstances in Libya. The results indicate that CL infection was more prevalent in men (57.60 %) than in women (42.40 %), although the difference was not statistically significant (p < 0.05). Similar findings have been reported in other studies, suggesting a semi-equal distribution of infection between males and females (Abdellatif et al., 2013; Abo Assara et al., 2015, Elammari et al., 2015). Various risk factors, such as clothing habits, travel to endemic areas, and environmental factors, may contribute to the higher prevalence in men, increasing their exposure to sand cracking and direct contact with vectors. Additionally, behaviors like sleeping outside on hot nights may increase the susceptibility of males to blood-sucking arthropods, further explaining the observed pattern of infection distribution between genders. These findings align with previous studies in Pakistan (Jamal et al., 2013), Iraq (AlSamarai et al., 2009), and Iran (Soltani et al., 2019). In the current study, CL was observed across all age groups, with a higher prevalence noted in the 10–19 years age group, followed by the 1–9 years age group, consistent with previous research indicating a higher infection rate in children. These age groups correspond to school and work ages for both genders, potentially increasing exposure to sand fly-related environmental conditions due to outdoor activities (Almaasi et al., 2008; Feiz-Haddad et al., 2015; Fakhar et al., 2022). Conversely, the infection rate was 6.31% in the age group ≤60 years, possibly attributed to the development of lifelong immunity to CL infections, leading to a lower incidence in the elderly (Mendonãa, 2016). Lesions of CL can appear on any part of the body, and there are varying reports of unusual locations (Kevric et al., 2015; Mir et al., 2023). We observed the presence of both single and multiple lesions in a variety of cases, with 74.68 % having a single lesion and 25.32 % having two or more lesions (Mir et al., 2023). The distribution of CL lesions primarily on the legs, face, hands, and arms, rather than other body areas, may be attributed to patients sleeping habits, with increased exposure of hands and legs to sand fly bites during the insects’ nocturnal activity. This observation aligns with previous studies (Alsamarai and Alobaidi, 2009; Abdellatif et al., 2013) that emphasize the impact of patient behavior on the localization of leishmaniasis lesions. The data presented in Figure 2 indicate a peak in the number of CL cases during January and February, followed by a decrease in April and May, before rising again in autumn. This seasonal trend suggests a higher prevalence during autumn to winter, likely attributed to the incubation period of 4 to 12 weeks post-sand fly bite (El-Buni et al., 2000; Al-Jawabreh et al., 2017). Variations in the incidence across seasons and regions may be influenced by distribution and bionomic differences, as highlighted by Bounoua et al. (2013). Treatment options for CL vary according to the type, location, and symptoms of the disease. The WHO recommends starting with local therapies for CL and progressing to systemic treatment as needed. Pentavalent antimonials are commonly used to treat CL. These include sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime). These drugs have been the mainstay of treatment for decades. They are effective against many species of Leishmania. However, adverse effects limit their use. These conditions include cardiotoxicity and pancreatitis (Mitropoulos et al., 2010; WHO, 2010). Alternative systemic treatments include liposomal amphotericin B and miltefosine. The agents are effective against antimonial-resistant Leishmania. This drug is well tolerated. It has fewer adverse effects than antimonials (WHO, 2010; Mosimann et al., 2018). Local therapies are the first-line treatment. These treatments include cryotherapy, intralesional antimonials, thermotherapy, and topical treatments for small, uncomplicated lesions. These treatments are less invasive and have fewer adverse effects. They are therefore appropriate for patients with limited disease (Mohammed et al., 2024). Cryotherapy is often used in combination with intralesional antimonials to improve the efficacy of CL (Brito et al., 2017). The treatment regimen preferences for CL in this study included liquid nitrogen (cryotherapy) at 26.16%, Glucantime I/L at 17.29%, and Pentostam I/L at 10.08%, with limited use of I/M Pentostam or I/M Glucantime. This is in contrast with a study conducted in Sirte (Abdel-Magied et al., 2016), where pentavalent antimony compounds were the primary treatment due to their efficacy against all forms of leishmaniasis. In Libya’s endemic regions, cryotherapy is a prevalent first-line treatment for CL, especially in pediatric cases. This approach is often favored due to its simplicity, effectiveness, and reduced risk of adverse reactions compared with traditional injection treatments. The use of cryotherapy for managing CL reflects the practical challenges and healthcare limitations faced in endemic regions where access to more complex treatments may be constrained. This practice aligns with other Libyan studies (Abdellatif et al., 2013; Mosleh et al., 2018). Although CL is typically resolved on its own, medications are used to minimize scar formation at the site of the lesion. Treatment outcomes are affected by various factors, including the characteristics of the parasite, host immune response, and the pharmacological properties of the drugs used pharmacodynamics and pharmacokinetics. The array of drugs utilized for CL treatment is extensive in the literature; however, determining the most effective treatment approach remains challenging (Goto and Lindoso, 2010). The ongoing obstacles to developing effective therapies for CL emphasize the need for rigorous screening of potential drugs using suitable models and standardized clinical trials. According to the WHO, the obstacles faced in managing CL in Libya, such as ongoing wars, population displacement, overcrowding, lack of sanitation, and limited healthcare services, underscore the need for comprehensive strategies that encompass both medical interventions and broader societal improvements (PAHO/WHO, 2019).
Fig. 4. Map of Libya showing the distribution of cutaneous leishmaniasis (CL) cases/incidences/100,000 by Health area in 2019–2022. ConclusionThe study of CL in Libya between 2019 and 2022 highlights the persistent public health challenge posed by the disease. With 13,625 reported cases during the study period, fluctuations in case numbers, demographic associations, and seasonal variations underscore the need for targeted interventions and continuous surveillance. The impact of ongoing conflicts on disease control efforts emphasizes the urgency of comprehensive strategies that integrate medical interventions and community enhancements to effectively manage CL and alleviate its burden on public health in Libya. AcknowledgmentsThe authors would like to express their gratitude to the Administration of Zoonotic Disease Control at the National Center for Disease Control (NCDC) in Tripoli, Libya, for their data collection collaboration. Conflicts of interestThe authors declare that this study was conducted without any financial relationships that could be perceived as a potential conflict of interest. FundingThe research detailed in this paper was conducted independently and Cutaneous leishmaniasis continues to pose a substantial public health challenge in Libya, intensified by the ongoing socio-political instability affecting disease surveillance and management systems. Comprehensive strategies integrating medical interventions and community-based initiatives are essential for mitigating the adverse medical and social impacts of CL in the country without external funding. Authors’ contributionAll authors contributed to this study, and all authors read, and approved the final manuscript. Data availabilityThe data supporting the findings of this study are not publicly available. However, they can be obtained from the Administration of Zoonotic Disease Control at the National Center for Disease Control (NCDC) in Tripoli, Libya upon reasonable request. ReferencesAbdellatif, M.Z., El-Mabrouk, K. and Ewis, A.A. 2013. An epidemiological study of cutaneous leishmaniasis in Al-Jabal Al-Gharbi, Libya. Korean J. Parasitol. 51(1), 75–84; doi:10.3347/kjp.2013.51.1.7. Abdel-Magied, A., Abou-El-Wafa, H. and El-Henawy, A. 2016. Clinical and demographic criteria for cutaneous leishmaniasis in Sirte, Libya. Parasitol United J. 9, 18–23. AboAssara, A., Buishi, I., Fares, A., Gusbi, M. and Annajar, B. 2015. Retrospective study for cutaneous leishmaniasis in Jadu area, Libya. Libyan J. Vet. Med. Sci. 1(1), 24–32. Al-Buni, A.A., Jabeal, I. and Ben-Darif, A.T. 2000. Cutaneous leishmaniasis in the Libyan Arab Jamahiriya: a study of the Yafran area. East Mediterr. Health J. 6(5–6), 884–887. Al-Dwibe, H., Gashout, A., Morogum, A.M., El-Zubi, S. and Amro, A. 2014. Contact dermatitis-like cutaneous leishmaniasis in a Libyan HIV patient. Parasites Vectors 7, 401; doi:10.1186/s13071-014-0401-2. Al-Jawabreh, A., Dumaidi, K., Ereqat, S., Al-Jawabreh, H., Nasereddin, A., Azmi, K., Barghuthy, F., Sawalha, S., Salah, I. and Abdeen, Z. 2017. Molecular epidemiology of human cutaneous leishmaniasis in Jericho and its vicinity in Palestine from 1994 to 2015. Infect. Genet. Evol. 50, 95–101; doi:10.1016/j.meegid.2016.06.007. Almaasi, A., Shhirdar, M.R. and Emadi, J. 2008. Epidemiologic study of cutaneous leishmaniasis in the city of Shiraz, Fars Province, 2006-2008. J. Fars. Med. Sci. 3(4), 15–34. AlSamarai, A.M and AlObaidi, H.S. 2009. Cutaneous leishmaniasis in Iraq. J Infect Dev Ctries. 3(2), 123–129; doi: 10.3855/jidc.59. Amro, A., Gashout, A., Al-Dwibe, H., Zahangir Alam, M., Annajar, B., Hamarsheh, O., Shubar, H. and Schönian, G. 2012. First molecular epidemiological study of cutaneous leishmaniasis in Libya. PLoS Negl. Trop. Dis. 6(6), e1700; doi:10.1371/journal.pntd.0001700. Aoun, K., Bousslimi, N., Haouas, N., Babba, H., El-Buni, A. and Bouratbine, A. 2006. First report of Leishmania (L) killicki Rioux, Lanotte & Pratlong, 1986 in Libya. Parasite 13(1), 87–88. Available via https://pubmed.ncbi.nlm.nih.gov/16605075/ Aronson, N. and Joya, C. 2019. Cutaneous leishmaniasis: updates in diagnosis and management. Infect. Dis. Clin. North Am. 33(1), 101–117; doi:10.1016/j.idc.2018.08.001. Ashford, R.W., Chance, M.L., Ebert, F., Schnur, L.F., Bushwereb, A.K. and Drebi, S.M.. 1976. Cutaneous leishmaniasis in the Libyan Arab Republic: distribution of the disease and identity of the parasite. Ann. Trop. Med. Parasitol. 70, 401–409. Ashford, R.W., Schnur, L.F., Chance, M.L., Samaan, S.A. and Ahmed, H.N. 1977. Cutaneous leishmaniasis in the Libyan Arab Republic: preliminary ecological findings. Ann. Trop. Med. Parasitol. 71(3), 265–271; doi:10.1080/00034983.1977.11687190. Ashour, A., Atia, A., Akash, N., Jumaa, E. and Alkhishrabi, A. 2022. Cutaneous leishmaniasis in Al-Jabal Al-Gharbi, Libya: incidence and epidemiology. Khalij-Libya J. Dent. Med. Res. 6(1), 81–85. Belal, U.S., Abdel-Hafeez, E.H., Naoi, K. and Norose, K. 2012. Cutaneous leishmaniasis in the Nalut District, Libyan Arab Jamahiriya: a clinico-epidemiologic study and Leishmania species identification. J. Parasitol. 98(6), 1251–1256; doi:10.1645/GE-3086.1. Bounoua, L., Kahime, K., Houti, L., Blakey, T., Ebi, K.L., Zhang, P., Imhoff, M.L., Thome, K.J., Dudek, C., Sahabi, S.A. and Messouli, M. 2013. Linking climate to incidence of zoonotic cutaneous leishmaniasis (L. major) in pre-Saharan North Africa. Int. J. Environ. Res. Public Health 10(8), 3172–3191; doi:10.3390/ijerph10083172. Brito, N., Rabello, A. and Cota, G. 2017. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: a systematic review. PLoS One 12, e0188051; doi:10.1371/journal.pone.0188051. Chin, J.C., Dai, Y. and Watts, J.E. 1995. Antibody response against Pseudomonas aeruginosa membrane proteins in experimentally infected sheep. Vet. Microbiol. 43, 21–32. de Souza, M.L., Dos Santos, W.M., de Sousa, A.L.M.D., Ferraz, L.R.D.M., da Costa, L.A.G., Silva, E.O. and Rolim Neto, P.J. 2021. Cutaneous leishmaniasis: new oral therapeutic approaches under development. Int. J. Dermatol. 61, 55–61; doi:10.1111/ijd.14906. Elammari, N., Elhadar, J. and Annajar, B.B. 2015. Trends in zoonotic cutaneous leishmaniasis (ZCL) in eight endemic municipalities of North-West Libya during 2004 – 2008. Glob. J. Biol. Agric. Health Sci. 4(4), 1–6. El-Badry, Ayman Al-Dwibe, Hamida Basyoni, Maha Al-Antably and Abeer Al-Bashier, Wafaa. 2016. Molecular prevalence and estimated risk of cutaneous leishmaniasis in Libya. J. Microbiol. Immunol. Infect. 50. doi: 10.1016/j.jmii.2015.12.004 El-Buni, A.A., Jabeal, I. and Ben-Darif, A.T. 2000. Cutaneous leishmaniasis in the Libyan Arab Jamahiriya: a study of the Yafran area. East Mediterr. Health J. 6(5–6), 884–887. Fakhar, M., Mikaeili, F., Hatam, G., Habibi, P., Karamian, M., Motazedian, M. and Banimostafavi, E. 2022. A molecular epidemiology survey of cutaneous leishmaniasis in patients referring to Parasitology Lab at Shiraz School of Medicine and the importance of PCR assay. Pars J. Med. Sci. 8(1), 2–6; doi: 10.29252/jmj.8.1.2 Fathy, F.M., El-Kasah, F. and El-Ahwal, A.M. 2009. Emerging cutaneous leishmaniasis in Sirte-Libya: epidemiology, recognition and management. J. Egypt Soc. Parasitol. 39(3), 881–905. Feiz-Haddad, M.H., Kassiri, H., Kasiri, N., Panahandeh, A. and Lotfi, M. 2015. Prevalence and epidemiologic profile of acute cutaneous leishmaniasis in an endemic focus, southwestern Iran. J. Acute Dis. 4(4), 292–297; doi:10.1016/j.joad.2015.05.003. Gonzãlez, U., Pinart, M., Reveiz, L. and Alvar, J. 2017. Interventions for old world cutaneous leishmaniasis. Cochrane Database Syst. Rev. 4, CD005067; doi:10.1002/14651858.CD005067.pub3. Goto, H. and Lindoso, J.A. 2010. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti Infect. Ther. 8(4), 419–433; doi:10.1586/eri.10.29. Heidari-Kharaji, M., Taheri, T., Doroud, D., Habibzadeh, S., Badirzadeh, A. and Rafati, S. 2016. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite Immunol. 38(10), 599–608; doi:10.1111/pim.12340. Jamal, Q., Shah, A., Ali, N., Ashraf, M., Awan, M.M. and Lee, C.M. 2013. Prevalence and comparative analysis of cutaneous leishmaniasis in Dargai region in Pakistan. Pak. J. Zool. 45(2), 537–541. Kevric, I., Cappel, M.A. and Keeling, J.H. 2015. New world and old world Leishmania infections: a practical review. Dermatol. Clin. 33(3), 579–593; doi:10.1016/j.det.2015.03.018. Mehabresh, M.I. 1994. Visceral leishmaniasis: new foci of infection in Libya. J. Trop. Med. Hyg. 97(5), 282–285. Mendonãa, S.C. 2016. Differences in immune responses against Leishmania induced by infection and by immunization with killed parasite antigen: implications for vaccine discovery. Parasit Vectors 9(1), 492; doi:10.1186/s13071-016-1777-x. Mir, F., Mushtaq, N., Ihsanullah, R., Noor, Z. and Abbas, A.Z. 2023. Body locations of leishmaniasis in the targeted population of Pakistan. Pak. J. Med. Dent. 12(4), 75–79; doi:10.36283/PJMD12-4/014. Mitropoulos, P., Konidas, P. and Durkin-Konidas, M. 2010. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J. Am. Acad. Dermatol. 63(2), 309–322; doi:10.1016/j.jaad.2009.06.088. Mohammed, O.M., Mohsen, K.K., Rivera, G., Kamel, N.A., Noori, S.S., Khatlan, S.A.D.M. and Hamad, A.A. 2024. Comparative efficacy of current and emerging therapies for cutaneous and visceral leishmaniasis: a global perspective. South Asian Res. J. Biol. Appl. Biosci. 6(5), 194–203; doi:10.36346/sarjbab.2024.v06i05.006. Morizot, G., Kendjo, E., Mouri, O., Thellier, M., Pérignon, A., Foulet, F., Cordoliani, F., Bourrat, E., Laffitte, E., Alcaraz, I. and Bodak, N. 2013. Travelers with cutaneous leishmaniasis cured without systemic therapy. Clin. Infect. Dis. 57(3), 370–380; doi:10.1093/cid/cit269. Mosimann, V., Neumayr, A., Paris, D. and Blum, J. 2018. Liposomal amphotericin B treatment of Old World cutaneous and mucosal leishmaniasis: a literature review. Acta Trop. 182, 246–250; doi:10.1016/j.actatropica.2018.03.016. Mosleh, I.M., Geith, E., Natsheh, L., Schönian, G., Abotteen, N. and Kharabsheh, S. 2008. Efficacy of a weekly cryotherapy regimen to treat Leishmania major cutaneous leishmaniasis. J. Am. Academy Dermatol. 58(4), 617–624. 10.1016/j.jaad.2007.12.032 PAHO/WHO, 2019: Leishmaniasis, epidemiological report in the Americas. Washington, D.C.: PAHO. Available via http://iris.paho.org/xmlui/handle/123456789/50505. Pan American Health Organization. 2019. Leishmaniasis 2019: epidemiological report in the Americas. Washington, D.C.: PAHO. Available via http://iris.paho.org/xmlui/handle/123456789/50505 Postigo, J.A. 2010. Leishmaniasis in the World Health Organization eastern mediterranean region. Int. J. Antimicrob. Agents 36(Suppl 1), S62–S65; doi:10.1016/j.ijantimicag.2010.06.023. Sadeghi-Nejad, B and Saki, J. 2014. Effect of Aqueous Allium cepa and Ixora brachiata root extract on Leishmania major promastigotes. Jundishapur J. Nat. Pharm. Prod. 9(2), e15442; doi:10.17795/jjnpp-15442 Soltani, S., Foroutan, M., Hezarian, M., Afshari, H. and Kahvaz, M.S.. 2019. Cutaneous leishmaniasis: an epidemiological study in southwest of Iran. J. Parasit Dis. 43(2), 190–197; doi:10.1007/s12639-018-1073-0. World Health Organization. 2009. Report on the Programme managers meeting on leishmaniasis control in the Eastern Mediterranean Region in Sharm el Sheikh, Egypt, from 27 to 29 October 2009. Available via https://applications.emro.who.int/docs/who_em_ctd_059_e_en.pdf World Health Organization. 2010. Report of a meeting of the WHO Expert Committee Control of Leishmaniases. Geneva, Switzerland: WHO; 22–26 March 2010. Available via https://iris.who.int/bitstream/handle/10665/44412/WHO_TRS_949_ara.pdf?sequence=2 WHO/EMRO. 2009. Report on the program managers meeting on leishmaniasis control in the Eastern Mediterranean Region in Sharm el Sheikh, Egypt, from 27 to 29 October 2009. Document WHO-EM/CTD/059/E/05.100/80. | ||
| How to Cite this Article |
| Pubmed Style Saad KA, Ageehal HA, Elgrari AS, Ammar SA. The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Vet. J.. 2025; 15(4): 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 Web Style Saad KA, Ageehal HA, Elgrari AS, Ammar SA. The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. https://www.openveterinaryjournal.com/?mno=233440 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.20 AMA (American Medical Association) Style Saad KA, Ageehal HA, Elgrari AS, Ammar SA. The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Vet. J.. 2025; 15(4): 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 Vancouver/ICMJE Style Saad KA, Ageehal HA, Elgrari AS, Ammar SA. The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 Harvard Style Saad, K. A., Ageehal, . H. A., Elgrari, . A. S. & Ammar, . S. A. (2025) The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Vet. J., 15 (4), 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 Turabian Style Saad, Kaula A., Hanan A. Ageehal, Ahmed S. Elgrari, and Saleh A. Ammar. 2025. The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Veterinary Journal, 15 (4), 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 Chicago Style Saad, Kaula A., Hanan A. Ageehal, Ahmed S. Elgrari, and Saleh A. Ammar. "The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices." Open Veterinary Journal 15 (2025), 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 MLA (The Modern Language Association) Style Saad, Kaula A., Hanan A. Ageehal, Ahmed S. Elgrari, and Saleh A. Ammar. "The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices." Open Veterinary Journal 15.4 (2025), 1685-1694. Print. doi:10.5455/OVJ.2025.v15.i4.20 APA (American Psychological Association) Style Saad, K. A., Ageehal, . H. A., Elgrari, . A. S. & Ammar, . S. A. (2025) The burden of cutaneous leishmaniasis in Libya (2019–2022): Epidemiological insights and treatment practices. Open Veterinary Journal, 15 (4), 1685-1694. doi:10.5455/OVJ.2025.v15.i4.20 |