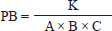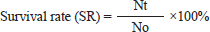| Research Article | ||
Open Vet. J.. 2025; 15(4): 1654-1662 Open Veterinary Journal, (2025), Vol. 15(4): 1654-1662 Research Article Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia)Dwi Kurniawati1, Evi Fitriana1, Wahju Tjahjaningsih2, Mohammad Faizal Ulkhaq1,3*, Maria Agustina Pardede1,3 and Jiun-Yan Loh41Aquaculture Study Program, Faculty of Health, Medicine and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 2Marine Department, Faculty of Fisheries and Marine, Universitas Airlangga, Surabaya, Indonesia 3Sustainable Aquaculture and Environment Research Group, Universitas Airlangga, Banyuwangi, Indonesia 4Tropical Futures Institute, James Cook University Singapore, Singapore *Corresponding Author: Mohammad Faizal Ulkhaq. Aquaculture Study Program, Faculty of Health, Medicine and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: m-faizalulkhaq [at] fpk.unair.ac.id Submitted: 15/12/2024 Accepted: 24/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
AbstractBackground: Catfish farming is often performed with high stocking densities, which can result in environmental degradation, stress, and increased vulnerability to infections such as Edwardsiellosis caused by Edwardsiella tarda. Infection by E. tarda occurs through horizontal transmission during cohabitation between infected and healthy fish. Aim: This study aimed to investigate hematological alterations and abundance of E. tarda in catfish following cohabitation with carrier silver rasbora. Methods: A total of 160 silver rasboras (length: 6.02 ± 0.36 cm, weight: 2.065 ± 0.565 g) were immersed in an E. tarda suspension at a concentration of 1013 colony forming unit (CFU) /ml for 2 weeks. Subsequently, they were cohabitate with 240 catfish (length: 6.9 ± 2.35 cm, weight: 2.78 ± 1.37 g) at different density ratios: 1:1 (P1), 1:2 (P2), and 2:1 (P3), along with negative control (without E. tarda, NC), and with E. tarda at 1013 CFU/ml (positive control) for 5 days. The observed parameters included hematological profiles (total erythrocytes, leukocytes, hemoglobin (Hb) levels, and leukocyte differentials) and the density of E. tarda in various organs (liver, kidneys, and spleen). Results: Catfish cohabiting with E. tarda-infected silver rasbora exhibited significant hematological alterations, including elevated percentages of neutrophils, lymphocytes, and monocytes, alongside decreased erythrocyte counts, Hb levels, and total leukocyte counts. Furthermore, the highest density of E. tarda was detected in the liver (1.15 ± 0.11 × 104 CFU/ml) compared with the other organs. Conclusion: Cohabitation between healthy catfish and E. tarda carrier silver rasbora resulted in bacterial infection in the catfish. Keywords: Catfish, Cohabitation, Edwardsiellosis, Fisheries, Silver Rasbora. IntroductionCatfish is a widely cultivated freshwater aquaculture commodity in almost 90 countries globally, recognized for its affordability and high nutritional value, as demonstrated by a marked increase in consumer demand. This rising interest is reflected in the high demand for catfish in the community (Segaran et al., 2023). According to the Indonesian Ministry of Marine Affairs and Fisheries (2024), catfish production increased by 4.27% from 2018 to 2022, representing the highest average growth rate among all aquaculture commodities in Indonesia. The intrinsic characteristics of catfish, including rapid growth and resilience to environmental fluctuations, contribute to its status as a preferred species for aquaculture, supported by established cultivation techniques and technologies (Salamah and Zulpikar, 2020). Nonetheless, the prevalence of disease is a significant challenge in catfish farming, resulting in high mortality rates and compromised harvests. The pathogen Edwardsiella tarda, a Gram-negative, rod-shaped, flagellated bacterium, is the causative agent of Edwardsiellosis (Goh et al., 2023). In addition to catfish, infections caused by E. tarda have been documented in various freshwater and marine fish species, including channel catfish (Ictalurus punctatus) (Armwood et al., 2022), Nile tilapia (Oreochromis niloticus) (Peng et al., 2022), iridescent shark catfish (Pangasianodon hypophthalmus) (Hoque et al., 2020), and Labeo rohita (Sattanathan et al., 2020), often resulting in elevated mortality rates during the grow-out phase. This phenomenon is attributed to the virulence factors of E. tarda, including adhesins, hemolysins, and type III and VI secretion systems, which facilitate the bacterium’s invasion, survival, and replication within host epithelial and phagocytic cells (Samir et al., 2021). The clinical manifestation of Edwardsiellosis in fish is characterized by symptoms such as depigmentation, exophthalmia, and red lesions on the skin and fins, as well as internal hemorrhages affecting organs such as the spleen, liver, and kidneys (Pandey et al., 2021). The emergence of Edwardsiellosis is exacerbated by factors such as stress, fluctuating water temperatures, and elevated organic matter levels in aquatic environments (Miniero Davies et al., 2018). Furthermore, Janda and Duman (2024) reported that Edwardsiellosis also infects reptiles, birds, and mammals, categorizing it as a zoonotic disease with potential implications for human health. The horizontal transmission of E. tarda through water from infected fish to healthy fish has been extensively documented (Bujãn et al., 2015). Instances of cohabitation-associated Edwardsiella infections have been reported in species such as striped catfish (P. hypophthalmus), resulting in abdominal swelling and the enlargement of the liver and lymph nodes (Phuoc et al., 2020). Additionally, da Costa et al. (2021) reported horizontal transmission of Edwardsiella infections in Pseudoplatystoma corruscans originating from two invasive fish species (O. niloticus and Clarias gariepinus), leading to mortality rates of up to 50%. The silver rasbora (Rasbora argyrotaenia) is a member of the family Cyprinidae and is distributed across India, China, and Southeast Asia. This species typically inhabits flowing waters, such as rivers, and calm environments, such as swamps (Anggararatri et al., 2023). The silver rasbora can serve as a vector for E. tarda. Findings reported by Husna et al. (2022) indicate that E. tarda can infect silver rasbora, with the bacteria accumulating in the blood, liver, and kidneys at densities ranging from 1.85 – 8.8 × 103 colony forming unit (CFU)/ml. Furthermore, fish infected with E. tarda exhibited decreased erythrocyte and hemoglobin (Hb) levels, along with an increase in white blood cell counts (Tirani et al., 2024). However, information regarding the horizontal transmission of E. tarda from silver rasbora carrier to healthy catfish remains undocumented. Therefore, this study aimed to analyze the hematological profile and total bacterial load of E. tarda in catfish following cohabitation with silver rasboras carrier. These results are anticipated to serve as a reference for preventing outbreaks of E. tarda in aquatic environments, particularly those caused by horizontal transmission by infected fish. Materials and MethodsFish and bacterial preparationThe study utilized 240 catfish (length 6.9 ± 2.35 cm, weight 2.78 ± 1.37 g) sourced from the Fish Hatchery Center Genteng in Banyuwangi, East Java, and 160 silver rasboras (length 6.02 ± 0.36 cm, weight 2.63 ± 1.5 g) obtained from the Freshwater Aquaculture Development Center Umbulan, Pasuruan, East Java. All fish were maintained separately in cylindrical fiberglass tanks with a capacity of 450 l (contains sterilized freshwater with 30 ppm chlorine and neutralized with sodium thiosulphate in the same concentration) and allowed to acclimatize for a duration of 7 days. During the acclimation period, the fish were fed a commercial pellet diet (Matahari Sakti, Indonesia; nutrient estimation: 30% protein, 6% fat, 3% carbohydrates) at 8:00 PM and 4:00 AM until satiation. Water quality parameters, including temperature, pH, and dissolved oxygen (DO), were measured bi-daily every morning (08.00 AM) and evening (04.00 PM) and maintained at optimal levels (26°C–28°C, pH 6–7, and DO at 5–6 ppm, respectively) to minimize stress in the fish. The E. tarda bacteria used in this study were obtained from the Microbiology Laboratory of the Faculty of Health Sciences, Medicine, and Natural Sciences at Airlangga University and were previously identified in Husna et al. (2022). The bacterial isolates were recultured on selective Xylose-Lysine Deoxycholate (XLD) agar (Himedia, India) and biochemically characterized according to Indonesian Standard number 7663:2011. Experimental design and in vivo challengeThis study was conducted using a completely randomized design with five treatments and four replications at the Instrument and Wet Laboratories, Airlangga University. The treatments consisted of: catfish immersed in an E. tarda suspension with a density of 1013 CFU/ml (positive control/PC), catfish maintained without exposure to E. tarda (negative control/NC), and silver rasbora carriers with E. tarda cohabitate with catfish in ratios of 1:1 (T1), 1:2 (T2), and 2:1 (T3). For all treatments, experiments were conducted in glass aquariums (H 40 × W 30 × L 30 cm) with an overall water volume of 160 l. Prior to cohabitation, healthy silver rasbora were immersed in E. tarda at a concentration of 1013 CFU/ml for 2 weeks, excluding the control treatments (PC and NC), to establish them as bacterial carriers. The presence of E. tarda in the organs (liver, kidney, and spleen) of the silver rasbora was verified through colony counting in XLD medium using the plate count method (Madigan et al., 2012). Following confirmation, E. tarda-carrier silver rasbora were cohabitate with healthy catfish according to established treatment ratios for a duration of 5 days. Throughout the maintenance period, the fish were fed commercial feed (Matahari Sakti, Indonesia) at satiation in the 8.00 PM and 4.00 AM. Every morning (8.00 AM), all solid waste was cleared from each aquarium. Water quality parameters, including temperature, pH, and dissolved oxygen (DO), were monitored bi-daily (8.00 AM and 4.00 PM) and were maintained within optimal ranges (26–28°C, pH 6–7, and DO 5–6 ppm). The temperature and pH were measured using a thermometer (Resun, Indonesia) and pH indicator (Merck, Germany). The DO levels were determined using a DO meter (Horiba, Poland), whereas the ammonia concentration was assessed using a SERA-ammonium/ammonia test kit (SERA, Germany). Hematological and bacterial samplingBlood and organ samples from catfish were collected before (0 dpi) and after (5 dpi) cohabitation with E. tarda carrier silver rasbora. The hematological parameters evaluated included total erythrocytes/red blood cells (RBCs), total leukocytes/white blood cells (WBCs), Hb levels, and the percentages of neutrophils, monocytes, and lymphocytes. Two catfish were randomly selected from each aquarium and transferred to an anesthetic solution containing 100-ppm eugenol for 1 minute. Blood was then drawn from the caudal vein using a syringe and transferred to a tube containing 10% ethylenediaminetetraacetic acid. Additionally, hematological profiles were analyzed according to the method described by Blaxhall and Daisley (1973). The density of E. tarda was assessed by dissecting catfish to obtain liver, kidney, and spleen samples. Subsequently, 1 g of each organ was weighed, crushed using a tissue grinder (Axygen, USA), and homogenized in 9 ml of a sterile phosphate buffered saline solution. An aliquot of 0.1 ml was collected and subjected to serial dilution using the plate count method. Each dilution (0.1 ml) was inoculated onto XLD medium (Himedia, India), incubated at 37°C for 24 hours, and the colonies were counted using the following formula (Madigan et al., 2012):
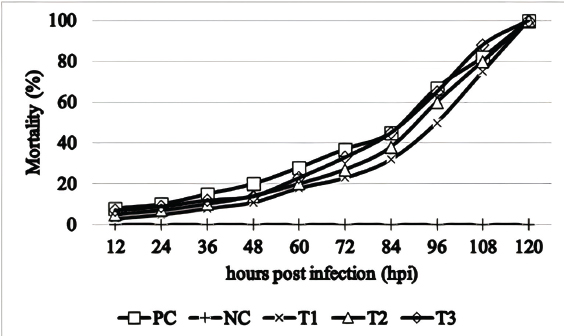
where: PB =bacterial population (CFU/ml). K =number of colonies. A =inoculum volume in diluent (ml). B =dilution factor at which the colonies are counted. C =inoculum volume transferred to the agar medium (ml). The survival rate of the catfish was assessed at the conclusion of the experiment using the following formula (Bera et al., 2020):
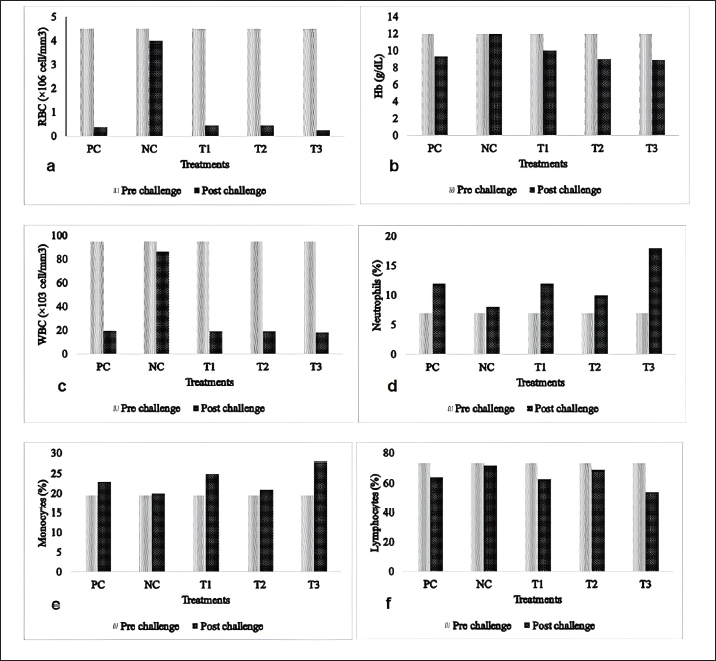
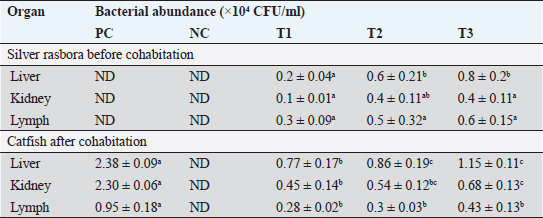
Nt=number of fish after the experiment. N0=number of fish at the start of the experiment. Data analysisThe hematological profiles and survival rates of the catfish were analyzed descriptively and compared to normal values. In addition, the density of E. tarda bacteria in various organs was analyzed using a one-way analysis of variance, followed by Duncan’s multiple range test when the significance level was set at p < 0.05. Ethical approvalThis study was conducted under the supervision and approval of the Faculty of Health, Medicine, and Natural Sciences at Airlangga University, with ethical clearance certificate number 85/UN3.1.16/2022. ResultsThe hematological profile of catfish following cohabitation with E. tarda-carrier exhibited alterations compared to pre-challenge assessments (Fig. 1), particularly within treatment group T3 (2:1 ratio) when compared to other treatments. The total red blood cell counts (Fig. 1a), Hb concentrations (Fig. 1b), and total white blood cell counts (Fig. 1c) demonstrated a decline after cohabitation. Conversely, the percentages of neutrophils (Fig. 1d), monocytes (Fig. 1e), and lymphocytes (Fig. 1f) increased after cohabitation compared with pre-challenge values. The hematological profiles of the NC group also exhibited changes although these were more stable than those observed in the other treatment groups. Bacterial counts in the liver, kidney, and spleen of silver rasbora showed E. tarda infection with bacterial densities ranging from 0.1 – 0.8 ×104 CFU/ml across all organs examined (Table 1). In the study, the density of bacteria in the liver, kidney, and spleen of post-cohabitation catfish with silver rasbora carriers was observed, as shown in Table 1. The highest bacterial density was observed in treatment T3 (2:1), whereas the lowest bacterial density was observed in treatment T1 (1:2) for all catfish organs. Catfish in all treatments, except the NC, exhibited 100% mortality by the 5th-day post-cohabitation (Fig. 2). Mortality began at 12 host infection (hpi) and increased exponentially starting at 72 hpi, reaching 100% at 120 hpi with E. tarda. DiscussionEdwardsiella tarda is a bacterial pathogen that causes Edwardsiellosis, which results in various alterations in hematological parameters and severe fish mortality. Edwardsiella tarda can adapt to many habitats and infect several hosts, resulting in significant mortality rates (Goh et al., 2023). Edwardsiellosis in fish causes several alterations in total RBCs, WBCs, Hb levels, and differential leucocyte counts (Tirani et al., 2024). Catfish hematological parameters and bacterial abundance changed significantly after cohabiting with silver rasbora carrying E. tarda. RBCs/erythrocytes, which are the most abundant blood cells in fish, play a vital role in the transportation of various substances (such as gases, water, minerals, nutrients, hormones, toxins, and metabolic waste products) within the body (Seibel et al., 2021). The decrease in the number of erythrocytes in catfish cohabitation with silver rasboras carrying E. tarda, particularly in treatment T3 (2:1), indicates that the catfish were affected by Edwardsiellosis. This finding is supported by the fact that the erythrocyte levels were significantly below the reference range of 3.02 ± 0.01 ×106 cells/mm3 (Fazio, 2019). Tjampakasari et al., (2024) reported that E. tarda secretes hemolysin toxins (EthA and EthB), which cause damage to the erythrocyte cell walls and result in cell lysis. Furthermore, Kalindamar et al. (2021) suggested that E. tarda hemolysin toxins facilitate adhesion and invasion within fish bodies, enabling intracellular bacterial survival. These findings are consistent with previous studies on tilapia (Sherif et al., 2021) and yellow catfish ( Pelteobagrus fulvidraco (Chen et al., 2020) infected with E. tarda. The decrease in erythrocyte count corresponds to a reduction in Hb levels in catfish following cohabitation with E. tarda carrier silver rasbora in all treatments (except for the NC), dropping below the normal Hb level of (12.423 ± 2.21 g/dl) (Al-Deghayem et al., 2017). This decline can be attributed to the positive correlation between the total erythrocyte count and Hb level, with pathogen infections inducing anemia (Ahmed et al., 2020). Edwardsiella tarda is believed to encode the fur (ferric uptake regulator) gene, which enables the bacteria to exploit iron from Hb released during erythrocyte lysis (Swain et al., 2020). Additionally, Lemos and Balado (2020) noted that E. tarda produces vibrioferrin, a compound similar to siderophore, which captures and extracts iron from heme-binding proteins in the host. Similar results were observed in yellow catfish (Pelteobagrus fulvidraco) (Chen et al., 2020) and Japanese flounder (Paralichthys olivaceus) (Sun et al., 2020) infected with E. tarda.
Fig. 1. Mean values for hematological parameters in catfish (Clarias sp.) pre- and postcohabitation with E. tarda carrier silver rasbora (R. argyrotaenia). PC: positive control; NC: negative control; E. tarda carrier silver rasbora cohabitate with catfish at ratios of 1:1 (T1); 1:2 (T2), and 2:1 (T3). a. RBCs, b. Hb, c. WBCs, d. Neutrophils, e. Monocytes, and f. Lymphocytes. Table 1. Bacterial abundance of different organs in silver rasbora and catfish before and after cohabitation. Different superscript showed significant different (p < 0.05).
Fig. 2. Mortality graph of catfish (Clarias sp.) pre- and postcohabitation with E. tarda carrier silver rasbora (R. argyrotaenia) . PC: positive control; NC: negative control; E. tarda carrier silver rasbora cohabitate with catfish at ratios of 1:1 (T1); 1:2 (T2), and 2:1 (T3). WBCs/leukocytes play a crucial role in fish immunity and overall health. Increased leukocyte levels are positively associated with the release of cytokines, phagocytosis activity, and inflammatory responses against pathogens (Mokhtar et al., 2023). In this study, we observed that leukocyte counts in catfish decreased following infection with E. tarda in all treatment groups, except for the NC. These leukocyte levels fell below the reference range of 20–150 ×103 cells/mm3 (Yanuhar et al., 2021), resulting in mortality by day 5 postinfection. This suggests the failure of the immune system to effectively combat E. tarda infection, leading to disease and death (Firdaus-Nawi and Zamri-Saad, 2016). Similar findings were reported in goldfish (Carassius auratus) experiencing Edwardsiellosis, where death occurred on day 5 post-challenge, accompanied by an initial leukocyte increase on day 1 post-infection (dpi), followed by a decline by day 5 (Choe et al., 2017). Transcriptomic analysis of Japanese flounder (Paralichthys olivaceus) revealed 21 genes associated with E. tarda infection, leading to disease and death (Sun et al., 2020). Neutrophils, the first leukocytes to be activated at sites of infection, are responsible for pathogen neutralization through mechanisms such as the production of reactive oxygen species, neutrophil extracellular traps (NETs) (Buchmann, 2022), antimicrobial peptides, and proteolytic enzymes (Mokhtar et al., 2023). The percentage of neutrophils in catfish increased following cohabitation with E. tarda carrier silver rasbora in all treatment groups, especially in T3 (2:1). However, these values remained below the reference range for neutrophil levels in catfish, which is 26.2 ± 4.9% (Adamu and Solomon, 2015). This indicates that neutrophils were unable to effectively control E. tarda infection in catfish. This condition is suspected to be caused by premature activation of neutrophils, compromising their ability to engage in phagocytosis, NETs formation, and intracellular killing (Leliefeld et al., 2016). Boucontet et al. (2018) also suggested that a phenomenon known as neutrophil paralysis could further weaken the ability of neutrophils to neutralize invading pathogens in fish. Monocytes play a critical role in the immune system of fish by acting as phagocytes and serving various functions during infection, inflammation, and tissue repair (Yang et al., 2021). They are found in bone marrow, blood, and spleen and can differentiate into macrophages upon entering tissues. Observations of monocyte percentages in catfish after cohabitation with E. tarda carrier silver rasbora showed an increase in all treatments, particularly in T3 (2:1), compared with pre-challenge values. However, this increase did not provide benefits to the catfish because E. tarda can survive within monocytes and reduce their phagocytic capacity. Tjampakasari et al. (2024) reported that the type VI secretion system (T6SS) of E. tarda plays a crucial role in adhesion, penetration, and replication within fish phagocytes. Ma et al. (2024) also reported that the qseB and qseC genes contribute to the intracellular replication of E. tarda, leading to systemic infection in fish. Fish lymphocytes are generally similar to those in mammals, consisting of T and B cells that can express immunoglobulins (IgM, IgT, and IgD) (Sakai et al., 2021). Morphologically, fish lymphocytes are small, round cells with a thin, narrow rim of homogeneous basophilic cytoplasm surrounding a large, round, oval, or irregularly shaped nucleus (Witeska et al., 2022). The percentage of lymphocytes in catfish increased after cohabitation with the E. tarda carrier silver rasboras. However, this increase was still lower than the reference range for catfish lymphocytes (68%–76%) (Adamu and Solomon, 2015). The low lymphocyte percentage suggests that the catfish immune system failed to provide adequate protection against E. tarda infection. This condition is thought to result from immune suppression caused by the pathogen, leading to reduced cytokine production and decreased cytotoxic activity, particularly in T cells (Davoodzadeh Gholami et al., 2017) . Additionally, impaired B-cell maturation due to toxin activity from the pathogen hinders the production of immunoglobulins essential for defense against infections (Oakes et al., 2016). Bacterial examination demonstrated the presence of E. tarda in silver rasbora without causing mortality, indicating a robust immune system in these fish, preventing E. tarda from establishing and enhancing its virulence (Ben Hamed et al., 2018) . This is supported by the fact that silver rasbora are not susceptible hosts for E. tarda. Bujãn et al. (2018) noted that E. tarda infections are more prevalent in catfish, eels, flounders, and turbots. The presence of E. tarda in catfish organs suggests that the immune system, particularly phagocytic cells, was unable to eradicate the pathogen. Kupffer cells also known as macrophages, are found in the liver and play a crucial role in the early immune response to liver infection. They release proinflammatory cytokines and chemokines (Slevin et al., 2020; Hussein et al., 2023). Macrophages in the kidneys and spleens are organized into melanomacrophage centers (MMCs) that act as primary phagocytic cells (Bjãrgen and Koppang 2024). MMCs can also serve as biomarkers for environmental contamination (Marteja and Modina 2021). Edwardsiella tarda is an intracellular pathogen that can survive and coexist within macrophages. It enters cells through mechanisms such as macropinocytosis and endocytosis pathways involving cholesterol and dynamin compounds in the cell membrane (Hu et al., 2019). In phagocytic cells, E. tarda penetrates cells through similar pathways mediated by clathrin and caveolin compounds (Sui et al., 2017). Additionally, E. tarda regulates pro-apoptotic and anti-apoptotic genes by enhancing its survival and replication within host cells, preventing apoptosis, which is an immune mechanism for eliminating pathogens (Zhou and Sun 2016). Horizontal transmission occurs from infected fish to healthy fish that cohabit in aquatic medium (Zaheen et al., 2022). Infected fish will excrete the bacteria through the skin, bile, feces, and urine and can infect other fish by ingestion or through the skin and gills. Furthermore, pathogens can also penetrate the physical defenses of the skin, gastrointestinal tract, and gills (Quintanilla et al., 2021). Horizontal transmission of E. tarda has been documented to exist in both the intraspecies (Phuoc et al., 2020) and different species of fish (da Costa et al., 2021). This condition is due to E. tarda is an opportunistic pathogen in water that can infect stressed and immunocompromised fish (Manzoor et al., 2023). Moreover, catfish are also susceptible to infection by E. tarda (Janda and Duman 2024). The mortality of catfish on the 5th day post-cohabitation indicates that the catfish were infected with edwardsiellosis and are susceptible hosts for E. tarda. These findings are consistent with Butar Butar et al. (2020), who reported E. tarda infections in catfish in North Sumatra, Indonesia. Additionally, Algammal et al. (2022) documented E. tarda infections in O. niloticus and C. gariepinus in Dakhlia Governore, Egypt, with clinical symptoms including excessive mucus production, abdominal swelling, operculum reddening, and fin and tail rot. ConclusionCatfish cohabitating with silver rasbora infected with E tarda experienced 100% mortality by the fifth day postinfection (dpi). Moribund fish exhibited altered hematological profiles, including increased percentages of neutrophils, lymphocytes, and monocytes, as well as decreased total erythrocyte, Hb levels, and total leukocyte counts. Furthermore, the liver was detected to have the highest concentration of E. tarda compared with other organs. AcknowledgmentsThe authors would like to express their gratitude to the Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga for providing the necessary facilities for this study. Conflict of interestThe author(s) declare(s) that they have no conflict of interest. FundingThis research received no specific grant. Authors’ contributionsMFU, DK, EF: Supervision, Conceptualization, Formal analysis, Investigation, Methodology, Writing-original draft, writing review, and editing. WT: validation, investigation, and formal analysis. MAP: Formal analysis, investigation, writing original draft, writing review, and editing. JYL: writing-review and editing. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAdamu, N.M. and Solomon, R.J. 2015. Effect of weight and length on full blood count of catfish (Clarias gariepinus). Rep. Opin. 7, 72–87. Ahmed, I., Reshi, Q.M. and Fazio, F. 2020. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquac. Int. 28, 869–899. Al-Deghayem, W.A., Al-Balawi, H.F., Kandeal, S.A. and Suliman, E.A.M. 2017. Gonadosomatic index and some hematological parameters in African catfish Clarias gariepinus (Burchell, 1822) as affected by feed type and temperature level. Brazilian Arch. Biol. Technol. 60, 1–10. Algammal, A.M., Mabrok, M., Ezzat, M., Alfifi, K.J., Esawy, A.M., Elmasry, N. and El-Tarabili, R.M. 2022. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture 548, 737643. Anggararatri, Y., Rukayah, S. and Lestari, W. 2023. Study of population dynamics silver rasbora ( Rasbora argyrotaenia Bleeker, 1849) in PB. Soedirman Reservoir, Banjarnegara. Genbinesia J. Biol. 2, 81–92. Armwood, A.R., Griffin, M.J., Richardson, B.M., Wise, D.J., Ware, C. and Camus, A.C. 2022. Pathology and virulence of Edwardsiella tarda, Edwardsiella piscicida, and Edwardsiella anguillarum in channel (Ictalurus punctatus), blue (Ictalurus furcatus), and channel × blue hybrid catfish. J. Fish Dis. 45, 1683–1698. Ben Hamed, S., Tavares Ranzani-Paiva M.J., Tachibana, L., de Carla Dias D., Ishikawa, C.M. and Esteban, M.A. 2018. Fish pathogen bacteria: adhesion, parameters influencing virulence and interaction with host cells. Fish Shellfish Immunol. 80, 550–562. Bera, K.K., Kumar, S., Paul, T., Prasad, K.P., Shukla, S.P. and Kumar, K. 2020. Triclosan induces immunosuppression and reduces survivability of striped catfish Pangasianodon hypophthalmus during the challenge to a fish pathogenic bacterium Edwardsiella tarda. Environ. Res. 186, 109575. Bjãrgen, H. and Koppang, E.O. 2024. The melano-macrophage: the black leukocyte of fish immunity. Fish Shellfish Immunol. 148, 109523. Blaxhall, P.C. and Daisley, K.W. 1973. Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–781. Boucontet, L., Passoni, G., Thiry, V., Maggi, L., Herbomel, P., Levraud, J.P. and Colucci-Guyon, E. 2018. A model of superinfection of virus-infected zebrafish larvae: increased susceptibility to bacteria associated with neutrophil death. Front. Immunol. 9, 01084. Buchmann, K. 2022. Neutrophils and aquatic pathogens. Parasite Immunol. 44, 1–11. Bujãn, N., Hernãndez-Haro, C., Monteoliva, L., Gil, C. and Magariãos, B. 2015. Comparative proteomic study of Edwardsiella tarda strains with different degrees of virulence. J. Proteomics. 127, 310–320. Bujãn, N., Toranzo, A.E. and Magariãos, B. 2018. Edwardsiella piscicida: a significant bacterial pathogen of cultured fish. Dis. Aquat. Organ. 131, 59–71. Butar-Butar, O.D., Suryanto, D. and Ilyas, S. 2020. Detection of Edwardsiella tarda infection of catfish (Clarias gariepinus) in Central Tapanuli Regency, North Sumatra, Indonesia. IOSR J. Agric. Vet. Sci. 13, 6–13. Chen, H., Yuan, G., Su, J. and Liu, X. 2020. Hematological and immune genes responses in yellow catfish (Pelteobagrus fulvidraco) with septicemia induced by Edwardsiella ictaluri. Fish Shellfish Immunol. 97, 531–539. Choe, Y., Yu, J.E., Park, J., Park, D., Oh, J. Il., Kim, S., Moon, K.H. and Kang, H.Y. 2017. Goldfish, Carassius auratus, as an infection model for studying the pathogenesis of Edwardsiella piscicida. Vet. Res. Commun. 41, 289–297. da Costa, A.R., de Abreu DC, Torres Chideroli, R., Santo, K., Dib Gonãalves, D., Di Santis, G.W. and Pãdua Pereira U. 2021. Interspecies transmission of Edwardsiella ictaluri in Brazilian catfish (Pseudoplatystoma corruscans) from exotic invasive fish species. Dis. Aquat. Organ. 145,197–208. Davoodzadeh Gholami, M., Kardar, G.A., Saeedi, Y., Heydari, S., Garssen, J. and Falak, R. 2017. Exhaustion of T lymphocytes in the tumor microenvironment: significance and effective mechanisms. Cell. Immunol. 322, 1–14. Fazio, F. 2019. Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500, 237–242. Firdaus-Nawi, M. and Zamri-Saad, M. 2016. Major components of fish immunity: a review. Pertanika J. Trop. Agric. Sci. 39, 393–420. Goh, K.W., Abdul Kari, Z., Wee, W., Zakaria, N.N.A., Rahman, M.M., Kabir, M.A., Abdul Hamid, N.K., Tahiluddin, A.B., Kamarudin, A.S. and Téllez–Isaías, G. 2023. Exploring the roles of phytobiotics in relieving the impacts of Edwardsiella tarda infection on fish: a mini-review. Front. Vet. Sci. 10:1149514. Hoque, F., Pawar, N., Pitale, P., Dutta, R., Sawant, B., Chaudhari, A. and Sundaray, J.K. 2020. Pathogenesis and expression profile of selected immune genes to experimental Edwardsiella tarda infection in iridescent shark Pangasianodon hypophthalmus. Aquac. Reports. 17, 100371. Hu, T., Zhang, L., Wang, W., Yang, D., Xiao, J., Zhang, Y. and Liu, X. 2019. Edwardsiella piscicida enters nonphagocytic cells via a macropinocytosis-involved hybrid mechanism. J. Bacteriol. 201, 1–13. Husna, N., Kusdarwati, R. and Ulkhaq, M.F. 2022. Bacterial viability of Edwardsiella tarda from silver rasbora (Rasbora argyrotaenia) after infection with immmersion methods. IOP Conf. Ser. Earth Environ. Sci. 1036, 012005. Hussein, M.M., Sayed, R.K.A. and Mokhtar, D.M. 2023. Structural and immunohistochemical analysis of the cellular compositions of the liver of molly fish (Poecilia sphenops), focusing on its immune role. Zool. Lett. 9, 1–13. Janda, J.M. and Duman, M. 2024. Expanding the spectrum of diseases and disease associations caused by Edwardsiella tarda and related species. Microorganisms 12, 1–40. Indonesia Ministry of Fisheries and Marine Affairs. 2024. Kelautan dan Perikanan dalam angka. Pusat Data, Statistik dan Informasi Kementerian Kelautan dan Perikanan. Indonesian National Standard. 2011. Identifikasi Edwardsiella tarda secara morfologis, fisiologis dan biokimia. SNI: 7664:2011. Kalindamar, S., Abdelhamed, H., Kordon, A.O., Pinchuk, L.M. and Karsi, A. 2021. Hemolysin co-regulated family proteins Hcp1 and Hcp2 contribute to Edwardsiella ictaluri pathogenesis. Front. Vet. Sci. 8, 681609. Leliefeld, P.H.C., Wessels, C.M., Leenen, L.P.H., Koenderman, L. and Pillay, J. 2016. The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care. 20, 1–9. Lemos, M.L. and Balado, M. 2020. Iron uptake mechanisms as key virulence factors in bacterial fish pathogens. J. Appl. Microbiol. 129, 104–115. Ma, J., Ahmed, M.A.H., Shao, S., Zhang, Y., Wang, Q. and Yin, K. 2024. The QseE-QseF two-component system: a key mediator of epinephrine-regulated virulence in the marine pathogen Edwardsiella piscicida. Microbiol. Res. 279, 127561. Madigan, M.T., Martinko, J.M., Stahl, D.A. and Clark, D.P. 2012. Brock biology of microorganisms. London, UK: Pearson Education. Manzoor, K., Rasool, F., Khan, N., Anjum, K.M. and Parveen, S. 2023. Prevalence and molecular detection of Edwardsiella tarda in cultured tilapia species of fish farms of Punjab in Pakistan and their postmortem examination. Pak. Vet. J. 43, 309–314. Marteja, J.C. and Modina, R.M.R. 2021. Melanomacrophage centers in nile tilapia (Oreochromis niloticus L.) as biomarker for carbamate exposure. J. Environ. Sci. Manag. 24, 25–35. Miniero Davies, Y., Xavier de Oliveira, M.G., Paulo Vieira Cunha, M., Soares Franco, L., Pulecio Santos, S.L., Zanolli Moreno, L., Túlio de Moura Gomes, V., Zanolli Sato, M.I., Schiavo Nardi, M. and Micke Moreno A. 2018. Edwardsiella tarda outbreak affecting fishes and aquatic birds in Brazil. Vet. Q. 38, 99–105. Mokhtar, D.M., Zaccone, G., Alesci, A., Kuciel, M., Hussein, M.T. and Sayed, R.K.A. 2023. Main components of fish immunity: an overview of the fish immune system. Fishes. 8, 020093. Oakes, C.C., Seifer, M., Assenov, Y., Gu, L., Przekopowitz, M., Ruppert, A.S., Wang, Q., Imbusch, C.D., Serva, A. and Koser, S.D. 2016. DNA methylation dynamics during B cell maturation underlie a continuum of disease phenotypes in chronic lymphocytic leukemia. Nat. Genet. 46, 253–264. Pandey, V., Hussain Bhat, R.A., Chandra, S., Tandel, R.S., Dubey, M.K., Sharma, P., Gehlot, B., Dash, P. and Joshi, R. 2021. Clinical signs, lethal dose and histopathological lesions in grass carp, Ctenopharyngodon idella experimentally infected with Edwardsiella tarda. Microb. Pathog. 161, 105292. Peng, L., Li, D., Yang, D. and Xiao, Peng B. 2022. Taurine promotes Oreochromis niloticus survival against Edwardsiella tarda infection. Fish Shellfish Immunol. 129, 137–144. Phuoc, N.N., Richards, R. and Crumlish, M. 2020. Establishing bacterial infectivity models in striped catfish Pangasianodon hypophthalmus (Sauvage) with Edwardsiella ictaluri. J. Fish Dis. 43, 371–378. Quintanilla, J.C., Gonzãlez, M.P., García, J.P., Olmos, P. and Contreras-Lynch, S. 2021. Horizontal transmission of Piscirickettsia salmonis from the wild sub-Antarctic notothenioid fish Eleginops maclovinus to rainbow trout (Oncorhynchus mykiss) under experimental conditions. J. Fish Dis. 44, 993–1004. Sakai, M., Hikima, J. and Kono, T. 2021. Fish cytokines: current research and applications. Fish. Sci. 87, 1–9. Salamah, S. and Zulpikar, Z. 2020. Pemberian probiotik pada pakan komersil dengan protein yang berbeda terhadap kinerja ikan lele (Clarias sp.) menggunakan sistem bioflok. Acta Aquat. Aquat. Sci. J. 7(1):21–29. Samir, S., Awad, A. and Younis, G. 2021. Prevalence, virulence determinants and antimicrobial-rsistant profile of Edwardsiella tarda isolated from Nile Tilapia (Oreochromis niloticus) in Egypt. Adv. Anim. Vet. Sci. 10, 1031–1038. Sattanathan, G., Tamizhazhagan, V., Padmapriya, S., Liu, W.C. and Balasubramanian, B. 2020. Effect of green algae Chaetomorpha antennina extract on growth, modulate immunity, and defenses against Edwardsiella tarda infection in Labeo rohita. Animals. 10, 1–16. Segaran, T.C., Azra, M.N., Piah, R.M., Lananan, F., Téllez-Isaías, G., Gao, H., Torsabo, D., Kari, Z.A. and Noordin, N.M. 2023. Catfishes: a global review of the literature. Heliyon. 9, e20081. Seibel, H., Baãmann, B. and Rebl, A. 2021. Blood will tell: what hematological analyses can reveal about fish welfare. Front. Vet. Sci. 8, 1–21. Sherif, A.H., Gouda, M.Y., Al-Sokary, E.T. and Elseify, M.M. 2021. Lactobacillus plantarum enhances immunity of Nile tilapia Oreochromis niloticus challenged with Edwardsiella tarda. Aquac. Res. 52, 1001–1012. Sui, Z.H., Xu, H., Wang, H., Jiang, S., Chi, H. and Sun, L. 2017. Intracellular trafficking pathways of Edwardsiella tarda: From clathrin- and caveolin-mediated endocytosis to endosome and lysosome. Front. Cell. Infect. Microbiol. 7, 00400. Sun, B., Li, X., Ning, X. and Sun, L. 2020. Transcriptome analysis of Paralichthys olivaceus erythrocytes reveals profound immune responses induced by Edwardsiella tarda infection. Int. J. Mol. Sci. 21(9), 21093094. Swain, B., Powell, C.T. and Curtiss, R. 2020. Pathogenicity and immunogenicity of Edwardsiella piscicida ferric uptake regulator (fur) mutations in zebrafish. Fish Shellfish Immunol. 107, 497–510. Tirani, E., Maftuch, M., Fadjar, M. and Awaluddin, M. 2024. Effect of Hermetia illucens larvae on the hematology of Tilapia (Oreochromis niloticus) infected Edwardsiella tarda. J. Aquac. Fish Heal. 13, 144–158. Tjampakasari, C.R., Scania, A.E. and Rediyanto, D.K. 2024. New development of Edwarsiella tarda infection. Clin. Med. Heal. Res. J. 4, 997–1004. Witeska, M., Kondera, E., Augowska, K. and Bojarski, B. 2022. Hematological methods in fish – Not only for beginners. Aquaculture 547, 737498. Yang, D.X., Yang, H., Cao, Y.C., Jiang, M., Zheng, J. and Peng, B. 2021. Succinate promotes phagocytosis of monocytes/macrophages in teleost fish. Front. Mol. Biosci. 8, 1–13. Yanuhar, U., Raharjo, D.K.W.P., Caesar, N.R. and Junirahma, N.S. 2021. Hematology response of catfish (Clarias sp.) as an indicator of fish health in tuban regency. IOP Conf. Ser. Earth Environ. Sci. 718, 012059. Zaheen, Z., War, A.F., Ali, S., Yatoo, A.M., Ali, M.N., Ahmad, S.B., Rehman, M.U. and Paray BA. 2022. Common bacterial infections affecting freshwater fish fauna and impact of pollution and water quality characteristics on bacterial pathogenicity. In Bacterial fish disease. Eds., Dar, G.H., Bhat, R.A., Qadri, H., Al-Ghamdy, K., Hakeem, K,R. Chennai, India: Academic Press, pp: 133–145. Zhou Z.J. and Sun, L. 2016. Edwardsiella tarda-induced inhibition of apoptosis: a strategy for intracellular survival. Front. Cell. Infect. Microbiol. 6, 1–10. | ||
| How to Cite this Article |
| Pubmed Style Kurniawati D, Fitriana E, Tjahjaningsih W, Ulkhaq MF, Pardede MA, Loh J. Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Vet. J.. 2025; 15(4): 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 Web Style Kurniawati D, Fitriana E, Tjahjaningsih W, Ulkhaq MF, Pardede MA, Loh J. Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). https://www.openveterinaryjournal.com/?mno=233029 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i4.17 AMA (American Medical Association) Style Kurniawati D, Fitriana E, Tjahjaningsih W, Ulkhaq MF, Pardede MA, Loh J. Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Vet. J.. 2025; 15(4): 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 Vancouver/ICMJE Style Kurniawati D, Fitriana E, Tjahjaningsih W, Ulkhaq MF, Pardede MA, Loh J. Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Vet. J.. (2025), [cited January 24, 2026]; 15(4): 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 Harvard Style Kurniawati, D., Fitriana, . E., Tjahjaningsih, . W., Ulkhaq, . M. F., Pardede, . M. A. & Loh, . J. (2025) Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Vet. J., 15 (4), 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 Turabian Style Kurniawati, Dwi, Evi Fitriana, Wahju Tjahjaningsih, Mohammad Faizal Ulkhaq, Maria Agustina Pardede, and Jiun-yan Loh. 2025. Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Veterinary Journal, 15 (4), 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 Chicago Style Kurniawati, Dwi, Evi Fitriana, Wahju Tjahjaningsih, Mohammad Faizal Ulkhaq, Maria Agustina Pardede, and Jiun-yan Loh. "Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia)." Open Veterinary Journal 15 (2025), 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 MLA (The Modern Language Association) Style Kurniawati, Dwi, Evi Fitriana, Wahju Tjahjaningsih, Mohammad Faizal Ulkhaq, Maria Agustina Pardede, and Jiun-yan Loh. "Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia)." Open Veterinary Journal 15.4 (2025), 1654-1662. Print. doi:10.5455/OVJ.2025.v15.i4.17 APA (American Psychological Association) Style Kurniawati, D., Fitriana, . E., Tjahjaningsih, . W., Ulkhaq, . M. F., Pardede, . M. A. & Loh, . J. (2025) Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia). Open Veterinary Journal, 15 (4), 1654-1662. doi:10.5455/OVJ.2025.v15.i4.17 |