| Research Article | ||
Open Vet. J.. 2025; 15(4): 1637-1644 Open Veterinary Journal, (2025), Vol. 15(4): 1637-1644 Research Article Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional studyWidodo Suwito1*, Andriani2 Eni Kusumaningtyas2, Roza Azizah Primatika3 and Raden Bambang Heryanto41Research Center Processing Food Technology, National Research and Innovation Agency (BRIN), Yogyakarta, Indonesia 2Veterinary Research Center, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Research Center for Geospatial, Research Organization for Earth Science and Maritime, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Widodo Suwito. Research Center Processing Food Technology, National Research and Innovation Agency (BRIN), Yogyakarta, Indonesia. Email: widodo.suwito [at] yahoo.com Submitted: 11/12/2024 Accepted: 18/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
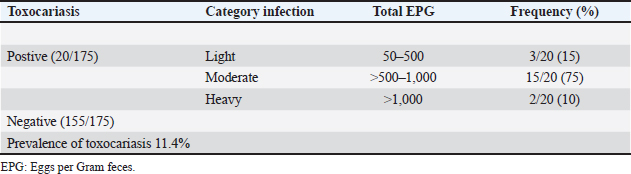
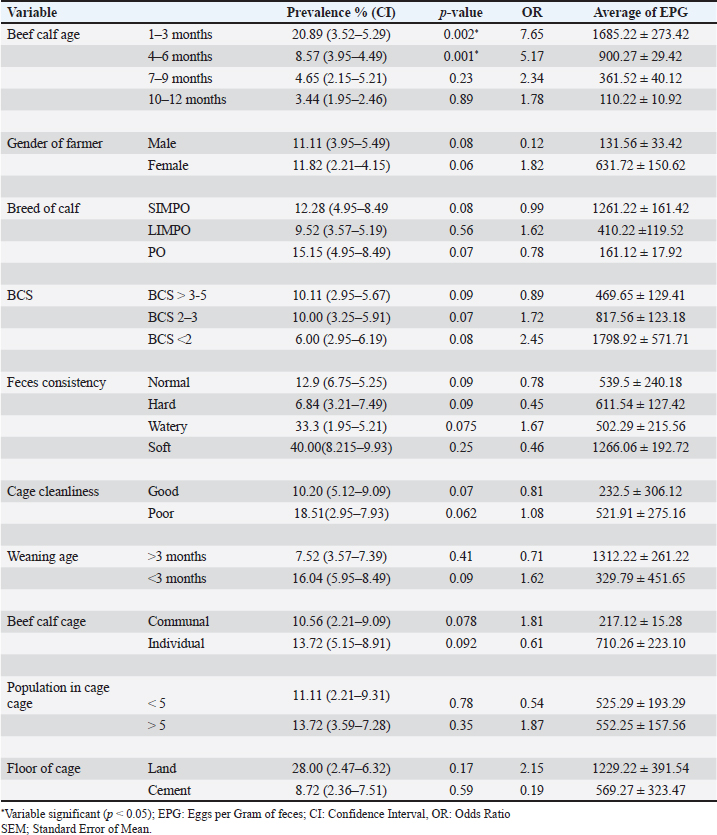
AbstractBackground: Toxocariasis, caused by Toxocara vitulorum, is economically detrimental to beef calves. Despite its potential impact on cattle productivity, toxocariasis remains underrecognized by local farmers in Bantul, Yogyakarta, Indonesia. Aim: This study aimed to determine the prevalence and risk factors associated with toxocariasis in beef calves in the Pandak subdistrict of Bantul Regency, Yogyakarta, Indonesia. Methods: The study employed a cross-sectional design, and 175 beef calf feces samples were collected for analysis. Risk factors such as age, sex, breed, body condition score, feces consistency, type of cage, weaning age, number of beef cattle per cage, and cage flooring material were collected using a structured questionnaire. Eggs per gram of feces (EPG) were quantified using the McMaster technique, and the total EPG count was categorized as light, moderate, or heavy. Data were analyzed using univariate, bivariate, and multivariate methods with the Statistical Package for the Social Sciences, version 16.0 for Windows. Results: The prevalence of toxocariasis in beef calves in the Pandak subdistrict, Bantul Regency, Yogyakarta, Indonesia was 11.4%. Ages 1–3 months and 4–6 months are identified as risk factors for toxocariasis in beef calves. Conclusion: Toxocariasis is an endemic parasitic disease in beef calves aged under 6 months in Pandak subdistrict of Bantul Regency, Yogyakarta, Indonesia. Consequently, anthelmintic treatment should be routinely administered to beef calves aged below 6 months. Keywords: Beef calves, Bantul regency, Toxocariasis, Prevalence, Risk factors. IntroductionToxocariasis is a parasitic disease caused by Toxocara vitulorum (T. vitulorum) in cattle. It is one of the most significant endoparasites affecting cattle, primarily residing in the small intestine (Rast et al., 2014; Bowman, 2020). This leads to substantial economic losses, particularly in the tropical and subtropical regions. The disease causes considerable economic impact, primarily due to weight loss, poor meat quality, and increased mortality rates (CDC, 2023). Toxocara vitulorum is particularly detrimental to cattle and buffalo, with a high mortality rate of up to 37.3% (Rast et al., 2014). In cases of uncontrolled infections, mortality rates can reach as high as 80% (Biswas et al., 2022). Calves affected by toxocariasis may experience up to 16 kg weight loss by 12 weeks of age in severe cases compared with uninfected calves (Radostits et al., 2000; Bowman, 2020). The estimated economic losses due to T. vitulorum infection in the grazing dairy cattle population in Mexico were US$ 189,212,709 (Rodríguez-Vivas et al., 2017). In buffalo calves aged 1–3 months, toxocariasis is the leading cause of morbidity and mortality (Rodríguez-Vivas et al., 2017). If left uncontrolled, the infection prevalence can approach 100%, with mortality rates reaching up to 50% (Srivastava and Sharma, 1981; Radostits et al., 2000; Rast et al., 2013). Calf toxocariasis is highly prevalent, particularly in calves under 6 months of age (Bowman, 2020; Wyrosdick et al., 2025). Beef cattle become infected with T. vitulorum by ingesting grass contaminated with infected worm eggs or larvae. These eggs remain in the small intestine until they hatch into first-stage larvae (L1), which then progress to second-stage larvae (L2), and eventually to third-stage larvae (L3) (Starke-Buzetti, 2006; Bowman, 2020). L3 larvae penetrate the intestinal wall and migrate to the liver and lungs via the bloodstream. L3 larvae are subsequently coughed up by the infected animal, ingested, and mature into adult worms in the intestine. Adult worms begin laying eggs 3–5 weeks after infection (Starke-Buzetti, 2006; Bowman, 2020). The severity of disease is correlated with the number of worm eggs present in the animal’s feces. Toxocara vitulorum can be transmitted to newborn calves through both the placenta and breast milk (Starke-Buzetti, 2006; Bowman, 2020). Postnatal infection in calves, typically through the ingestion of L3 larvae present in colostrum or milk, is common on farms and contributes to increased morbidity and mortality. Because L3 larvae are secreted in colostrum and milk for up to 12 days after birth, beef calves are often infected while suckling. L3 larvae have been detected in both colostrum and milk samples (Starke-Buzetti, 2006). Microscopic examination revealed the presence of L3 larvae in 4% of samples, whereas 10% of the samples tested positive using molecular techniques (Urhan et al., 2023). L3 larvae were found in colostrum and milk samples collected between 2 and 28 days postpartum. As calves suckle, they ingest L3 larvae, which mature into adult worms in the intestine (Starke-Buzetti, 2006). The adult worms began producing eggs daily, starting on the 12th day postinfection. The presence of T. vitulorum eggs in feces is associated with the prepatent period, which is defined as the time between infection and the onset of egg production (Bowman, 2020). Toxocara vitulorum has a prepatent period of 22.3 ± 1.6 days, with peak egg production occurring at 5 weeks (Starke-Buzetti, 2006; Bowman, 2020). After this peak, egg output gradually declines to zero after 3 months. The topography of Pandak subdistrict consists of 10% hilly plains and 90% undulating lowlands. The area encompasses 1,344,785 hectares of paddy fields, 1,231,587 hectares of dry land, 0.8186 hectares of wetlands, 21,840 hectares allocated for public facilities, and 219,104 hectares of barren or sandy land (BPS, 2023). Pandak subdistrict is a lowland region characterized by numerous rivers that provide irrigation for rice fields. It also has the highest population of beef cattle in the area compared with other subdistricts in the area. Located in Bantul Regency, Yogyakarta, Indonesia, Pandak subdistrict experiences an average rainfall of 90.76 mm and a temperature of approximately 30°C (BMKG, 2020). Due to its favorable topography and efficient irrigation system, agriculture is the primary source of income for local residents. In 2023,the beef cattle population in Pandak subdistrict is projected to reach 2,809 heads (BPS, 2023). Most residents rely on farming and livestock breeding for their livelihoods, benefiting from the region’s abundant water resources and extensive land availability. The primary breeds of beef cattle raised in the study area include the Ongole crossbreed, Limousin-Ongole crossbreed (LIMPO), and Simmental-Ongole crossbreed (SIMPO) (Suwito, 2020). In the Bantul sub-district, beef cattle are raised using three distinct methods: traditional, intensiv and semiintensive. Cattle are typically housed in communal or private enclosures, depending on the management system. The primary source of feed consists of agricultural waste, while King grass, harvested from adjacent agricultural land, is also commonly used. Additionally, beef farmers generally wean calves at three months of age to strengthen them and introduce them to green fodder, such as field grass (Suwito, 2020). Toxocariasis in beef cattle has been reported in Indonesia, particularly in areas with extensive irrigation (Davila et al., 2010; Purwandani et al., 2021). Considering that Pandak sub-district in Bantul Regency is well-irrigated, and local beef farmers frequently encounter confusion when calves, either still suckling or newly born, exhibit signs of worm infestation in their feces, it is essential to conduct research on the prevalence and risk factors of toxocariasis in beef calves in this region. Materials and MethodsEthical approvalThe authors complied with all applicable international, national, and institutional guidelines for the care and use of animals. The ethical protocols followed during sample collection and animal handling are outlined as follows: The beef calf farmer provided a restraint device, and latex gloves were worn during the collection of feces samples, which were obtained either directly from the rectum or from freshly excreted feces. After collection, feces samples were placed in labeled ziplock plastic bags, which included essential information such as the calf’s identification number, gender, age, date of collection, breed, farm address, and farmer’s name. Pattern and description area researchThis research employed a cross-sectional study conducted from May to June 2022 in the Pandak subdistrict of Bantul Regency, Yogyakarta, Indonesia. Calf feces samples were collected from the study area, and their locations were recorded using a Garmin Handheld global positioning system (GPS) eTrex 10. Sampling sizeA total of 175 beef calf feces samples were collected, with the sample size estimated using the formula n=4PQ/L2, where P represents the apparent prevalence, Q is 1-P, and L denotes the desired precision (Martin et al., 1987). Risk factorRisk factor data were collected through interviews and feces sampling using a structured questionnaire (Thrusfield, 2007). The questionnaire included inquiries regarding various variables, such as age of beef calf, gender of calf owner, breed of calf, body condition score (BCS), feces consistency, cage cleanliness, weaning age, type of cage, total beef cattle populationper cage, and type of cage flooring. Feces examinationFecal samples were sent to the Parasitology Laboratory at Veterinary Center for Investigation, Directorate General of Animal Husbandry and Health, Ministry of Agriculture, Wates, Indonesia, where they were stored in a refrigerator prior to testing. The beef calf feces were classified as normal, hard, watery, or soft. A 2 g sample from each specimen was examined using the flotation method with a saturated NaCl solution. The eggs per gram (EPG) of feces were determined using the McMaster technique, which has a sensitivity of 50 EPG. Based on conventional EPG counts, the severity of toxocariasis in beef calves was classified into three categories: light, moderate, and heavy (Johnson et al., 2022). Statistical analysesData were analyzed using univariate, bivariate, and multivariate methods. Prevalence was calculated by dividing the number of T. vitulorum-positive feces samples by the total number of examined samples and then multiplying the result by 100% (Thrusfield, 2007). To determine the relationship between risk factors and T. vitulorum prevalence, bivariate analysis was performed using the chi-squared test, with a significance level set at p < 0.05 (Dahoo et al., 2014). The variables that exhibited statistical significance (p < 0.05) in the bivariate analysis were selected as candidate variables for logistic regression. Statistical analysis was conducted using the Statistical Package for the Social Sciences, version 16.0 for Windows. ResultsAccording to GPS data, Pandak Subdistrict in Bantul District is located at an elevation of 27 m above sea level. The geographic coordinates range from 07° 44’ 04”S to 08° 00’ 27” Slatitude and from 110° 12’ 34” E to 110° 31’ 08”E longitude. The administrative boundaries of Pandak Sub-district are as follows: to the west, it borders Srandakan District; to the north, it borders Pajangan and Bantul Districts; to the east, it borders Bambanglipuro and Bantul Districts; and to the south, it borders Sanden District (Fig. 1). Toxocariasis in beef calves was diagnosed based on the presence of eggs, with conventional EPG counts performed on positive samples. The results indicated that 10% of the calves had heavy infections, 75% had moderate infections, and 15% had light infections (Table 1). Bivariate chi-square test analysis revealed that the age of beef calves had a significant (p < 0.05) effect on the prevalence of toxocariasis (Table 2). In contrast, factors such as calf owner gender, breed of beef calf, BCS, cage cleanliness, calf weaning age, feces consistency, type of cage, number of beef cows per cage, and type of cage flooring did not show a significant (p > 0.05) effect on the occurrence of toxocariasis. Of the 10 variables hypothesized as potential risk factors, only calf age was identified as a significant (p < 0.05) risk factor and was included in the final logistic regression model (Table 3). DiscussionMany beef cattle farmers in Bantul Regency engage in agriculture, which enables them to use agricultural waste as feed for their cattle. Additionally, the region’s adequate water supply ensures consistent availability of green animal feed. As part of a free cattle health inspection program offered by veterinarians at local animal health centers, farmers conduct health checkups on their cattle every 3 months. However, this schedule is insufficient for effectively managing toxocariasis in beef calves. Toxocariasis can be transmitted to beef calves both transplacentally and through breastfeeding, indicating that calves may become infected either at birth or during suckling (Starke-Buzetti, 2006; Bowman, 2020). Toxocariasis has been reported in breastfeeding crossbred Jersey beef calves, and its symptoms include intermittent diarrhea, poor growth, weakness, moderate dehydration, and anorexia (Ahmed et al., 2016). The prevalence and risk factors of toxocariasis in beef calves are crucial for developing effective control strategies for parasitic diseases, particularly in Pandak Sub-district of Bantul Regency, Yogyakarta, Indonesia, where control measures for toxocariasis have been insufficient or inefficient. Toxocariasis is widespread globally, with significant implications for livestock, especially in tropical and subtropical regions (Rast et al., 2013). Additionally, Ninditya et al. (2024) noted that Indonesia, as a tropical country with a humid climate and year-round rainfall, continues to face challenges in controlling and eradicating toxocariasis. The protective shell of T. vitulorum eggs enables their persistence for many years, as it provides resistance to harsh environmental conditions (Mahapatra et al., 2022). Continuous grazing by cattle on land can increase the risk of the area becoming a potential source of T. vitulorum infection. Controlling toxocariasis in beef cattle is challenging because of the high reproductive capacity of adult female T. vitulorum, which produces large numbers of eggs daily (Starke-Buzetti, 2006; Bowman, 2020; Dey et al., 2020; Healy et al., 2022). Various factors, including geography, season, age, sex, BCS, feces consistency, nutritional status, and cattle practices, may influence the prevalence of toxocariasis. Numerous studies have identified factors such as age, sex, BCS, season, and feces consistency as potential contributors to T. vitulorum infection in different geographical regions worldwide (Woodbury et al., 2012; Biswas et al., 2022).
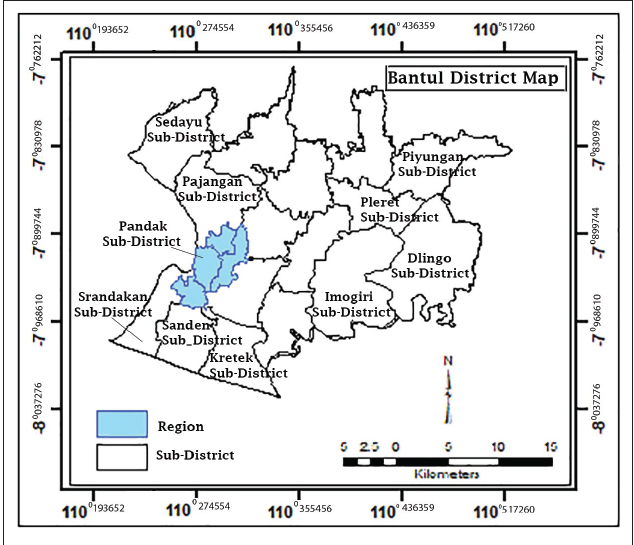
Fig. 1. Study area collection beef calf feces. Table 1. Toxocariasis in beef calves based on T. vitulorum eggs and total of EPG feces count.
The presence of T. vitulorum eggs in the feces of beef calves of different age groups, particularly those under 6 months of age, indicates that toxocariasis is highly prevalent in calves under 6 months. This suggests that age is a potential risk factor for infection. Dewair and Bess (2020) reported that toxocariasis predominantly affects beef calves older than 6 months, a finding consistent with Suwito (2020), who observed that the prevalence of toxocariasis decreases with age. This reduction is likely due to the maturation of the immune system in older animals, which reduces their susceptibility to infection (Starke-Buzetti, 2006; Bowman, 2020; Hernãndez et al., 2020). A similar study conducted in East Java, Indonesia, also noted a decrease in the prevalence of T. vitulorum with age (Nugroho et al., 2020). Ridwan et al. (2019) also reported that toxocariasis was more prevalent in beef calves than in adult beef cattle in the Kasiman District, Indonesia. The highest prevalence was reported in the 30-day age group (49.23%), followed by 39.38% in 31-60-day group, 29.82% in 61-90-day group, 18.85% in 91-120-day group, 12.93% in 121-150-day group, 9.23% in 151-180-day group, and 2.33% in calves older than 180 days. Toxocariasis has been reported in beef calves in the Siak Sriindrapura area, Riau, Indonesia, with a prevalence of 20% (Susana et al., 2019). In contrast, in Ada’a District, Central Ethiopia, the prevalence rate was 2.79% in urban and peri-urban areas (Alkadir et al., 2024). Table 2. Risk factors associated with toxocariasis in beef calves.
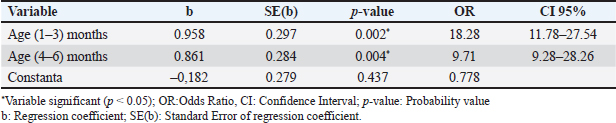
Table 3. Logistic regression model used to identify risk factors toxocariasis in beef calves.
The sex of beef calves does not influence their susceptibility to toxocariasis. Similarly, Kozan et al (2021) and Waindok et al (2021) found no correlation between the prevalence of T. vitulorum and the breed or sex of beef calves. This study also demonstrated that beef calf breeds, including SIMPO, LIMPO, and PO, do not exhibit a higher prevalence of toxocariasis. Although the prevalence of toxocariasis varies among different beef calf breeds, the breed does not appear to be a significant contributing factor. This is consistent with the findings of Kozan et al. (2021), who concluded that neither breed nor sex influences the occurrence of toxocariasis in cattle. Hiranmoy et al. (2021) reported differences in toxocariasis prevalence between buffalo calves and indigenous river buffalo calves. Toxocariasis in beef calves typically presents with clinical symptoms such as weight loss and anorexia, resulting in a BCS score of less than 2. However, toxocariasis can also occur in beef calves with a BCS greater than 2, as observed in calves from the Pandak subdistrict in Bantul. In contrast, Hiranmoy et al. (2021) found that toxocariasis was associated with BCS and was more prevalent in calves with poor or medium BCS compared with those with adequate BCS. The low BCS observed in affected animals may be attributed to poor farming practices, malnutrition, and various comorbidities, such as toxocariasis, which could compromise the immune response to the infective stage of the parasite (Hernãndez et al., 2020). Toxocariasis affects beef calves with varying stool consistencies. Our findings contrast those from China, where a significant association between toxocariasis and soft feces in calves was reported (Li et al., 2016). However, in Cambodia, buffalo calves aged 1–3 months with soft feces were not identified as risk factors for toxocariasis (Johnson et al., 2022). There is an ongoing debate regarding whether soft feces influence the prevalence of toxocariasis in beef calves. In a study by Ifqiyyah et al. (2021), a link was suggested between soft feces and increased susceptibility to toxocariasis in calves in Jombang District, Indonesia, whereas others found no such association in buffalo or calf populations in North America (Johnson et al., 2022). The cleanliness of cages can potentially be a source of various infectious agents, including toxocariasis. However, this was not observed in beef calves in the Pandak subdistrict of Bantul Regency, Yogyakarta, Indonesia. In contrast, studies by Ridwan et al. (2019) and Purwandani et al. (2021) have demonstrated that toxocariasis is significantly more prevalent in unclean cages, as the eggs, worms, or larvae thrive and develop in such environments, leading to contamination of the environment such as feed or drink. Therefore, clean cage floors can help reduce worm infestations in cattle. In the Pandak subdistrict of Bantul Regency, beef calves are typically weaned by farmers aged 3 months or older. This approach aims to ensure calves’ strength and reduce the risk of toxocariasis. However, our research indicates that beef calves weaned at 3 months of age are not immune to toxocariasis because the infection can occur through the placenta or during breastfeeding. This finding is consistent with those of Kozan et al. (2021) and Urhan et al. (2023), who reported that toxocariasis is more prevalent in calves aged 1–6 months. Calves can become infected through colostrum, and the worms may be expelled spontaneously by the time the calf reaches 5 months of age (Starke-Buzetti, 2006; Bowman, 2020). Beef cattle in the Pandak subdistrict of Bantul Regency, whether housed in private or communal pens, can become infected with T. vitulorum. Geographical conditions and cattle forage contaminated with L3 larvae are believed to be the primary factors contributing to the onset of toxocariasis in beef cattle. In contrast, Purwandani et al. (2021) reported that the placement of cattle cages influences the occurrence of toxocariasis, with cattle in lowland areas being more affected than those in highland regions. This difference is likely due to the humidity and temperature conditions in lowland areas, which favor the development of worm egg larvae (Hiranmoy et al., 2021). Additionally, Li et al. (2016) suggested that the geographical origin of beef calves is a risk factor for T. vitulorum infection. Our research indicates that housing multiple beef cattle in a single cage has a minimal impact on the prevalence of toxocariasis. However, the rapid development of T. vitulorum eggs may occur when the cage floor is not cemented, and this issue is further exacerbated when multiple cattle are housed together in the same cage. The regression analysis indicates that age is a risk factor for toxocariasis in beef calves. Calves aged 1–3 months and 4–6 months are significantly more likely to be affected by toxocariasis, with Odds ratios of 18.28 (95% CI=11.78–27.54) and 9.71 (95% CI=9.28–28.26), respectively. Specifically, calves aged 1–3 months have an 18.28-fold higher risk of toxocariasis compared to older calves, whereas calves aged 4–6 months have a 9.71-fold higher risk. These findings suggest that the risk of toxocariasis decreases with calves age. This is consistent with previous studies (Tamire and Bedore, 2019; Nugroho et al., 2020; Ifqiyyah et al., 2021; Kozan et al., 2021; Biswas et al., 2022; Urhan et al., 2023; Alkadir et al., 2024), which demonstrated that younger calves are at higher risk for toxocariasis. One contributing factor is the immature immune system of younger calves. Hernãndez et al. (2020) observed that as calves age, their immune system gradually strengthens. Additionally, factors such as semiintensive feeding systems and inadequate calf management also play a role in the development of toxocariasis. ConclusionToxocariasis in beef calves in Pandak Sub-district, Bantul Regency, Yogyakarta, Indonesia is endemic, with a higher prevalence observed in calves under 6 months of age. Therefore, beef calves younger than 6 months should be promptly treated with anthelmintics. AcknowledgmentsThe authors wish to express their sincere gratitude to the beef cattle farmers in Pandak Subdistrict of Bantul Regency, Yogyakarta, Indonesia. They also extend their thanks to Mrs Wahyu Ananingsih and Ms Sulasih for their technical suppor and Ms Dewi from the English Language Training Center at Universitas Gadjah Mada (UGM), Yogyakarta, Indonesia, for her assistance with manuscript copyediting. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThe authors declare that no funding was received for this research. Authors’ contributionsAll authors contributed equally to the planning, laboratory work, manuscript preparation, and statistical analysis. Data availabilityAll relevant data are presented in the text. ReferencesAhmed, R., Wani, Z.A., Allaie, I.M., Bushra, M.S. and Hussain, H.A. 2016. Toxocara vitulorum in a suckling calf: a case study. J. Parasit. Dis. 40, 1330–1331. Alkadir, G., Ayana, D., Wakjira, G. and Fatalo, T. 2024. Helminth parasites transmission between species of ruminants in urban and peri-urban areas of Ada’a district of Central Ethiopia. J. Vet. Med. Anim Health. 16, 1–11. Badan Meteorologi Klimatologi dan Geofisika. 2020. Profil Daerah Kabupaten Bantul. Profil daerah kabupaten Bantul. Badan Perencanaan Pembangunan Kabupaten Bantul. Yogyakarta, Indonesia: Bapeda Press. Badan Pusat Statistik. 2023. Badan Pusat Statistik Kabupaten Bantul. Kabupaten Bantul dalam Angka. Bantul Regency. Yogyakarta, Indonesia: BPS Kabupaten Bantul Press. Biswas, H., Roy, B.C., Hasan, M.M., Ahmed. N., Dutta, P.K., Begum, N. and Talukder, Md. H. 2022. Efficacy of clinically used anthelmintics against toxocariasis of buffalo calves in Bangladesh. J. Parasit. Dis. 46, 988–997. Bowman, D.D. 2020. History of Toxocara sp. Adv. Parasit. 109, 17–380. Centers for Disease Control. 2023. Toxocariasis. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Dahoo, I., Martin, W. and Stryhn, H. 2014. Veterinary epidemiologic research. Charlottetown, CA: VER, Inc. Davila, G., Irsik, M. and Greiner, E.C. 2010. Toxocara vitulorum in beef calves in north central Florida. Vet. Parasitol. 168, 261–268. Dewair, A. and Bessat, M. 2020. Molecular and microscopic detection of natural and experimental infections of Toxocara vitulorum in bovine milk. PLoS One. 20, 1–12. Dey, A.R., Begum, N., Alim, Md.A., Malakar, S., Islam, Md.T. and Alam, M.Z. 2020. Gastrointestinal nematodes in goats in Bangladesh: a large-scale epidemiological study on the prevalence and risk factors. Parasit. Epid. Control. 4, 1–10. Healy, S.R., Morgan, E.R., Prada, J.M. and Betson, M. 2022. First report demonstrating the presence of Toxocara spp. eggs on vegetables grown in community gardens in Europe. Food Waterborne. Parasit. 27, 1–7. Hernãndez, D.A.J., Garcés, L.F.S., Baquero-Parra, M.M., da Silva-Pinheiro, C. and Alcantara-Neves, N.M. 2020. Toxocariasis and toxocara vaccine: a review. Orinoquia. 24, 79–95. Hiranmoy, B., Chandra, R.B., Dutta, P.K., Hassan, M.M., Parvin, S., Choudhury, D.K., Begum, N. and Talukder, Md. H. 2021. Prevalence and risk factors of Toxocara vitulorum in buffalo calves in cthe coastal northeastern, and northwestern regions of Bangladesh. Vet. Parasit. Regional Stud. Rep. 26, 59–72. Ifqiyyah, M.M., Setiawan, B. and Wijaya, A. 2021. Prevalence of gastrointestinal parasites on beef cattle in Jombang district. J. Parasit. Sci. 2, 45–50. Johnson, W.L., Reynolds, S., Adkins, C.L., Wehus-Tow, B., Brennan, J., Krus, C.B., Buttke, D., Martin, J.M. and Chelladurai, J.R.J.J. 2022. A comparison of mini-FLOTAC and McMaster techniques, overdispersion and prevalence of parasites in naturally infected North American bison (Bison bison) in the USA. Curr. Res. Parasit. Vect. Borne. Dis. 8, 1–10. Kozan, E., Birdane, F.M., Erez, M.S. and Goksu, A. 2021. Prevalence of Toxocara vitulorum in calves in Afyonkarahisar, Turkey. Koc. Vet. J. 2, 225–230. Li, K., Lan, Y., Luo, H., Zhang, H., Liu, D., Zhang, L., Gui, R., Wang, L., Shahzad, M., Sizhu, S., Li., J. and Chamba, Y. 2016. Prevalence, associated risk factors, and phylogenetic analysis of Toxocara vitulorum infection in Yaks the Qinghai Tibetan Plateau, China. Korea. J. Parasitol. 54, 645–652. Mahapatra, S., Ali, Md. H., Samal, K. and Moulick, S. 2022. Diagnostic and treatment technologies for detection and removal of helminth in wastewater and sludge. Energy. Nexus. 8, 1–16. Martin, S.W., Meek, A.H. and Willeberg, P. 1987. Veterinary epidemiology: principles and methods. Ames IA: Iowa State University Press. Ninditya, V.I., Ekawasti, F., Prastowo, J., Widiyono, I. and Nurcahyo, W. 2024. Incidence and risk factors of Toxocara vitulorum infection in beef cattle of Yogyakarta, Indonesia. World Vet. J. 14, 592–599. Nugroho, W., Aditya, S., Swastomo, R. and Aulanni’am, A. 2020. Productivity, absence of a bull and endoparasitic nematodiosis in beef cattle farms in an upland area of East Java, Indonesia. Vet. World. 13, 1982–1987. Purwandani, C.E.P., Kuncorojakti, S. and Suwanti, L.T. 2021. Prevalence of helminths in digestive tract of cows in Indonesia. World’s. Vet. J. 4, 658–662. Radostits, O., Gay, C., Blood, D. and Hinchcliff, K.W. 2000. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats and horses. New York, NY:WB Saunders CO. Rast, L., Lee, S., Nampanya, S., Toribio, J.A., Khounsy, S. and Windsor, P.A. 2013. Prevalence and clinical impact of Toxocara vitulorum in cattle and buffalo calves in Northern Lao PDR. Trop. Anim. Health. Prod. 45, 539–546. Rast, L., Toribio, J.A., Dhand, N.K., Khounsy, S. and Windsor, P.A. 2014. Why are simple control options for Toxocara vitulorum not being implemented by cattle and buffalo smallholder farmers in South East Asia?. Prev. Vet. Med. 113, 211–218. Ridwan, Y., Satrija, F. and Winarso, A. 2019. Prevalence and risk factor of strongyloidosis in beef cattle in Kasiman Subdistrict, Bojonegoro. J. Ked. Hewan. 13, 105–108. Rodríguez-Vivas, R.I., Grisi, L., Angel Pérez de Leãnc, A., Silva Villelad, H., Felipe de Jesús, J., Torres-Acostaa, Sãncheze, H.F., Salasf, D.R., Cruzg, R.R., Saldiernah, F. and Carrasco, D.D. 2017. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev. Mex. Cienc. Pecu. 8, 61–74. Srivastava, A.K. and Sharma, D.N. 1981. Studies on the occurrence, clinical features and pathomorphological aspects of ascariasis in buffalo calves. Vet. Res. J. 4, 160–162. Starke-Buzetti, W.A. 2006. Toxocara vitulorum in livestock. C.V. Holland, C.V. and Smith, H.V. (Eds.). Toxocara: the Enigmatic Parasite. Wallingford, UK: CAB International, pp: 260–277. Susana, Y., Suwanti, L.T. and Suprihati, E. 2019. Identification and prevalence of gastrointestinal parasites in beef calf in Siak Sriindrapura, Riau, Indonesia. Ind. J. Trop. Infec. Dis. 7, 155–160. Suwito, W. 2020. Kejadian toxocariasis pedet lepas sapih pada UPSUS SIWAB di DIY. Badan Penelitian dan Pengembangan Pertanian. Jakarta, Indonesia: IAARD Press. Tamire, M. and Bedore, B. 2019. Study on prevalence of Toxocara vitulorum in bovine of Senkale Faris Peasant Association of Ambo districts, west Shewa zone, Ethiopia. Am. J. Epid. Pub. Health. 3, 1–6. Thursfiled, M. 2007. Veterinary Epidmiology; describing disease Ocurrence. Third. Hoboken, NJ: Blackwell Publishing Ltd. Urhan, O.F., Erol, U. and Altay, K. 2023. Molecular detection and phylogenetic analysis of Toxocara vitulorum in feces and milk samples from naturally infected water buffaloes. Res. Vet. Sci. 9, 1–7. Waindok, P., Kann, S., Aristizabal, A., Dib, J.C. and Strube, C. 2021. Toxocara seroprevalence and risk factor analysis in four communities of the Wiwa, an indigenous tribe in Colombia. Micro. 8, 1–8. Wyrosdick, H., Johnson, A., Riese, K., Miller, D. and Gerhold, R. 2025. First report of Toxocara vitulorum infection in a dairy calf in Tennessee. Vet. Parasitol: Reg. Stud. Rep. 57, 101184. Woodbury, M.R., Copeland, S., Wagner, B., Fernando, C., Hill, J.E. and Clemence, C. 2012. Toxocara vitulorum in a bison (bison bison) herd from Western Canada. Canadian Vet. J. 7, 791–794. | ||
| How to Cite this Article |
| Pubmed Style Suwito W, Andriani A, Kusumaningtyas E, Primatika RA, Heryanto RB. Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Vet. J.. 2025; 15(4): 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 Web Style Suwito W, Andriani A, Kusumaningtyas E, Primatika RA, Heryanto RB. Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. https://www.openveterinaryjournal.com/?mno=232497 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.15 AMA (American Medical Association) Style Suwito W, Andriani A, Kusumaningtyas E, Primatika RA, Heryanto RB. Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Vet. J.. 2025; 15(4): 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 Vancouver/ICMJE Style Suwito W, Andriani A, Kusumaningtyas E, Primatika RA, Heryanto RB. Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 Harvard Style Suwito, W., Andriani, . A., Kusumaningtyas, . E., Primatika, . R. A. & Heryanto, . R. B. (2025) Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Vet. J., 15 (4), 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 Turabian Style Suwito, Widodo, Andriani Andriani, Eni Kusumaningtyas, Roza Azizah Primatika, and Raden Bambang Heryanto. 2025. Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Veterinary Journal, 15 (4), 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 Chicago Style Suwito, Widodo, Andriani Andriani, Eni Kusumaningtyas, Roza Azizah Primatika, and Raden Bambang Heryanto. "Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study." Open Veterinary Journal 15 (2025), 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 MLA (The Modern Language Association) Style Suwito, Widodo, Andriani Andriani, Eni Kusumaningtyas, Roza Azizah Primatika, and Raden Bambang Heryanto. "Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study." Open Veterinary Journal 15.4 (2025), 1637-1644. Print. doi:10.5455/OVJ.2025.v15.i4.15 APA (American Psychological Association) Style Suwito, W., Andriani, . A., Kusumaningtyas, . E., Primatika, . R. A. & Heryanto, . R. B. (2025) Prevalence and risk factors of toxocariasis in beef calves in Pandak, Indonesia: A cross-sectional study. Open Veterinary Journal, 15 (4), 1637-1644. doi:10.5455/OVJ.2025.v15.i4.15 |