| Research Article | ||
Open Vet. J.. 2025; 15(5): 1969-1981 Open Veterinary Journal, (2025), Vol. 15(5): 1969-1981 Original Research Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogsMamoru Onuma1,2*, Kai Ataka3 and Akiyoshi Murakami11Oosagami Animal Clinic, Koshigaya-shi, Japan 2Department of Animal Risk Management, Faculty of Risk and Crisis Management, Chiba Institute of Science, Choshi, Japan 3Anicom Pafe Inc., Tokyo, Japan *Corresponding Author: Mamoru Onuma. Oosagami Animal Clinic, Koshigaya-shi, Japan. Email: monuma [at] cis.ac.jp Submitted: 11/12/2024 Revised: 06/04/2025 Accepted: 19/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
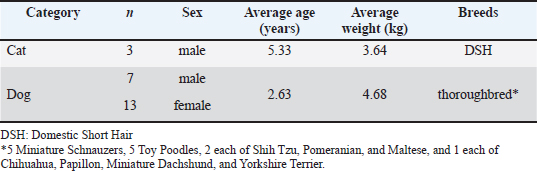
ABSTRACTBackground: Although various prebiotics, probiotics, and postbiotics are available, their safety and efficacy in combination are unknown. Aim: We investigated the safety and functionality of a newly developed supplement, previously unreported in pet animals, containing 26 types of biotic material bacteria (2 prebiotics, 1 probiotic, and 23 postbiotics) in cats and dogs. The biotic materials included were selected based on current evidence from cats and dogs. Methods: A new supplement developed using species tested in cats and dogs was administered. One-way analysis was used for data obtained from 3 cats (7 days of treatment and 7 days of nontreatment), and a parallel, controlled study was performed in 20 dogs (n=10 each in control and test groups, for 27 days). Results: In cats, no abnormal values were observed in complete blood count or blood chemistry tests, whereas significant decreases in blood glucose and total cholesterol were confirmed (p < 0.05 each). In the feline lymphocyte subset test, significant increases were observed in T and B cells (p < 0.05). A significant difference in fecal pH was observed in the test group (p < 0.01). In addition, 60% (9/15) of the test group had an increase in total organic acids. In dogs, only indole showed a consistent decrease among putrefactive products (p=0.055). Regarding analyses of intestinal flora from feces using a gene sequencer at the genus level, no changes were observed in cats. Conversely, Lachnospira and Anaeroplasma genera tended to be decreased in the control group but increased by 23.1% and 45%, respectively, in the test group. In addition, Escherichia-Shigella and Tyzzerella genera showed slight increases or changes in the control group but significant decreases in the test group. Regarding the Firmicutes/Bacteroidetes ratio, an increase in the control group and a decrease in the test group were observed in all cats, whereas no differences were observed in dogs. Conclusion: The supplement is safe for both cats and dogs. Results of comprehensive analyses suggested that the supplement improved the intestinal environment by regulating the gastrointestinal microbiota. Keywords: Cat, Dog, Postbiotic, Prebiotic, Probiotic. IntroductionThe intestinal flora has been the focus of much attention in recent years in the fields of both feline and canine health, particularly gut health. The gastrointestinal microbiota contributes to the maintenance of gastrointestinal and host homeostasis and health through multiple mechanisms, including defense against intestinal pathogens, provision of nutrients, promotion of nutrient digestion and absorption, improvement of barrier function, promotion of gastrointestinal development, and regulation of the immune system immune system (Yang and Wu, 2023). Among the important mediators of host-microbiota interactions are bacterial metabolites such as short-chain fatty acids (SCFAs), secondary bile acids, and tryptophan metabolites (Gasaly et al., 2021). SCFAs include acetic acid, propionic acid, and butyric acid and play a particularly important role in intestinal and host health by acting as the energy substrate for intestinal epithelial cells, maintaining the integrity of the epithelial barrier, regulating energy metabolism, and exerting anti-inflammatory effects (Zhang et al., 2021). Abnormalities in the intestinal flora (dysbiosis) result in gut-related diseases, such as chronic enteropathy, inflammatory bowel disease (IBD), and acute diarrhea in humans, dogs, and cats (Pilla and Suchodolski, 2021). Dysbiosis is associated with various diseases in humans, such as IBD, antibiotic-related diarrhea, colorectal cancer, allergies, type 2 diabetes, and atopic dermatitis; however, live probiotics reportedly exhibit preventive and therapeutic activities against these pathologies (Yang and Wu, 2023). The benefits of probiotics in health promotion and disease prevention have also been demonstrated in dogs and cats (Pilla and Suchodolski, 2021). In addition to probiotics, there are other types of compounds called prebiotics and postbiotics. Prebiotics are indigestible food compounds that selectively alter the growth and activity of specific bacteria in the colon and improve host health (Tashiro et al., 2006). Postbiotics are dead bacteria, their components, products, and compounds not directly involved in intestinal bacteria (Tashiro et al., 2006). Probiotics commonly used in functional foods include species and strains belonging to the genera Lactobacillus, Bifidobacterium, Enterococcus, and Saccharomyces (Gu et al., 2022). Furthermore, complex microbial groups of bacteria, fungi, archaea, protozoa, and viruses constitute the microbiota of the digestive tract (Berg et al., 2020). Twelve dead species of bifidobacteria and lactobacilli (Bifidobacterium animalis subsp. lactis bifidum, Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus paracasei, Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus helveticus, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus rhamnosus, and Streptococcus thermophilus) were shown to be effective as probiotics for dogs (Rossi et al., 2020a). Notably, studies demonstrated that in the intestinal tracts of cats and dogs, these bacterial species increased the counts of bifidobacteria and lactobacilli, decreased the counts of anaerobic bacteria, and elevated intestinal mucosal immunoglobulin (Ig)A and blood IgG levels (Rossi et al., 2020a). Natural killer cells in peripheral blood as well as peripheral blood mononuclear cells were also shown to have cytotoxic functions against canine tumor cells, and a high concentration of Bacillus subtilis variant natto elevated the expression of tumor necrosis factor α (Mikawa et al., 2021). Notably, these effects are known to vary between cats and dogs (Wallis et al., 2017), as well as among various breeds (Simpson et al., 2002) and with changes in diet (Lee et al., 2022). The effects of probiotics also depend on the genus and species and specific strains of bacteria (Karaseva et al., 2023). Thus, the varieties and combinations of probiotic strains are important determinants of their effects (Hmar et al., 2024). In addition, one study demonstrated that when dogs were administered a single bacterium or a mixture of three bacteria, levels of SCFAs increased only in those dogs administered the mixture. Similarly, counts of “good” bacteria increased and counts of “bad” bacteria decreased in the group of dogs administered the mixture of bacteria (Zubillaga et al., 2024). Another report noted that kittens can regulate changes in intestinal metabolites and alleviate intestinal inflammation (Zhu et al., 2025). Since approximately 1,000 different types of intestinal bacteria have been identified (Atuahene et al., 2024), the administration of as many types of bacteria as possible may be beneficial, even for probiotics. In the case of prebiotics, evidence suggests that only materials that are effective for cats and dogs should be selected. Notably, fructooligosaccharide kestose has been reported to be effective in many cases and has been shown to increase “good” bacteria and decrease “bad” bacteria in dogs (Ide et al., 2020). Other studies have also demonstrated that the combination of L. paracasei and kestose was effective against canine atopic dermatitis (Kawano et al., 2023). Psyllium was also shown to be effective in healthy and constipated cats (Keller et al., 2024) and in dogs with acute colitis (Rudinsky et al., 2022). Beta-glucan in prebiotics enhances the bioavailability of probiotics, whereas fructo-oligosaccharides and galacto-oligosaccharides encapsulate and protect probiotics, thereby increasing their effectiveness (Chen et al., 2024). Symbiotics, as a combination of prebiotics and probiotics, are reportedly more effective than antibacterial drugs for treating chronic diarrhea in dogs (Jensen and Bjørnvad, 2019). Furthermore, the combination of prebiotic and postbiotic was more effective than the administration of either prebiotic or postbiotic alone in significantly promoting the restoration of healthy gut microbiota (Yang et al., 2022). Postbiotic and probiotic supplements may help improve abnormal intestinal flora in dogs (Belà et al., 2024). In addition, studies have reported that the combination of prebiotics and postbiotics alleviates immunosenescence in healthy, vaccinated older dogs (Wambacq et al., 2024). Based on this evidence, we sought to identify combinations of probiotics, prebiotics, and postbiotics that demonstrate efficacy in cats and dogs. The following were selected to develop a combination of 26 species for the purpose of our study: 1) prebiotics—kestose (Ide et al., 2020; Kawano et al., 2023) and psyllium (Rudinsky et al., 2022; Keller et al., 2024); 2) probiotics—high-concentration, spore-forming B. subtilis variant natto (Mikawa et al., 2021); and 3) postbiotics—12 species of bacteria that exist in the intestinal flora of cats and dogs and have been reported to improve intestinal balance, produce SCFAs, and stimulate immunity (Rossi et al., 2020a,b), two species of bacteria that are effective against canine atopic dermatitis (Ural et al., 2021), Ligilactobacillus johnsonii and Ligilactobacillus salivarius, as known canine intestinal bacteria (Kang et al., 2024), Enterococcus faecalis with antiviral activity in cats (Ukita et al., 2023), and an additional seven species of lactic acid bacteria. This study aimed to evaluate both the safety and functional benefits of this supplement in cats and dogs, focusing on its potential to support digestive health and overall well-being. Specifically, we assessed the impacts on the composition of the gut microbiota, nutrient absorption, and potential adverse effects to determine the suitability for long-term use in cats and dogs. Materials and MethodsA mixture of 23 postbiotic lactic acid bacteria and bifidobacteria (dead bacteria; CHORI Co., Osaka, Japan, and Pharma Foods International Co., Kyoto, Japan), the probiotic B. subtilis var. natto (viable bacteria; G.K. Hokulea, Tokyo, Japan), and two prebiotics (kestose; B Food Science Co., Aichi, Japan, and psyllium; Bizen Chemical Co., Okayama, Japan) were used. The product was formulated as a powder with the following composition: 31.14% dextrin, 30% reduced maltose syrup, 10% oligosaccharides, 17% lactic acid bacteria and bifidobacteria, 8.34% B. subtilis var. natto, 0.34% kestose, 0.34% psyllium, and 2.84% other ingredients, including seasoning. This product was prepared as a powder (1.5 g/package). The dosage was set at two packets per dog and three packets per cat. This adjustment was made to accommodate dogs weighing less than 3 kg. Study subjects included 3 healthy adult cats raised at the Narita Laboratory of the NAS Research Institute (Chiba, Japan) and 20 healthy adult dogs raised at Koinu no Mori Hesoten (Ishikawa, Japan). All cats were Domestic Short Hair (DSH), and all were males with an average weight of 3.64 kg (range, 3.05–4.26 kg) and an average age of 5.33 years (range, 4.91–6.1 years). The 7 male and 13 female dogs included the following breeds: Miniature Schnauzer (n=5), Toy Poodle (n=5), Shih Tzu (n=2), Pomeranian (n=2), Maltese (n=2), Chihuahua (n=1), Papillon (n=1), Miniature Dachshund (n=1), and Yorkshire Terrier (n=1). The average age was 2.63 years (range, 2–7 years; age unknown for 1 dog), and the average weight was 4.68 kg (range, 2.5–7.4 kg) at the start of administration of this compound (Table 1). For the study of cats, we selected a one-way design with a 7-day control period followed by a 7-day test period, as Zubillaga et al. (2024) demonstrated that a change in the intestinal flora in dogs could be observed 7 days after starting probiotic administration (Zubillaga et al., 2024). We did not use a washout period because we did not use any control solvent. The 20 dogs were randomly assigned to either a test group (n=10) or a control group (n=10), and a parallel control study was conducted for 27 days. The compound was administered orally, with both animal groups fed a softened solid feed (for cats, CS-A; Oriental Yeast Co., Tokyo, Japan, and for dogs, Mini-Adult; Royal Canin Japon, Tokyo, Japan) with the test substance mixed in, and animals had free access to the food to ensure that they ate all the food provided. We observed the general condition of the animals and monitored their feed intake and body weight. We also collected feces to analyze the intestinal flora. Feces were collected three times before the control period and before and after the test period in cats and before and after administration in both the test and control groups for dogs. Fecal examinations were performed for the following: SCFAs (lactic acid, acetic acid, butyric acid, propionic acid, and valeric acid; TechnoSuruga Laboratory Co., Shizuoka, Japan), intestinal flora analysis (Anicom Specialty Medical Institute Inc., Tokyo, Japan), and putrefactive products in feces (phenols [p-cresol, phenol, 4-ethyl phenol], indole, and skatole (TechnoSuruga Laboratory Co.). In addition, safety was evaluated in cats using complete blood count (Vetscan HM5 multipurpose automated hematology analyzer; Zoetis Japan, Tokyo, Japan) and blood biochemistry (Fuji DRI-CHEM 3000V clinical chemistry analyzer; FUJIFILM Medical Co., Tokyo, Japan). The following blood counts were measured: white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC), platelet count (PLT), neutrophil count (NEUT#), lymphocyte count (LYMPH#), monocyte count (MONO#), eosinophil count, and basophil count (BASO#). Similarly, the following were measured in blood biochemistry tests: blood glucose (GLU), total cholesterol, triglycerides (TG), total bilirubin, blood urea nitrogen (BUN), creatinine (CRE), glutamate oxaloacetate transaminase (GOT/AST), glutamate pyruvate transaminase (GPT/ALT), alkaline phosphatase (ALP), γ-glutamyltranspeptidase (GGT), sodium (Na), potassium (K), chlorine (Cl), calcium (Ca), and inorganic phosphorus (IP). In addition, a cat lymphocyte subset panel test (Animal Allergy Clinical Laboratories, Kanagawa, Japan) was performed to measure the counts of T, B, CD4-positive, and CD8-positive T cells. Table 1. Cat and dog participant profiles.
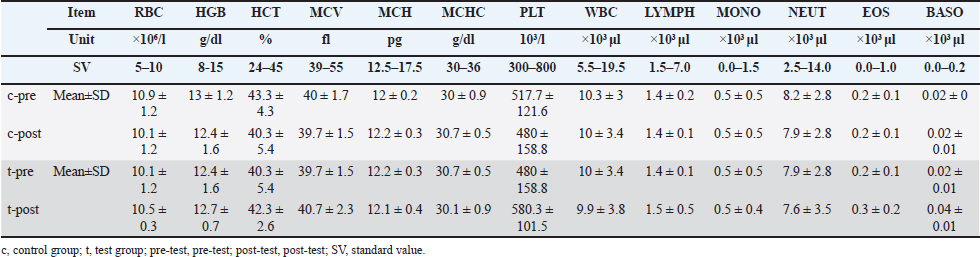
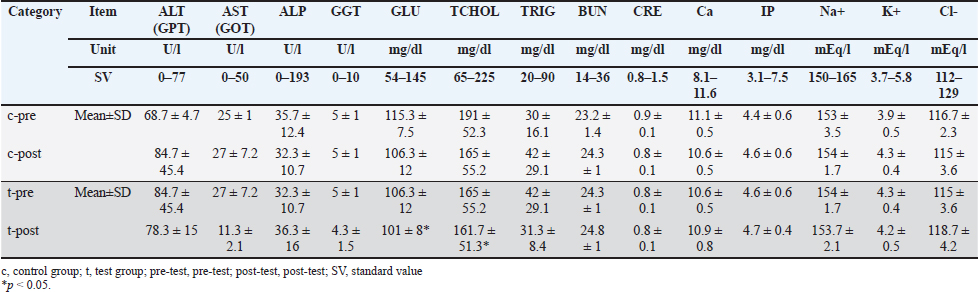
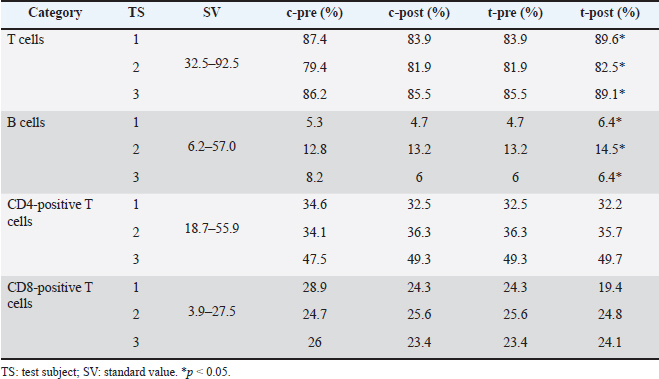
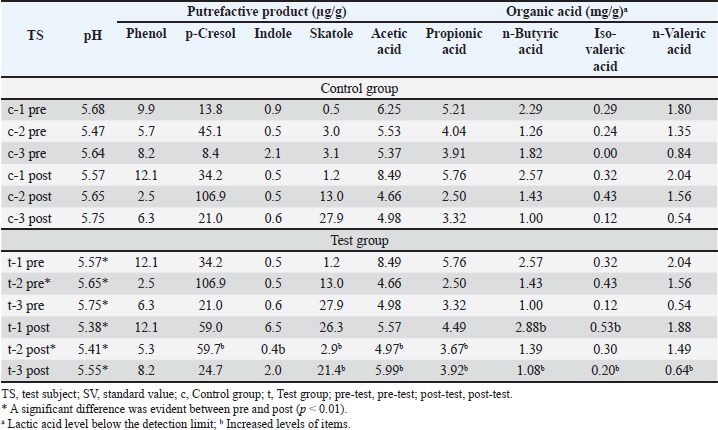
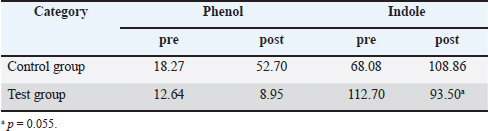
For statistical analyses, data were analyzed using Bell Curve for Excel (Social Survey Research Information Co., Tokyo, Japan), with Tukey’s test for multiple comparisons and paired Student’s t-test for comparisons between two groups. Values of p < 0.05 were considered statistically significant. Ethical approvalFor ethical considerations, cats were from a laboratory animal facility that does not perform euthanasia, and dogs were from a breeding facility. Animal welfare and pain management were conducted in accordance with the “Act on Welfare and Management of Animals” and “Standards for the Care and Keeping of Laboratory Animals and the Alleviation of Pain” with the approval of their owners. In addition, experiments on cats were conducted in accordance with the “Guidelines for the Management and Use of Laboratory Animals in NAS Laboratories” (approval no. 24-F047). ResultsWhile we observed soft stools in some dogs early in the treatment (mean, 5 days; range, 3–7 days), this symptom resolved spontaneously in all cases. No other abnormalities were observed in dogs in terms of general condition, with no significant changes in body weight. Fecal changes were transient and no soft stools were observed by the end of the study, although soft stools were transiently observed during the course of the study in some animals. No safety issues were observed in cats, with no abnormalities in complete blood count or blood biochemistry (Tables 2 and 3). Furthermore, blood biochemistry showed that GLU was within the standard range, and a nonsignificant decrease in TCHOL was observed from above the standard range to within the standard range (p < 0.05). In the cat lymphocyte subset tests, the levels of T and B cells decreased in 2 of the 3 cats during the control period and increased in the test period (p < 0.05). In particular, Cats 1 and 3 had B cell counts below the standard range in the control period and above the standard range in the test period (Table 4). Tables 5 and 6 summarize data from cats and dogs, respectively, for fecal levels of putrefactive products and organic acids. A significant difference in pH was observed between before and after the start of administration in the test group (p < 0.01). Although the difference was not significant in the test group, 60% (9/15) of all organic acid samples tended to be elevated after supplement administration, particularly in all investigations of Cat 3. Overall, significant differences were also obtained in samples from Cat 2 before and after starting supplement administration, as analyzed by the Tukey method (p < 0.01). In dogs, only fecal levels of putrefactive products were measured (Table 4), with the results showing a decrease in indole only (p=0.055). Table 2. Results of feline complete blood count tests.
Table 3. Results of blood biochemistry tests for cats.
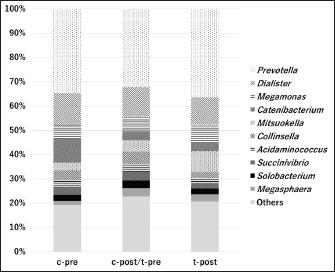
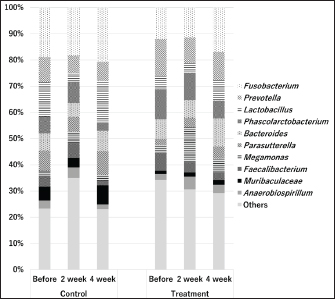
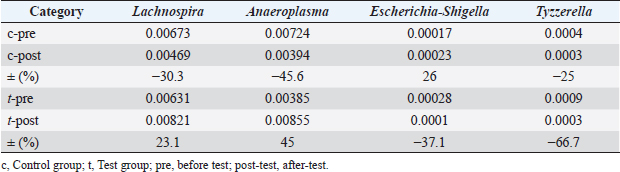
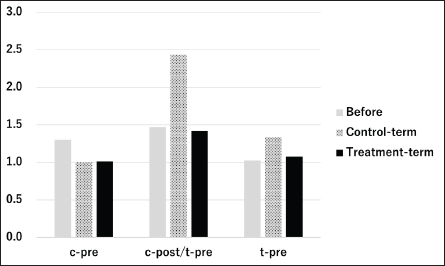
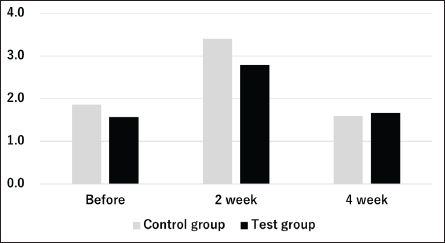
Figures 1 and 2 present the results of an intestinal flora analysis, highlighting the top 10 bacterial species identified through gene sequencing of fecal samples at the genus level for cats and dogs, respectively. No significant differences or trends were observed between cats and dogs. However, in dogs, four bacterial species (Lachnospira, Anaeroplasma, Escherichia-Shigella, and Tyzzerella) showed changes after treatment in the test group, but not in the control group, with a mean change of 34.6% (range: 23.1–45.0%) (Table 7). The results showed that while Lachnospira spp. and Anaeroplasma spp. showed a decreasing trend in the control group, the test group showed a 23.1% increase in Lachnospira spp. and a 45% increase in Anaeroplasma spp. Similarly, an increase or moderate change was observed in Escherichia-Shigella and Tyzzerella spp. in the control group, whereas Escherichia-Shigella spp. decreased by 35.7% and Tyzzerella spp. decreased by 66.7% in the test group. Firmicutes/Bacteroidetes (F/B) ratios are shown in Figures 3 and 4 for cats and dogs, respectively. In all cats, the F/B ratio increased during the control period and decreased during the test period. In dogs, trends toward increases were observed in both groups after 2 weeks, but these changes were not significant. In cats, mean alpha diversity was 5.526 before intervention and 5.703 after intervention in the control period and 5.703 and 5.539 in the test period. In dogs, the alpha diversity was 5.573 and 5.579 before and after treatment in the control group and 5.823 and 5.648 before and after treatment in the test group, respectively. The values varied even before treatment, and no significant changes were evident after treatment. DiscussionWe developed a test compound comprising 26 prebiotics, probiotics, and postbiotics, selected based on evidence from the current literature on cats and dogs. The present study comprehensively evaluated the test compound and demonstrated its potential effectiveness in regulating intestinal flora in cats and dogs. Efficacy of the test compoundStudies have shown that the use of probiotics in cats and dogs can improve the balance of intestinal flora, alleviate inflammation, improve immune function, and protect against infections caused by intestinal pathogens (Yang and Wu, 2023). The probiotics most commonly studied in cats and dogs are lactobacilli, bifidobacteria, and enterococci (Yang and Wu, 2023). In particular, probiotics combining several lactobacilli and bifidobacteria have been shown to exert immunostimulatory effects in healthy dogs by decreasing Clostridium perfringens levels in feces, increasing bifidobacteria and lactobacilli species, increasing IgA levels in feces and IgG levels in feces, increasing bifidobacteria and lactobacilli species, and increasing IgA in feces and IgG in plasma (Rossi et al., 2020a). As such, several studies have reported the effects of probiotics against IBD (Wernimont et al., 2020), colonic motility disorders (Rossi et al., 2020b), and acute hemorrhagic diarrhea (Ziese et al., 2018). However, the use of live bacteria as probiotics is a challenge; although some studies have confirmed the viability of candidate probiotic strains in canine stomach acid and the intestines, bacteria often die without engrafting after administration (Nakamura et al., 2015). Interest is therefore increasing in the use of prebiotics and postbiotics that do not die and remain effective even without engraftment, as well as probiotics made from spores resistant to gastric acid. Table 4. Results of feline lymphocyte subset analysis.
Table 5. Fecal levels of putrefactive products and organic acids in cats.
Table 6. Fecal levels of putrefactive products in dogs (μg).
Fig. 1. Results of intestinal flora analysis in cats at the genus level: results for the top 10 bacterial species in the control and test groups (averages). c, control group; t, test group; pre, before test; post-test, after-test. Bacillus subtilis variant natto is effective in dogs when used as a spore-forming probiotic (Mikawa et al., 2021). Other spore-forming bacteria, specifically Saccharomyces cerevisiae, have also been reported to reduce fecal pH and ammonia levels in humans (Bastos et al., 2023). In addition, B. subtilis and Bacillus licheniformis, which belong to the same Bacillus genus as B. subtilis variant natto, were shown to improve fecal scores and reduce fecal odor and nitrogen fermentation products in canine feces (Bastos et al., 2020). Thus, the efficacy of B. subtilis variant natto is promising. Postbiotics that have been shown to be effective can be classified into cellular components, such as cell wall fragments of bacterial lysates, filamentous hairs (SpaCBA pili), S-layer proteins, nuclei (CpG-rich DNA), cell surface mucosa (EPS/CPS/LPS), and β-galactosidase, and cell-free components, such as bacterial extracellular polysaccharides and low-molecular-weight metabolites (γKetoC ete) (Da et al., 2024). The postbiotics used in the present study were 12 dead bacteria species that have various effects, including improvement of intestinal balance and SCFA production and immunostimulatory effects (Rossi et al., 2020a,b). Furthermore, E. faecalis is effective against viral diseases in cats (Ukta et al., 2023). Collectively, these effects of postbiotics may have translated to the observed outcomes in our study. Studies have demonstrated that the combination of prebiotics and probiotics is effective in dogs (Fukushima et al., 2018) and that the combination of prebiotics or postbiotics is more effective than the administration of either prebiotics or postbiotics alone in restoring healthy gastrointestinal microbiota (Yang et al., 2022). In dogs, a combination of postbiotics and prebiotics has been shown to regulate the intestinal flora of dogs (Belà et al., 2024). The compounds used in the present study may be effective as a combination of prebiotics, probiotics, and postbiotics.
Fig. 2. Results of intestinal flora analysis in dogs at the genus level: results for the top 10 bacterial species in the control and test groups (averages). Control: control group. Treatment: test group. SafetyInterest in the quality and safety of foods given to pets has been growing in recent years, including dietary supplements (Nestle and Nesheim, 2010). In this study, we did not observe any changes in the general conditions of animals during the study period, and no abnormal test results were seen from complete blood counts or blood biochemistry tests in cats. Table 7. Results after testing for fungi in dogs.
Fig. 3. Average F/B ratio in cats (n=3). c, control group; t, test group; pre, before test; post-test, after-test.
Fig. 4. Average F/B ratio in dogs (n=10). ImmunityIn relation to gut microbiota and immunity, a study showed that inflammatory cytokines [interleukin (IL)-1, IL-8, IL-12] were elevated in cats with IBD due to increased levels of Enterobacteriaceae (Janeczko et al., 2008). In dogs, levels of anti-inflammatory cytokines and regulatory T cells are decreased in IBD, resulting in inflammation (Maeda et al., 2016). Probiotics have been reported to regulate immune function. Specifically, probiotics were shown to increase plasma IgG in dogs by stimulating fecal IgA and antibody-producing B cells (Xu et al., 2019; Rossi et al., 2020a,b), stimulating T-cell differentiation, regulating inflammatory and anti-inflammatory cytokine profiles, and inducing production of IgA secretory component (Yang and Wu, 2023). Although cytokine concentrations were not measured in the present study, the lymphocyte subset test in cats showed significant differences in T and B cells, with a significant decrease during the control period and a significant increase during the test intervention in 2 of the 3 cats (p < 0.05). In particular, Cats 1 and 3 had lower B cell counts than the reference value during the control period and higher B cell counts than the reference value during the test period. Thus, the compound used in this study may regulate immune function. Intestinal pHLowering intestinal pH inhibits the metabolism of harmful bacteria (Booth, 1985). A significant decrease in pH was observed in the test group (p < 0.01). In cats, a reduction in pH has been reported to result in increased levels of “good” lactic acid bacteria and decreased levels of “bad” bacteria such as E. faecalis and C. difficile (Bastos et al., 2020). Thus, decreases in pH may have been beneficial for improving intestinal flora diversity. Putrefactive productsAdministration of S. cerevisiae and Weissella cibaria JW15 reduces ammonia levels (Sun et al., 2019; Bastos et al., 2023), and administration of B. subtilis and B. licheniformis reduces nitrogen fermentation products in feces and consequently fecal odor (Bastos et al., 2020). These findings suggest that the administration of probiotics to dogs can improve fecal quality and suppress nitrogen fermentation products. In cats, we demonstrated that administration of the test compound significantly decreased putrefactive products in Cat 2 but had no significant effect overall. However, in dogs, a decreasing trend in mean indole concentration was observed in the test group (p=0.055). Therefore, the improvement of the intestinal flora may also inhibit putrefactive products. SCFAsIn dogs and cats, butyrate is a particularly important SCFA produced from dietary fiber and protein to promote healthy gut microbiota (Zubillaga et al., 2024). Dysbiosis in canine IBD is characterized by a decrease in the production of SCFAs, in addition to a decrease in the phylum Firmicutes and an increase in the phylum Pseudomonadota (proteobacteria) (Hernandez et al., 2022). SCFA-producing bacteria belonging to the genera Lactobacillus and Bifidobacterium elevate levels of SCFAs, such as butyric acid, by cross-feeding with other commensal bacteria (Liu et al., 2018; Zhang et al., 2021). The present study did not detect any significant differences in SCFA levels among cats. However, 60% (9/15) of all organic acids tested were elevated; notably, Cat 3 showed increases in acetic acid, propionic acid, n-butyric acid, and iso/n-valeric acid after the start of administration. Overall, a significant difference was observed between before and after administration of Cat 2 (p < 0.01). In the analysis of the canine intestinal flora, we observed an increasing trend in Firmicutes, which decreases under dysbiotic conditions, and a decreasing trend in Pseudomonadota, which increases during dysbiosis. These bacteria may be involved in the increase in SCFA levels. Changes in the intestinal floraThe composition of the gastrointestinal flora is generally similar between dogs and cats (Hoffmann et al., 2016; Hashimoto-Hill and Alenghat, 2021). The fecal microbiota of healthy dogs is dominated by the phyla Firmicutes, Bacteroidetes, and Fusobacteria, whereas the fecal microbiota of cats is dominated by Firmicutes, Pseudomonadota, Actinomycetota, Bacteroidetes, and Fusobacteria in decreasing significance (Pilla and Suchodolski, 2021; Lee et al., 2022). The diversity of the gut microbiota, including these bacteria, is important, and this diversity is inevitably reduced in dysbiosis. Diseases with reduced microbiota diversity include acute diarrhea in dogs (Guard et al., 2015), obesity in dogs (Kim et al., 2023), and diabetes in cats (Kieler et al., 2019). The gut microbiota is considered an ecosystem, and alpha diversity provides a measure of species diversity per sample (Inoue, 2019). We found no changes in alpha diversity in cats and dogs. This could be attributed to the fact that the animals used in this study were healthy, young animals, with a baseline average alpha diversity of 5.5 or more. In addition, the dilution curve used to evaluate alpha diversity may not be useful when the level of alpha diversity is maintained at a high plateau level (Inoue, 2019). Therefore, the interpretation of the findings of this study is difficult. In addition, no significant differences or trends were observed in the intestinal flora of cats, likely because the study period was relatively short. In the case of dogs, although no differences were significant, trends in some bacterial species were observed. Specifically, an increase in the test group and a decrease in the control group were observed for Lachnospira sp. in the butyrate- and acetate-producing phylum Firmicutes, as well as Anaeroplasma sp. in the phylum Pseudomonadota, which alleviates chronic inflammation (Beller et al., 2019). In addition, we observed an increasing trend in the control group and a decreasing trend in the study group for Escherichia-Shigella, a nondominant and nonbeneficial E. coli, and Tyzzerella (formerly C. piliforme) (Takewaki et al., 2024), a genus in the Bacillota phylum associated with multiple sclerosis. Chronic enteritis in dogs is associated with a decrease in Faecalibacterium prausnitzii, a beneficial species in the Firmicutes phylum, as well as overgrowth of the nonbeneficial C. perfringens (Gasaly et al., 2021). Similarly, canine IBD is associated with a decrease in the Firmicutes phylum and an increase in the Pseudomonadota phylum (Schmitz and Suchodolski, 2016; Hernandez et al., 2022), and feline IBD is associated with a decrease in beneficial Bifidobacterium spp. and an increase in nonbeneficial E. coli (Ziese and Suchodolski, 2021). Our findings showed opposing trends to these pathological states, suggesting the potential effectiveness of the compound. F/B ratioAlthough no significant changes were observed in the F/B ratio in dogs, we observed that the F/B ratio increased during the control period and decreased during the test period in all cats. The intestinal flora of cats is dominated by Firmicutes, followed by Bacteroidetes, Proteobacteria, and Actinobacteria, but cats infected with feline coronavirus have been shown to have more Firmicutes and fewer Bacteroidetes (Shi et al., 2024). In other words, the F/B ratio was higher in cats with infectious diseases. The observed decrease in the F/B ratio in our study indicates a positive effect on cats with infections. The other promising effects of the compound include improved metabolism and suppression of obesity. A recent study demonstrated that beagles fed a diet containing postbiotics displayed improved intestinal flora and reduced serum levels of TG and cholesterol (Xuan et al., 2024). The blood biochemistry tests performed in our study revealed that the GLU level was within the standard range and that the TCHOL level decreased from above the standard range (p < 0.05). Therefore, additional experiments should be conducted to identify the effects on metabolism and obesity. ConclusionIn summary, we performed a comprehensive evaluation of the test compound comprising 26 prebiotics, probiotics, and postbiotics selected based on evidence from the literature on cats and dogs and demonstrated that the test compound regulated the intestinal flora in these animals. This finding was based on data obtained for selective immune markers in blood, pH in feces, putrefactive products, SCFAs, the F/B ratio, and changes in the microbiota from gut microflora analysis. However, our study was limited by the relatively small sample size and the short duration of the feline study. Additional studies are needed to evaluate the safety and functionality of this compound over a long period of time in cats. In dogs, additional studies are necessary to further evaluate safety and functionality using various indicators. AcknowledgmentsWe would like to express our sincere gratitude to the dogs and staff of “Koinu no Mori Heso Ten (Ishikawa, Japan)” for their cooperation in the study. The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing. Conflict of interestThe authors declare no conflict of interest. FundingHundred Inc. (Fukushima, Japan) partially contributed to the financial costs of the present study. Author contributionsOnuma Mamoru was the primary researcher, the implementer of the study, and the writer of the manuscript. Dr. Ataka supervised the research and assisted with writing the manuscript, and Dr. Murakami played a role in helping to direct the study and revise some aspects of the manuscript. Data availabilityWith the exception of some data from intestinal flora analyses, data supporting the results of this research are available in the manuscript. ReferencesAtuahene, D., Mukarram, S.A., Balouei, F. and Antwiet, A. 2024. Gut health optimization in canines and felines: exploring the role of probiotics and nutraceuticals. Pets 1(2), 135–151; doi:10.3390/pets1020011 Bastos, T.S., de Lima, D.C., Souza, C.M.M., Maiorka, A., de Oliveira, S.G., Bittencourt, L.C. and Félix, A.P. 2020. Bacillus subtilis and Bacillus licheniformis reduce faecal protein catabolites concentration and odour in dogs. BMC. Vet. Res. 16, 116. Bastos, T.S., Souza, C.M.M., Legendre, H., Richard, N., Pilla, R., Suchodolski, J.S., de Oliveira, S.G., Lesaux, A.A. and Felix, A.P. 2023. Effect of yeast Saccharomyces cerevisiae as a probiotic on diet digestibility, fermentative metabolites, and composition and functional potential of the fecal microbiota of dogs submitted to an abrupt dietary change. Microorganisms 11, 506. Belà, B., Coman, M.M., Verdenelli, M.C., Gramenzi, A., Pignataro, G., Fiorini, D. and Silvi, S. 2024. In vitro assessment of postbiotic and probiotic commercial dietary supplements recommended for counteracting intestinal dysbiosis in dogs. Vet. Sci. 11(1), 19; doi:10.3390/vetsci11010019 Beller, A., Kruglov, A., Durek, P., von Goetze, V., Hoffmann, U., Maier, R., Heiking, K., Siegmund, B., Heinz, G.A., Mashreghi, M.F., Radbruch, A. and Chang, H.D. 2019. P104 Anaeroplasma, a potential anti-inflammatory probiotic for the treatment of chronic intestinal inflammation. ARD 78(1), A1–A83, P104. Berg, G., Rybakova, D., Fischer, D., Cernava, T., Verges, M.C., Charles, T., Chen, X., Cocolin, L., Eversole, K., Corral, G.H., Kazou, M., Kinkel, L., Lange, L., Lima, N., Loy, A., Macklin, J.A., Maguin, E., Mauchline, T., McClure, R., Mitter, B., Ryan, M., Sarand, I., Smidt, H., Schelkle, B., Roume, H., Kiran, G.S., Selvin, J., de Souza, R.S.C., van Overbeek, L., Singh, B.K., Wagner, M., Walsh, A., Sessitsch, A. and Schloter, M. 2020. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103. Booth, I.R. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49(4), 359–378. Chen, C., Su, Y., Li, S., Man, C., Jiang, Y., Qu, B., Yang, X. and Guo, L. 2024. Advances in oligosaccharides and polysaccharides with different structures as wall materials for probiotics delivery: a review. Int. J. Biol. Macromol. 277(4), 134468. Da, M., Sun, J., Ma, C., Li, D., Dong, L., Wang, L.S. and Chen, F. 2024. Postbiotics: enhancing human health with a novel concept. eFood 5(5), e108. Fukushima, K., Ohno, K., Odamaki, T., Takatsu, Z., Maeda, S. and Tsujimoto, H. 2018. Research on the effect of probiotics administration on gastrointestinal tract in healthy dogs. Jpn. J. Pet. Anim. Nutr. 18(41), 43. Gasaly, N., de Vos, P. and Hermoso, M.A. 2021. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 12, 658354. Gu, Q., Yin, Y., Yan, X., Liu, X., Liu, F. and McClements, D.J. 2022. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: a review. Adv. Colloid. Interface. Sci. 309, 102781. Guard, B.C., Barr, J.W., Reddivari, L., Klemashevich, C., Jayaraman, A., Steiner, J.M., Vanamala, J. and Suchodolski, J.S. 2015. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS One 10(5), e0127259; doi:10.1371/journal.pone.0127259 Hashimoto-Hill, S. and Alenghat, T. 2021. Inflammation-associated microbiota composition across domestic animals. Front. Genet. 12, 649599. Hernandez, J., Rhimi, S., Kriaa, A., Mariaule, V., Boudaya, H., Drut, A., Jablaoui, A., Mkaouar, H., Saidi, A., Biourge, V., Borgi, MA., Rhimi, M. and Maguin, E. 2022. Domestic environment and gut microbiota: lessons from pet dogs. Microorganisms 10(5), 949. Hmar, E.B.L., Paul, S. and Sharma, H.K. 2024. An insight into the combination of probiotics and their implications for human health. Endocr. Metab. Immune. Disord. Drug. Targets. 24(1), 1–12. Hoffmann, A.R., Proctor, L.M., Surette, M.G. and Suchodolski, J.S. 2016. The microbiome: the trillions of microorganisms that maintain health and cause disease in humans and companion animals. Vet. Pathol. 53, 10–21. Ide, K., Shinohara, M., Yamaguchi, S., Endo, A., Nishifuji, K. and Tochio, T. 2020. Kestose supplementation exerts bifidogenic effect within fecal microbiota and increases fecal butyrate concentration in dogs. J. Vet. Med. Sci. 82(1), 1–8; doi:10.1292/jvms.19-0071 Inoue, R. 2019. ABCs for analysis of gut microbiota. Jpn. J. Lactic. Acid. Bact. 30(1), 27–31. Janeczko, S., Atwater, D., Bogel, E., Greiter-Wilke, A., Gerold, A., Baumgart, M., Bender, H., McDonough, P., McDonough, S. and Goldstein, R. 2008. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet. Microbiol. 128, 178–193. Jensen, A.P. and Bjørnvad, C.R. 2019. Clinical effect of probiotics in prevention or treatment of gastrointestinal disease in dogs: a systematic review. J. Vet. Intern. Med. 33(5), 1849–1864. Kang, A., Kwak, M.J., Hye Choi, J., Son, S.H., Lim, S., Eor, J.Y., Song, M., Kim, M.K., Kim, J.N., Yang, J., Lee, M., Kang, M., Oh, S. and Kim, Y. 2024. Integrative analysis of probiotic-mediated remodeling in canine gut microbiota and metabolites using a fermenter for an intestinal microbiota model. Food. Sci. Anim. Resour. 44(5), 1080–1095. Karaseva, O., Ozhegov, G., Khusnutdinova, D., Siniagina, M., Anisimova, E., Akhatova, F., Fakhrullin, R. and Yarullina, D. 2023. Whole genome sequencing of the novel probiotic strain Lactiplantibacillus plantarum FCa3L. Microorganisms 11(5), 1234; doi:10.3390/microorganisms11051234 Kawano, K., Iyori, K., Kondo, N., Yamakawa, S., Fujii, T., Funasaka, K., Hirooka, Y. and Tochio, T. 2023. Clinical effects of combined Lactobacillus paracasei and kestose on canine atopic dermatitis. Pol. J. Vet. Sci. 26(1), 131–136. Keller, E., Laxalde, J., Tranier, N., von Kretschmann, P.B., Jackson, A. and van Hoek, I. 2024. Psyllium husk powder increases defecation frequency and faecal score, bulk and moisture in healthy cats. J. Feline Med. Surg. 26(4), 1098612X241234151; doi:10.1177/1098612X241234151 Kieler, I.N., Osto, M., Hugentobler, L., Puetz, L., Gilbert, M.T.P., Hansen, T., Pedersen, O., Reusch, C.E., Zini, E., Lutz, T.A. and Bjørnvad, C.R. 2019. Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci. Rep. 9, 4822. Kim, H., Seo, J., Park, T., Seo, K., Cho, H.W., Chun, J.L. and Kim, K.H. 2023. Obese dogs exhibit different fecal microbiome and specific microbial networks compared with normal weight dogs. Sci. Rep. 13, 723. Lee, D., Goh, T.W., Kang, M.G., Choi, H.J., Yeo, S.Y., Yang, J., Huh, C.S., Kim, Y.Y. and Kim, Y. 2022. Perspectives and advances in probiotics and the gut microbiome in companion animals. J. Anim. Sci. Technol. 64(2), 197–217. Liu, H., Wang, J., He, T., Becker, S., Zhang, G., Li, D. and Ma, X. 2018. Butyrate: a double-edged sword for health? Adv. Nutr. 9, 21–29. Maeda, S., Ohno, K., Fujiwara-Igarashi, A., Uchida, K. and Tsujimoto, H. 2016. Changes in Foxp3-positive regulatory T cell number in the intestine of dogs with idiopathic inflammatory bowel disease and intestinal lymphoma. Vet. Pathol. 53, 102–112. Mikawa, S., Matsuda, A., Kamemori, Y., Asanuma, S. and Kitagawa, H. 2021. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open. Vet. J. 11(3), 394–400. Nakamura, A., Ohnishi, Y., Shirotori, K. and Matsumoto, M. 2015. Evaluation of viability Bifidobacterium animalis subsp. lactis LKM512 in dogs. Benef. Microbes. 6, 791–797. Nestle, M. and Nesheim, M. 2010. Feed your pet right: the authoritative guide to feeding your dog and cat. New York, NY: Simon and Schuster. Pilla, R. and Suchodolski, J.S. 2021. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. N. Am. Small Anim. Pract. 51, 605–621. Rossi, G., Gioacchini, G., Pengo, G., Suchodolski, J.S., Jergens, A.E., Allenspach, K., Gavazza, A., Scarpona, S., Berardi, S., Galosi, L., Bassotti, G. and Cerquetella, M. 2020b. Enterocolic increase of cannabinoid receptor type 1 and type 2 and clinical improvement after probiotic administration in dogs with chronic signs of colonic dysmotility without mucosal inflammatory changes. Neurogastroenterol. Motil. 32, e13717. Rossi, G., Pengo, G., Galosi, L., Sara, B., TambellaAdolfo, A.M., Attili, A.R., Gavazza, A., Cerquetella, M., Jergens, A.E., Guard, B.C., Lidbury, J.A., Stainer, J.M., Crovace, A.M. and Suchodolski, J.S. 2020a. Effects of the probiotic mixture Slab51® (SivoMixx®) as food supplement in healthy dogs: evaluation of fecal microbiota, clinical parameters and immune function. Front. Vet. Sci. 7, 613; doi:10.3389/fvets.2020.00613 Rudinsky, A.J., Parker, V.J., Winston, J., Cooper, E., Mathie, T., Howard, J.P., Bremer, C.A., Yaxley, P., Marsh, A., Laxalde, J., Suchodolski, J. and Perea, S. 2022. Randomized controlled trial demonstrates nutritional management is superior to metronidazole for treatment of acute colitis in dogs. J. Am. Vet. Med. Assoc. 260, S3; doi: 10.2460/javma.22.08.0349 Schmitz, S. and Suchodolski, J. 2016. Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics -what is the evidence? Vet. Med. Sci. 2, 71–94; doi:10.1002/vms3.17 Shi, Y., Peng, G., Gebremariam, A.A., Iqbal, M.M., Daemi, H.B., Khan, M.A., Ullah, R. and Wang, D. 2024. Analytical insights, modulation and compositional dynamics of the feline gut microbiota: a review. Anim. Dis. 4, 36. Simpson, J.M., Martineau, B., Jones, W.E., Ballam, J.M. and Mackie, R.I. 2002. Characterization of fecal bacterial populations in canines: effects of age, breed and dietary fiber. Microb. Ecol. 44, 186–197. Sun, H.Y., Kim, K.P., Bae, C.H., Choi, A.J., Paik, H.D. and Kim, I.H. 2019. Evaluation of Weissella cibaria JW15 probiotic derived from fermented Korean vegetable product supplementation in diet on performance characteristics in adult beagle dog. Animals 9, 581. Takewaki, D., Kiguchi, Y., Masuoka, H., Manu, M.S., Raveney, B.J.E., Narushima, S., Kurokawa, R., Ogata, Y., Kimura, Y., Sato, N., Ozawa, Y., Yagishita, S., Araki, T., Miyake, S., Sato, W., Suda, W. and Yamamura, T. 2024. Tyzzerella nexilis strains enriched in mobile genetic elements are involved in progressive multiple sclerosis. Cell. Rep. 43(10), 114785; doi:10.1016/j.celrep Tashiro, Y., Oku, T. and Nakamura, Y. 2006. Chapter 5: Prebiotics. In Probiotics, prebiotics, and bio-genetics. Ed., Mitsuoka, T. Tokyo, Japan: The Society for Intestinal Bacteria, pp: 115–128. Ukita, S., Nawai, A., Sato, I., Murakami, A., Ohshima, S. and Onuma, M. 2023. Examination of antiviral activity in cats treated with high-density concentrated pasteurized lactic acid bacteria, EC-12®. Jpn. J. Pet Anim. Nutr. 26(1), 1–7. Ural, K., Erdoğan, S., Balikçi, C. and Erdoğan, H. 2021. Development of various methods in innovative gastroentero-dermatology I: does probiotic enema with Lactobacillus plantarum and Lactobacillus paracasei provide anti-pruritic efficacy in dogs with atopic dermatitis? Van. Vet. J. 32(2), 74–81. Wallis, C.V., Wallis, C.V., Marshall-Jones, Z.V., Deusch, O. and Hughes, K.R. 2017. Canine and feline microbiomes. In Understanding host-microbiome interactions - an omics approach. Eds., Singh, R.P., Kothari, R., Koringa, P.G. and Singh, S.P. Berlin, Germany: Springer Nature; pp:279–325. Wambacq, WA., Apper, E., Le Bourgot, C., Barbe, F., Lyu, Y., Pelst, M., Broeckx, B.J.G., Devriendt, B., Cox, E. and Hesta, M. 2024. A new combination of a prebiotic and postbiotic mitigates immunosenescence in vaccinated healthy senior dogs. Front. Vet. Sci. 11, 1392985. Wernimont, S.M., Radosevich, J., Jackson, M.I., Ephraim, E., Badri, D.V., MacLeay, J.M., Jewell, D.E. and Suchodolski, J.S. 2020. The effects of nutrition on the gastrointestinal microbiome of cats and dogs: impact on health and disease. Front. Microbiol. 11, 1266. Xu, H., Huang, W., Hou, Q., Kwok, L.Y., Laga, W., Wang, Y., Ma, H., Sun, Z. and Zhang, H. 2019. Oral administration of compound probiotics improved canine feed intake, weight gain, immunity and intestinal microbiota. Front. Immunol. 10, 666. Xuan, C.A.I., Shan, L.I.U., Ying, W., Ming-ke, F. and Ya-zhen, Y. 2024. Effect of postbiotic supplementation on fecal characteristics, serum biochemical indexes, and fecal flora of dogs. Feed Res. 47(1), 131. Yang, K., Wang, X., Huang, R., Wang, H., Lan, P. and Zhao, Y. 2022. Prebiotics and postbiotics synergistic delivery microcapsules from microfluidics for treating colitis. Adv. Sci (Weinh). 9(16), e2104089. Yang, Q. and Wu, Z. 2023. Gut probiotics and health of dogs and cats: benefits, applications, and underlying mechanisms. Microorganisms. 11(10), 2452; doi:10.3390/microorganisms11102452 Zhang, L., Liu, C., Jiang, Q. and Yin, Y. 2021. Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol. Metab. 32, 159–169. Zhu, S., Zha, M. and Xia, Y. 2025. Complex probiotics suppress inflammation by regulating intestinal metabolites in kittens. Animals 15(2), 272; doi:10.3390/ani15020272 Ziese, A.L. and Suchodolski, J.S. 2021. Impact of changes in gastrointestinal microbiota in canine and feline digestive diseases. Vet. Clin. N. Am. Small Anim. Pract. 51, 155–169. Ziese, A.L., Suchodolski, J.S., Hartmann, K., Busch, K., Anderson, A., Sarwar, F., Sindern, N. and Unterer, S. 2018. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS One 13, e0204691. Zubillaga, M.A.G., Jørgensen, J.N., Jørgensen, L. and Alaverdova, L. 2024. PSIX-7 A multi-strain probiotic modifies fecal microbial populations of healthy adult dogs. J. Anim. Sci. 102(3), 578–579. | ||
| How to Cite this Article |
| Pubmed Style Onuma M, Ataka K, Murakami A. Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Vet. J.. 2025; 15(5): 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 Web Style Onuma M, Ataka K, Murakami A. Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. https://www.openveterinaryjournal.com/?mno=232454 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.11 AMA (American Medical Association) Style Onuma M, Ataka K, Murakami A. Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Vet. J.. 2025; 15(5): 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 Vancouver/ICMJE Style Onuma M, Ataka K, Murakami A. Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 Harvard Style Onuma, M., Ataka, . K. & Murakami, . A. (2025) Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Vet. J., 15 (5), 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 Turabian Style Onuma, Mamoru, Kai Ataka, and Akiyoshi Murakami. 2025. Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Veterinary Journal, 15 (5), 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 Chicago Style Onuma, Mamoru, Kai Ataka, and Akiyoshi Murakami. "Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs." Open Veterinary Journal 15 (2025), 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 MLA (The Modern Language Association) Style Onuma, Mamoru, Kai Ataka, and Akiyoshi Murakami. "Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs." Open Veterinary Journal 15.5 (2025), 1969-1981. Print. doi:10.5455/OVJ.2025.v15.i5.11 APA (American Psychological Association) Style Onuma, M., Ataka, . K. & Murakami, . A. (2025) Evaluating the safety and functionality of a novel compound containing prebiotics, probiotics, and postbiotics in healthy cats and dogs. Open Veterinary Journal, 15 (5), 1969-1981. doi:10.5455/OVJ.2025.v15.i5.11 |