| Research Article | ||
Open Vet. J.. 2025; 15(4): 1663-1672 Open Veterinary Journal, (2025), Vol. 15(4): 1663-1672 Research Article Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studiesReski Amalia1, Takashi Ohama2, Ishwar Singh Parhar3, Claude Mona Airin4, Hiroshi Sato5, Agung Budiyanto6 and Pudji Astuti4*1Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 2Department of Veterinary Pharmacology, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 3Center Initiative for Training International Researchers, University of Toyama, Toyama, Japan 4Department of Physiology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 5Department of Veterinary Parasitology, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 6Department of Reproduction and Obstetrics, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia *Corresponding Author: Pudji Astuti. Department of Physiology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia. Email: pastuti2 [at] ugm.ac.id Submitted: 11/12/2024 Accepted: 15/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
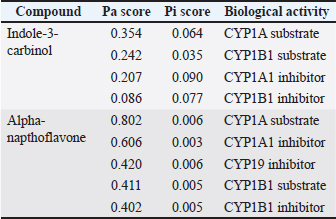
ABSTRACTBackground: Testosterone is a vital androgen hormone responsible for sex differentiation, producing male sex characteristics, spermatogenesis, and fertility. Abnormalities in testosterone levels, leading to reduced weight gain and reproductive capacity, pose a significant problem for livestock farmers. Although synthetic testosterone injection is a general strategy to restore hormone levels, it leads to the downregulation of testosterone production in livestock. Therefore, an alternative method that does not affect the safety of livestock products is needed. Studies have shown that abnormal conversion from testosterone to estradiol (aromatization) causes high levels of estradiol in male animals. In this study, we analyzed the aromatase blocker activity of I3C extracted from the stem part of broccoli, which has beneficial effects on the reproductive system via unknown molecular mechanisms. Aim: This study aimed to explore the potential of I3C as an aromatase blocker using in silico and in vivo approaches. Methods: Biological activity was predicted using PASS Online, and toxicity was assessed using Protox-3. Drug-likeness was determined using Lipinski’s Rule of Five using SWISS ADME. Molecular docking simulations in PyRx 0.8 analyzed binding interactions with CYP19, CYP1A1, and CYP1B1, using LigPlot for amino acid interactions and PyMOL for visualization. Molecular dynamics simulations were used to assess the binding stability over time. Broccoli was freeze-dried, blended, and analyzed for I3C using thin-layer chromatography. Male Sprague-Dawley rats were used to measure CYP19 levels, and blood samples were analyzed using the ELISA method. Results: I3C met the criteria as a drug candidate, binding to CYP19, CYP1A1, and CYP1B1 at the active site with similar poses and good affinity of -5.8, -7.8, and -8.2, respectively. However, the binding affinity was not as strong as that of the control (Alpha-Naphthoflavone), and the formed bonds were unstable. Consistent with the in vivo results, the CYP19 concentrations in the letrozole and broccoli powder groups were not significantly different. Conclusion: I3C can influence CYP19 expression, suggesting its potential as an aromatase-blocking agent. However, further research is required to gain a comprehensive understanding of its effects. Keywords: Aromatase blocker, Broccoli, Estradiol, Indole-3-carbinol, testosterone. IntroductionTestosterone is the primary male hormone that regulates sex differentiation and produces male sex characteristics, spermatogenesis, and fertility. Testosterone is responsible for the development of primary sexual development, which includes testicular descent, spermatogenesis, enlargement of the penis and testes, and increased libido. It is also involved in regulating the secondary male characteristics responsible for masculinity (Nassar and Leslie, 2023). Many factors, including diet, influence the concentration of testosterone in male animals (Kataoka et al., 2021). Studies have shown that the abnormality of the conversion process from testosterone to estradiol, known as aromatization, is one of the causes of high estradiol levels in male animals (Russell et al., 2014). The conversion of testosterone to estradiol can lower the testosterone to estradiol (T:E) ratio to abnormal levels (<10). which decreases semen parameters, and the administration of an aromatase inhibitor normalized the ratio and improved sperm concentration, motility, and morphology (Schulster et al., 2016). A synthetic hormone injection is a general strategy for restoring hormone levels in livestock. However, synthetic testosterone administration can disrupt testosterone biosynthesis by interfering with the expression of steroidogenic acute regulatory protein (StAR) and CYP17A1 in Leydig cells (Pomara et al., 2016), resulting in a decrease in testosterone production known as downregulation (Lin et al., 2020). Alternatively, natural aromatase blockers stabilize testosterone levels in canaries and increase testosterone levels in male chickens (Astuti et al., 2020; Yuneldi et al., 2021). Broccoli (Brassica oleracea var. italica) is a vegetable consumed worldwide. Broccoli stalks are often discarded and end up as household waste, categorized as organic waste from household activities, markets, and restaurant businesses. Many researchers have proven that broccoli contains a chemical compound called I3C (Jeffery and Araya, 2009; Li et al., 2022), which can influence hormonal processes (Katz et al., 2018). I3C negatively regulates estrogen receptor alpha (ERα) signaling. (Auborn et al., 2003). The mechanisms causing hormonal variations after I3C administration are uncertain, especially testosterone (Martín-Ruiz et al., 2018), and the effect of I3C on animals indicates that I3C has a beneficial impact on the reproductive system, but no reports on the effect of I3C on reproduction or development in humans were found. Therefore, it is reasonable to consider the potential of I3C as an alternative ingredient to inhibit the aromatization process. However, a comprehensive profile of the effects of I3C on the male reproductive system is lacking, and its regulatory mechanism remains unclear, which may restrict the utilization of broccoli waste. We designed this study to evaluate the influence of I3C in silico to predict its potential in regulating estradiol, thereby promoting the broader utilization of broccoli by-products for various purposes. Materials and MethodsCollection of compound and protein dataI3C and alpha-naphthoflavone were obtained from the PubChem database in SMILE and 3D SDF formats. The 3D structures of the CYP19, CYP1A1, and CYP1B1 proteins were retrieved from the PDB database. Analysis of the biological activity of the compoundsThe PASS Online web server predicted the biological activity of I3C, focusing on its Probability of Activity (Pa) and Probability of Inactivity (Pi) regarding aromatization. Toxicity was assessed using the Protox-3 web server to classify I3C toxicity. Analysis of compound drug-likenessI3C drug-likeness was evaluated using Lipinski’s Rule of Five through the SWISS ADME web server, examining the molecular weight, hydrogen bond donors/acceptors, LogP, and molar refractivity. Molecular docking simulation analysisBinding interactions between I3C and the target proteins were analyzed using the VinaWizard plugin in PyRx 0.8. Analysis of amino acid residue interactions in compound-protein complexesThe chemical bonding interactions within the compound-protein complexes, derived from docking simulations, were analyzed using LigPlot software. Visualization of compound-protein complex interactionsPymol software was used to visualize the 3D structures and interactions within the compound-protein complexes. Molecular dynamics simulationsThe binding stability between I3C and proteins was further evaluated using molecular dynamics simulations. Materials and animal preparationsThe broccoli was freeze-dried for 72 hours and then blended. I3C content was analyzed using Thin Layer Chromatography. Sixteen male Sprague-Dawley rats (2–3 months old) were divided into four groups: one control (letrozole) and three receiving varying doses of broccoli powder (0.117 g, 0.234 g, and 0.460 g). Blood collectionMicrohematocrit analysis was performed every 7 days for 1 month. Rats were anesthetized with ketamine and xylazine, and blood samples were collected from the eye’s medial canthus. Samples were centrifuged at 3,000 rpm for 15 minutes, and serum samples were stored at -20°C. CYP19 analyzedCYP19 levels were measured using an ABclonal Technology Aromatase ELISA kit (United States). The steps included reagent preparation, sample addition, incubation, and optical density measurement at 450 nm, with correction for optical imperfections. Ethical approvalThe study was approved by the Universitas Gadjah Mada Ethics Committee (certificate number 0103/EC-FKH/Int./2022). ResultsCollection of compound and protein dataThe SMILE notations for the compound of interest (I3C) and the control compound (alpha napthoflavone) were collected from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) with PubChem IDs 3712 and 11790. Alpha-naphthoflavone was chosen as the positive control because it is a synthetic aromatase inhibitor. Positive control means any known ligand with a reported ligand pose and activity. These controls support the process of verifying the docking methodology and validating the dependability of the docking outcomes. Positive control ligands bind to target proteins with high affinity (Elkhalifa et al., 2023). Analysis of the potential biological activities of the compoundsThe probability of activity (Pa) and probability of inactivity (Pi) values were analyzed as described in the Materials and Methods. The results suggest that I3C and alpha-naphthoflavone are substrates of CYP1A and function as inhibitors of CYP1A1. This is indicated by the Pa scores of each compound and control (Table 1). The Pa study showed the Pa values for I3C and alpha-naphthoflavone were close to one, supporting the biological activity of these compounds in in vivo studies (Pramely and Leon, 2012). Analysis of compound toxicity potentialProtox-3 showed that I3C and alpha-naphthoflavone have low toxicity levels based on the LD50 dosage, falling into classes four and five, respectively. This result indicates that both compounds are safe for oral consumption within the safe dosage threshold. Table 1. The predicted biological activity results of the compound of interest and the control compound.
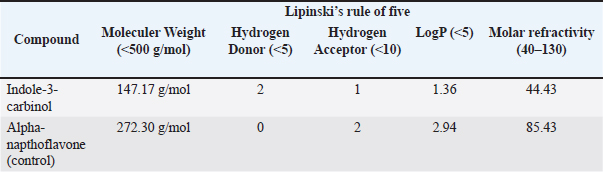
Table 2. Predicted drug-likeness of the compounds of interest and control.
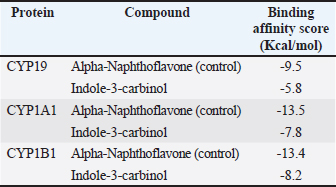
Table 3. The molecular docking simulation results of the compounds of interest and control against the CYP19 protein.
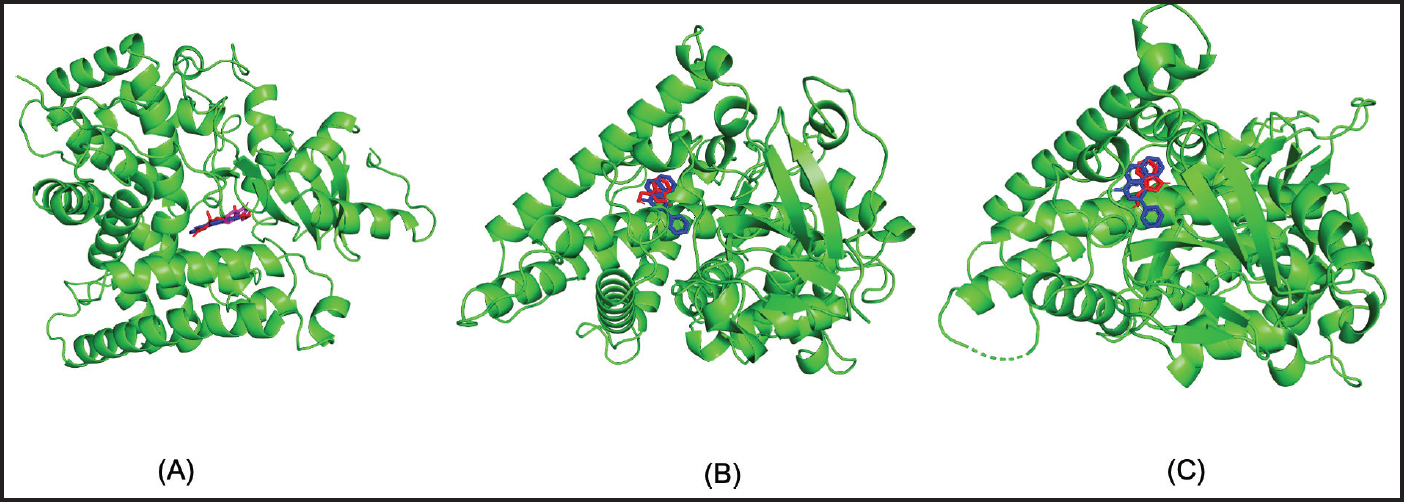
Fig. 1. The results of molecular docking simulation between the CYP19 protein (green) and the compounds Indole-3-Carbinol (Magenta), Alpha-Naphthoflavone (Blue), and Testosterone (Red). CYP19 (A), CYP1A1 (B), CYP1B1 (C).
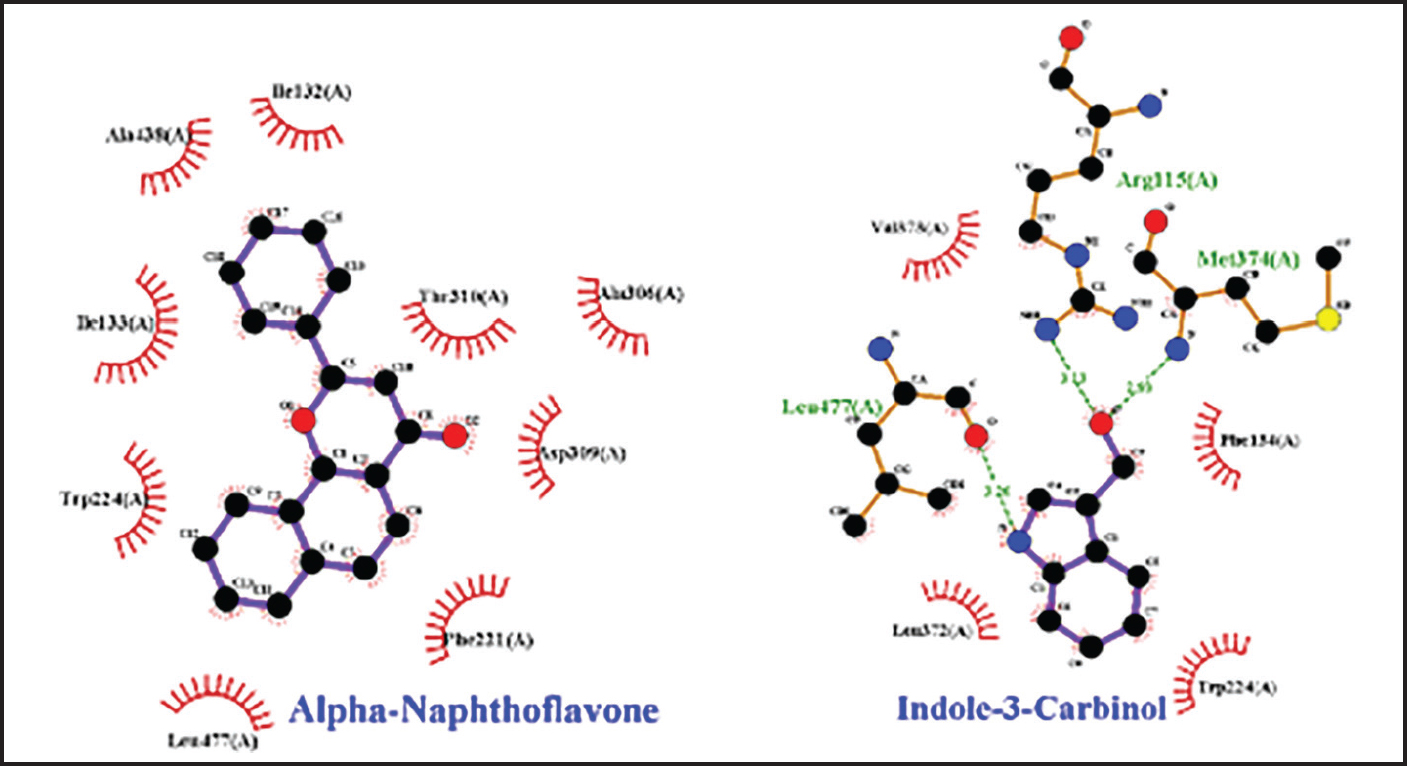
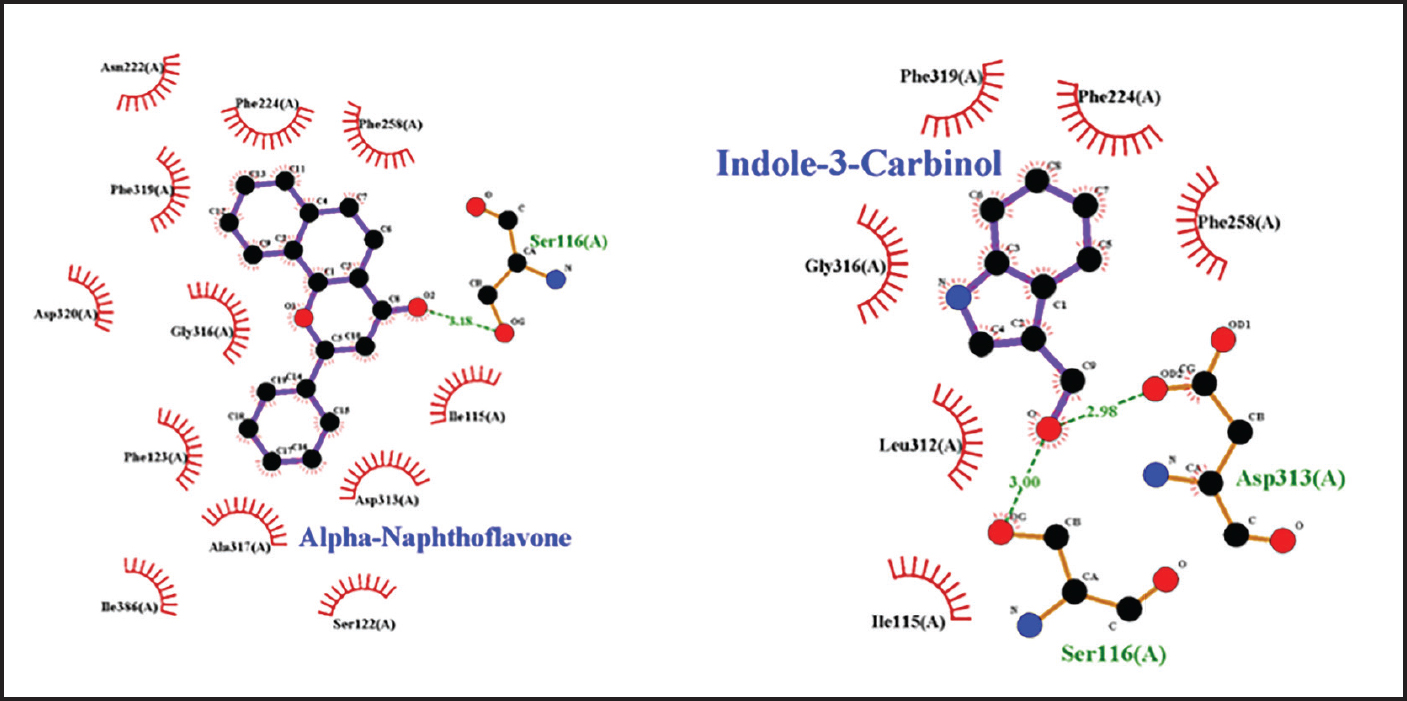
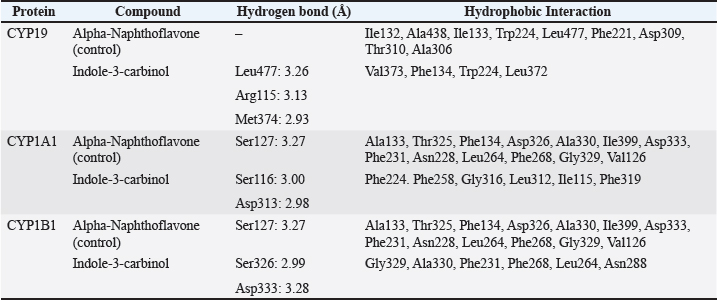
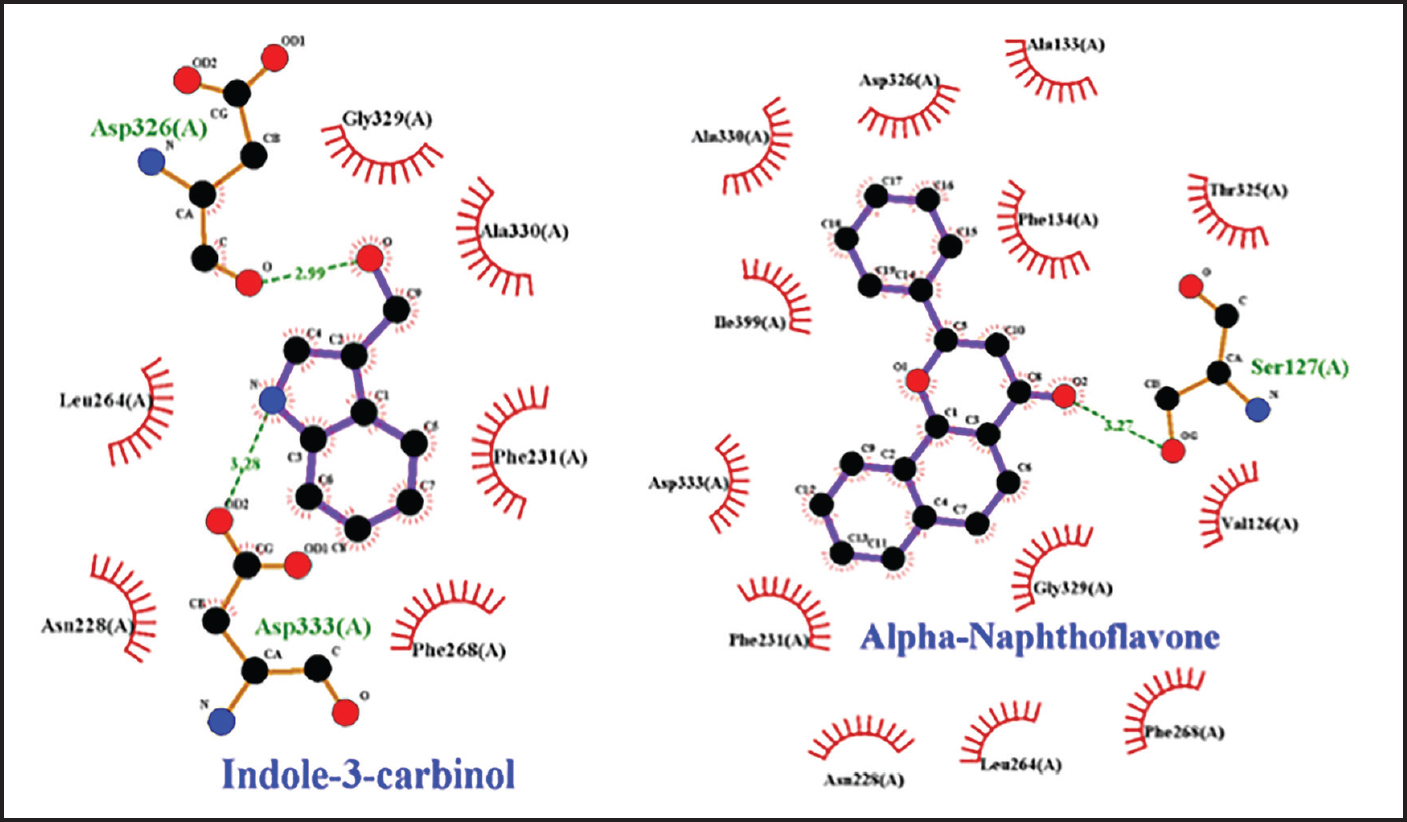
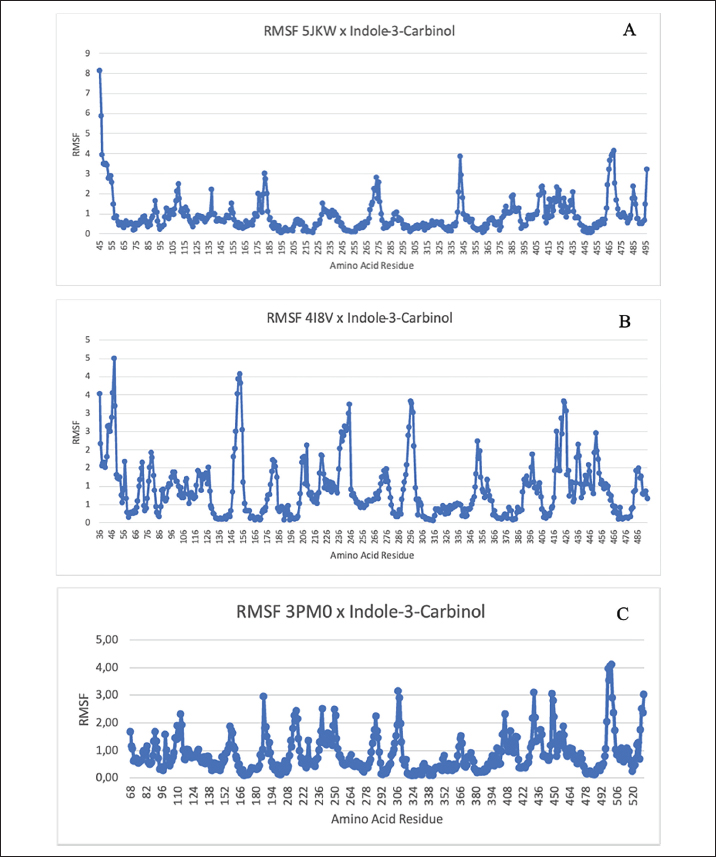
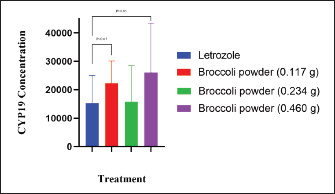
Fig. 2. Amino acid residues in the CYP19 protein complex with Indole-3-carbinol and alpha-napthoflavone. Analysis of compound drug-likenessBased on Lipinski’s Rule of Five, I3C and alpha-naphthoflavone do not violate any of the Lipinski Rule of Five parameters (Table 2). These results indicate that both compounds are easily absorbed and are potential candidates for oral therapy. Molecular dockingThe results of molecular docking were intended to assess the stability of the interaction between the ligand and the target protein. A lower binding affinity indicates a higher level of interaction stability, suggesting that the ligand has a stronger inhibitory effect on the target protein (Table 3). The docking results indicate that I3C, found in broccoli, binds to its target protein at the same site as the control (Fig. 1). I3C binds to the target protein (CYP19) at the same binding site with a similar pose as the control. I3C shares the same residues with the alpha-naphthoflavone, including Ile133, Trp224, Leu372, Phe134, Val373, Ala306, Met374, and Arg115 (Fig. 2). CYP1A1 interacted with the I3C and alpha-naphthoflavone at the grid position. This result suggested that CYP1A1 interacts with I3C at the same site as alpha-naphthoflavone (Fig. 3), possibly with a different pose from that of alpha-naphthoflavone (Table 4). This result can be inferred from the differences in amino acid residue interactions, as shown in Figure 3. Similarly, CYP1B1 interacted with I3C and alpha-naphthoflavone at the grid position. This result indicated that CYP1B1 interacts with I3C at the binding site of alpha-naphthoflavone. The hydrogen bonds and hydrophobic interactions with residues such as Ala330, Phe231, Leu264, Phe268, and Gly329 are shown in Figure 4. Molecular dynamics simulationsA molecular dynamics simulation was conducted to analyze the stability of the protein and its interaction with the ligan. The simulation results showed that the RMSF (Root Mean Square Fluctuation) value is above 3ã…. This indicates that most amino acids move during the simulation. Although the interactions between I3C and CYP19 (Fig. 5A) and between I3C and CYP1B1 (Fig. 5C) are comparable to those between I3C and CYP1A1 (Fig. 5B); overall, the interactions between I3C and CYP19, CYP1A1, and CYP1B1 are less stable. In vivo studyIn vivo studies revealed that I3C treatment affected CYP19 concentrations in male rats, although the inhibitory effect was less pronounced. As shown in Figure 6, treatment with letrozole (a synthetic aromatase blocker) and Broccoli powder did not significantly differ (p > 0.05). DiscussionIn this study, we found that all tested criteria indicate that I3C, found in broccoli, has the potential to be used as an aromatase blocker, although its effectiveness does not yet match that of the control. This was demonstrated by the drug safety criteria, docking results showing negative binding, and RMSF values indicating instability. The use of plants as medicine, known as phytotherapy, is based on the therapeutic benefits of secondary metabolites, which have health-related effects regardless of whether their use has been clinically proven. Broccoli inherently contains numerous bioactive compounds, such as flavonoids, polyphenols, and coumarins, found in the florets, leaves, and stems. Among these parts, the leaves and stems, which are considered byproducts, contain many unique active metabolites that may have potential biological functions for further research and utilization (Zhao et al., 2024).
Fig. 3. Amino acid residues in the CYP1A1 protein complex with Indole-3-carbinol and alpha-napthoflavone. Table 4. Amino acid interactions in the CYP19, CYP1A1, and CYP1B1 complex.
Our findings show that the binding of I3C to CYP19 has promising potential, although it has yet to surpass the control. However, this does not mean that broccoli cannot be used as an aromatase blocker. This is because broccoli is a species with polymorphism, which may alter the affinity of CYP19 for testosterone versus 16-hydroxyandrostenedione (Tworoger et al., 2004). Data indicate that 16-hydroxyandrostenedione has a separate binding site from testosterone and androstenedione, but each hormone inhibits the aromatization of the other (Auvray et al., 2002). The T: E ratio is critical, as circulating estrogens can compete with androgens for binding to sex hormone-binding globulin, and it is generally assumed that the synthesis of sex hormone-binding globulin is regulated and reflects the androgen–estrogen balance (Modugno et al., 2001). Therefore, we suggest that this be further investigated.
Fig. 4. Amino acid residues in the CYP1B1 protein complex with Indole-3-carbinol and alpha-napthoflavone. I3C and its metabolites can influence estrogen receptor (ER) signaling, a process mediated by cytochrome P450 enzymes (CYP) (Parkin and Malejka-Giganti, 2004), by altering estrogen metabolism through CYP1A1 (Chen et al., 1996). I3C can act as a phytoestrogen by inducing competing metabolic pathways that increase the production of 2-hydroxyestrone, thereby reducing the available substrate for the production of 16-hydroxyestrone (Greenwald, 2004). It is important to note that the pharmacological response of I3C in various cellular events may be partly due to its primary metabolite, diindolylmethane (DIM), as both indole derivatives exhibit a high degree of similarity in their modes of action (Weng et al., 2008). In this study, it is observed that CYP1B1 has the best binding affinity. CYP1B1 is a member of the Cytochrome P450 family, which plays a crucial role in the oxidative metabolism of xenobiotics (Shah et al., 2019). CYP1B1 is expressed in various normal tissue cells, including endocrine and reproductive organs (Murray et al., 2001). The CYP1B1 gene encodes an enzyme that catalyzes the addition of hydroxyl groups at positions 2 and 4 of estrone and estradiol (Tworoger et al., 2004). This gene can undergo mutations that result in the substitution of valine/leucine (Val/Leu) at codon 432 (Dupont and Parl, 1998), which is consistent with the findings of this study showing an interaction at Leu264 of the protein. In this study, we found that CYP19, CYP1A1, and CYP1B1 can bind to I3C, but their in silico effectiveness was unstable because the RMSF value was above 3ã… (Kumar et al., 2019). However, changes in the expression of these enzymes can depend on individual genes. This is consistent with previous studies indicating that variations in the aromatase CYP19 gene can alter hormone balance (Kachakova et al., 2016). In silico analysis revealed an unstable interaction between I3C and CYP19. However, our in vivo results (Fig. 6) demonstrated that CYP19 concentrations in rats administered letrozole were not significantly different from those in rats administered broccoli powder. This suggests that I3C in broccoli powder exerts a distinct regulatory effect on reproductive hormones in male rats. Additionally, I3C has been reported to exhibit chemical instability in in vitro assays (Williams, 2021). Previous studies (Amalia et al., 2024) have shown that I3C significantly reduces estradiol levels in vivo. These findings raise critical questions that necessitate further investigation into the mechanisms by which I3C modulates hormone levels, particularly in males. I3C is rapidly absorbed and distributed to various organs (Anderton et al., 2006). Furthermore, its swift oxidation, metabolism, and elimination result in a rapid decline in serum levels. Research (Reed et al., 2006) has indicated that I3C is quickly absorbed, distributed, and eliminated from plasma and tissues, often becoming undetectable within 1 hour after administration. This may elucidate how the unstable interactions between I3C and CYP19 can influence the aromatization process, thereby imparting an aromatase blocker effect. Moreover, naturally occurring compounds typically have multiple active constituents, allowing for diverse biological functions (Suresh et al., 2023). These compounds exhibit a wide array of structures and forms (Barba-Ostria et al., 2022), and their interactions with other chemicals can enhance their functional diversity and efficacy (Kelly and Bowers, 2018). In addition to their independent roles, natural compounds may serve as substrates for the synthesis of more complex molecules (Tharp et al., 2021).
Fig. 5. Root Mean Square Fluctuation (RMSF) results for molecular dynamics simulations of the compound of interest with the CYP19, CYP1A1, and CYP1B1 protein.
Fig. 6. CYPI9 concentrations in male rats in the presence of letrozole and broccoli powder The concentration of CYP19 may increase with higher concentrations of broccoli powder, as noted by Reed et al. (2006), who suggested that saturation could occur in the absorption and excretion processes. When the efflux transporter becomes saturated by the absorbed DIM, the efflux rate stabilizes; however, absorption via diffusion continues to increase with the dosage. This mechanism is attributed to the low bioavailability of DIM (Staub et al., 2006), whereas passive uptake of DIM is significant in cells (Anderton et al., 2004). The recommended dosage ranges from 400 to 600 mg, and the minimum effective dose is 300 mg per day (Wong et al., 1997). The maximum CYP19 level recorded in our study was 0.460 g of broccoli powder, which corresponded to an I3C dosage of 800 mg. Further research is necessary to determine the optimal maximum I3C dose for regulating reproductive hormones in male animals, confirm these findings, and establish the most effective long-term dosing schedule. Furthermore, other compounds that could enhance the effectiveness of broccoli powder should be explored. ConclusionOur study demonstrated that I3C can influence CYP19 concentrations, potentially regulating testosterone levels in male rats. This research shows that I3C has the potential to be a natural aromatase blocker, but it exhibits unstable results. However, this does not mean that I3C in broccoli cannot be used as an aromatase blocker. However, further research is necessary to elucidate the mechanisms by which I3C affects testosterone given its diverse properties depending on the target. This diversity complicates the identification of I3C contributions to in vivo activity. Further studies with more specific research are needed to explain the contribution of I3C in maintaining the stability of the T:E ratio. Moreover, our research is currently in the biomolecular prediction stage. It could yield comprehensive findings if extended to in vitro and in vivo studies incorporating a wider array of treatments. AcknowledgmentThe authors thank Universitas Gadjah Mada and the Ministry of Research and Technology of the Republic of Indonesia. FundingThis research was supported by the Ministry of Research and Technology of the Republic of Indonesia (grant number 2067/UN1/DITLIT/PT.01.03/2024). Author’s contributionsAll authors made significant contributions to this research. RA conducted the study, performed the analyses, and drafted the manuscript. TO, CMA, ISP, HS, AB, and PA assisted with data analysis, critically reviewed the draft, and offered valuable feedback to improve the manuscript. All authors have reviewed and approved the final version of the manuscript. Conflict of interestThe authors have not stated any conflicts of interest. Data availabilityAll data supporting the results of this study are accessible in the manuscript. ReferencesAmalia, R., Airin, C.M., Astuti, P. and Budiyanto, A. 2024. Indole-3-carbinol of broccoli powder can decrease the estrogen production of male rats. J. Anim. Health Prod. 12(4), 647–653. Anderton, M.J., Manson, M.M., Verschoyle, R.D., Gescher, A., Lamb, J.H., Farmer, P.B., Steward, W.P. and Williams, M.L. 2006. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer. Res. 10(15), 5233–5242. Astuti, P., Airin, C.M., Nurrurozi, A., Aidi, R., Hana, A., Hadi, S. and Harimurti, H. 2020. Potential natural aromatase blockers on enhance the frequency and sound quality of male canaries. In Proceedings of the 1st International Conference on Veterinary, Animal, and Environmental Sciences, Bengaluru, pp: 3. Auborn, K.J., Fan, S., Rosen, E.M., Goodwin, L., Chandraskaren, A., Williams, D.E., Chen, D. and Carter, T.H. 2003. Nutritional genomics in cancer processes indole-3-carbinol is a negative regulator of estrogen. Int. J. Nutr. 133, 2470S–2475S. Auvray, P., Nativelle, C., Bureau, R., Dallemagne, P., Séralini, G. E. and Sourdaine, P. 2002. Study of substrate specificity of human aromatase by site directed mutagenesis. Eur. J. Biochem. 269(5), 1393–1405. Barba-Ostria, C., Carrera-Pacheco, S.E., Gonzalez-Pastor, R., Heredia-Moya, J., Mayorga-Ramos, A., Rodríguez-Pãlit, C., Zúãiga-Miranda, J., Arias-Almeida, B. and Guamãn, L.P. 2022. Evaluation of biological activity of natural compounds: current trends and methods. Molecules. 27(14), 4490. Chen, I., Safe, S. and Bjeldanes, L. 1996. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in t47d human breast cancer cells. Biochem. Pharmacol. 51(8), 1069–1076. Dupont, W.D. and Parl, F. 1998. Association of cytochrome P450 1B1 (CYP1B1) polymorphism with steroid receptor status in breast cancer. Cancer Res. 58(22), 41–5038. Elkhalifa, A.E.O., Al-Shammari, E., Kuddus, M., Adnan, M., Sachidanandan, M., Awadelkareem, A.M., Qattan, M.Y., Khan, M.I., Abduljabbar, S.I., Baig, S.M. and Ashraf, S.A. 2023. Structure-based multi-targeted molecular docking and dynamic simulation of soybean-derived isoflavone genistin as a potential breast cancer signaling proteins inhibitor. Life. 13(8), 1739. Greenwald, P. 2004. Clinical trials in cancer prevention: current results and perspectives for the future 1. J. Nutr. 134, 3507S-3512S. Jeffery, E.H. and Araya, M. 2009. Physiological effects of broccoli consumption. Phytochem. Rev. 8(1), 283–298. Kachakova, D., Mitkova, A., Popov, E., Beltcheva, O., Vlahova, A., Dikov, T., Christova, S., Mitev, V., Slavov, C. and Kaneva, R. 2016. Polymorphisms in androgen metabolism genes AR, CYP1B1, CYP19, and SRD5A2 and prostate cancer risk and aggressiveness in Bulgarian patients. Turk. J. Med. Sci. 46(3), 40–626. Kataoka, T., Hotta, Y. and Kimura, K. 2021. A Review of foods and food supplements increasing testosterone levels. J. Men’s Health. 17(2), 4–14. Katz, E., Nisani, S., Chamovitz, D.A., Firestone, G.L. and Zapata, J.M. 2018. Indole-3-carbinol: a plant hormone combatting cancer [version 1; peer review: 2 approved]. F100Research 7689. Kelly, C.A. and Bowers, M.D. 2018. Host plant iridoid glycosides mediate herbivore interactions with natural enemies. Oecologia. 188(2), 491–500. Kumar, N., Sood, D., Tomar, R. and Chandra, R. 2019. Antimicrobial peptide designing and optimization employing large-scale flexibility analysis of protein-peptide fragments. ACS Omega. 4(25), 21370–21380. Li, H., Xia, Y., Liu, H.Y., Guo, H., He, X.Q., Liu, Y., Wu, D.T., Mai, Y.H., Li, H.B., Zou, L. and Gan, R.Y. 2022. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends. Food. Sci. Technol. 119, 288–308. Lin, P.H., Kuo, T.H., Chen, C.C., Jian, C.Y., Chen, C.W., Wang, K.L., Kuo, Y.C., Shen, H. Y., Hsia, S.M., Wang, P.S., Lieu, F.K. and Wang, S.W. 2020. Downregulation of testosterone production through luteinizing hormone receptor regulation in male rats exposed to 17α-ethynylestradiol. Sci. Rep. 10(1), 1576. Martín-Ruiz, A., Peãa, L., Gonzãlez-Gil, A., Díez-Cãrdova, L.T., Cãceres, S. and Illera, J.C. 2018. Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammary cancer. BMC Cancer, 18(1), 626. Modugno, F., Weissfeld, J.L., Trump, D.L., Zmuda, J.M., Shea, P., Cauley, J.A. and Ferrell, R.E. 2001. Allelic variants of aromatase and the androgen and estrogen receptors: toward a multigenic model of prostate cancer risk. Clin. Cancer. Res. 7(10), 3092–3096. Murray, G.I., Melvin, W.T., Greenlee, W.F. and Burke, D. 2001. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu. Rev. Pharmacol. Toxicol. 41, 297–316. Nassar, G.N. and Leslie, S.W. 2023. Physiology, testosterone. Treasure Island, FL: StatPearls [Internet]. Parkin, D.R. and Malejka-Giganti, D. 2004. Differences in the hepatic P450-dependent metabolism of estrogen and tamoxifen in response to treatment of rats with 3,3”′-diindolylmethane and its parent compound indole-3-carbinol. Cancer Detect. Prev. 28(1), 72–79. Pomara, C., Barone, R., Marino Gammazza, A., Sangiorgi, C., Barone, F., Pitruzzella, A., Locorotondo, N., Di Gaudio, F., Salerno, M., Maglietta, F., Sarni, A.L., Di Felice, V., Cappello, F. and Turillazzi, E. 2016. Effects of nandrolone stimulation on testosterone biosynthesis in leydig cells. J. Cell. Physiol. 231(6), 1385–1391. Pramely, R. and Raj, T.L.S. 2012. Prediction of biological activity spectra of a few phytoconstituents of Azadirachta indicia A. Juss. J. Biochem. Tech. 3(4), 357–379. Reed, G.A., Arneson, D.W., Putnam, W.C., Smith, H.J., Gray, J.C., Sullivan, D.K., Mayo, M. S., Crowell, J.A. and Hurwitz, A. 2006. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3”′-diindolylmethane. Cancer Epidemiol. Biomark. Prev. 15(12), 2477–2481. Russell, J.F., Wong, J.C. and Grumbach, M.M. 2014. Aromatase deficiency and aromatase excess. In Genetic Steroid Disorders, Eds., New, M.I., O. Lekaref, A. Parsa., T.T. Yuen., B.W. O’Malley. and G.D. Hammer. San Diego, Academic Press, pp: 165–190. Schulster, M., Bernie, A.M. and Ramasamy, R. 2016. The role of estradiol in male reproductive function. Asian. J. Androl. 18(3), 435–440. Shah, B.R., Xu, W. and Mraz, J. 2019. Cytochrome P450 1B1: role in health and disease and effect of nutrition on its expression. RSC Adv. 9(36), 21050–21062. Staub, R.E., Onisko, B. and Bjeldanes, L.F. 2006. The fate of 3,3’-diindolylmethane in cultured MCF-7 human breast cancer cells. Chem. Res. Toxicol. 19(3), 42-436. Suresh, P.S., Kumari, S., Sahal, D. and Sharma, U. 2023. Innate functions of natural products: a promising path for the identification of novel therapeutics. Eur. J. Med. Chem. 260, 115748. Tharp, J.M., Walker, J.A., Söll, D. and Schepartz, A. 2021. Initiating protein synthesis with noncanonical monomers in vitro and in vivo. Methods. Enzymol. 656, 495–519. Tworoger, S.S., Chubak, J., Aiello, E.J., Ulrich, C.M., Atkinson, C., Potter, J.D., Yasui, Y., Stapleton, P.L., Lampe, J.W., Farin, F.M., Stanczyk, F.Z. and Mctiernan, A. 2004. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 13(1), 94-101. Weng, J.R., Tsai, C.H., Kulp, S.K. and Chen, C.S. 2008. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer. Lett. 262(2), 153–163. Williams, D.E. 2021. indoles derived from glucobrassicin: cancer chemoprevention by Indole-3-Carbinol and 3,3’-Diindolylmethane. Front. Nutr. 8, 734334. Yuneldi, R.F., Airin, C.M., Saragih, H.T. and Astuti, P. 2021. Application of natural aromatase blocker towards the level of testosterone in rooster layer [gallus gallus gallus (Linn., 1758)]. Key Eng. Mater. 884, 251–255. Zhao, Y., Zhang, Y., Yang, H., Xu, Z., Li, Z., Zhang, Z., Zhang, W. and Deng, J. 2024. A comparative metabolomics analysis of phytochemicals and antioxidant activity between broccoli floret and by-products (leaves and stalks). Food. Chem. 443, 138517. | ||
| How to Cite this Article |
| Pubmed Style Amalia R, Ohama T, Parhar IS, Airin CM, Sato H, Budiyanto A, Astuti P. Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Vet. J.. 2025; 15(4): 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 Web Style Amalia R, Ohama T, Parhar IS, Airin CM, Sato H, Budiyanto A, Astuti P. Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. https://www.openveterinaryjournal.com/?mno=232414 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.18 AMA (American Medical Association) Style Amalia R, Ohama T, Parhar IS, Airin CM, Sato H, Budiyanto A, Astuti P. Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Vet. J.. 2025; 15(4): 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 Vancouver/ICMJE Style Amalia R, Ohama T, Parhar IS, Airin CM, Sato H, Budiyanto A, Astuti P. Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 Harvard Style Amalia, R., Ohama, . T., Parhar, . I. S., Airin, . C. M., Sato, . H., Budiyanto, . A. & Astuti, . P. (2025) Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Vet. J., 15 (4), 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 Turabian Style Amalia, Reski, Takashi Ohama, Ishwar Singh Parhar, Claude Mona Airin, Hiroshi Sato, Agung Budiyanto, and Pudji Astuti. 2025. Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Veterinary Journal, 15 (4), 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 Chicago Style Amalia, Reski, Takashi Ohama, Ishwar Singh Parhar, Claude Mona Airin, Hiroshi Sato, Agung Budiyanto, and Pudji Astuti. "Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies." Open Veterinary Journal 15 (2025), 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 MLA (The Modern Language Association) Style Amalia, Reski, Takashi Ohama, Ishwar Singh Parhar, Claude Mona Airin, Hiroshi Sato, Agung Budiyanto, and Pudji Astuti. "Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies." Open Veterinary Journal 15.4 (2025), 1663-1672. Print. doi:10.5455/OVJ.2025.v15.i4.18 APA (American Psychological Association) Style Amalia, R., Ohama, . T., Parhar, . I. S., Airin, . C. M., Sato, . H., Budiyanto, . A. & Astuti, . P. (2025) Potential of Indole-3-Carbinol compounds from broccoli (Brassica oleracea var. italica) as natural aromatase blockers: In silico prediction and in vivo studies. Open Veterinary Journal, 15 (4), 1663-1672. doi:10.5455/OVJ.2025.v15.i4.18 |