| Review Article | ||
Open Vet. J.. 2025; 15(5): 1895-1906 Open Veterinary Journal, (2025), Vol. 15(5): 1895-1906 Review Article Uncovering the truth about cat-scratch diseaseYulianna Puspitasari1*, Aswin Rafif Khairullah2, Hartanto Mulyo Raharjo1, Ima Fauziah2, Wiwiek Tyasningsih1, Dea Anita Ariani Kurniasih3, Muhammad Khaliim Jati Kusala2, Ikechukwu Benjamin Moses4, Bantari Wisynu Kusuma Wardhani5, Kartika Afrida Fauzia6,7, Katty Hendriana Priscilia Riwu8, Riza Zainuddin Ahmad2, Sheila Marty Yanestria9, Syahputra Wibowo10, Arif Nur Muhammad Ansori11,12,13 and Ilma Fauziah Ma’ruf51Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 5Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan 8Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 9Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 10Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 11Postgraduate School, Universitas Airlangga, Surabaya, Indonesia 12Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, India 13Medical Biotechnology Research Group, Virtual Research Center for Bioinformatics and Biotechnology, Surabaya, Indonesia *Corresponding Author: Yulianna Puspitasari. Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: yulianna-puspitasari [at] fkh.unair.ac.id Submitted: 28/11/2024 Revised: 31/03/2025 Accepted: 21/4/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTCat-scratch disease (CSD) is a systemic infection caused by the facultative, rod-shaped, nonmotile, Gram-negative, intracellular zoonotic bacillus Bartonella henselae. The bacteria responsible for CSD were not discovered until decades after the condition was first characterized in 1931. The prevalence of CSD is more common in warm, humid areas and is typically seasonal, peaking in the fall and winter. The pathogenesis of CSD starts when a tiny wound from an infected cat’s bite or scratch allows the bacteria B. henselae to enter the human body. The innate immune system, which includes neutrophils and macrophages, is activated as an initial reaction. Histological examination of skin lesions and lymph nodes in immunocompetent people early in the clinical phase of CSD revealed lymphoid hyperplasia and arteriolar proliferation. The best initial test for CSD is an enzyme-linked immunosorbent assay or an indirect fluorescence assay. Bacteremia is typically asymptomatic in cats that are naturally infected with B. henselae. In humans, B. henselae can spread and infect the liver, spleen, eyes, and central nervous system in certain people. Cat fleas (Ctenocephalides felis) are the main vectors of B. henselae transmission. The zoonotic nature of CSD makes it a public health concern because it can be transmitted from cats to people. Treatment strategies for Bartonella infections differ according to the patient’s immunological status and clinical signs. The research on the effectiveness of antibiotics in vitro and in vivo differs significantly. Eliminating fleas from cats and preventing severe injuries from cats are two ways to prevent CSD. Keywords: B. henselae, cat, CSD, public health, scratch. IntroductionFor more than 10,000 years, people have kept dogs and cats as pets. In contemporary metropolitan civilizations, they have become commonplace as “pets” and have shared our surroundings (Jaroš, 2021; Moreira et al., 2024). Healthy dogs and cats have hundreds of different harmful bacteria, including Bartonella species, in their oral cavities (Álvarez-Fernández et al., 2018). There are around 30 species of facultative intracellular bacteria in the genus Bartonella, which are found in mammals and other vertebrates, including humans (Okaro et al., 2017). Cat-scratch disease (CSD) is a systemic infection caused by the facultative, rod-shaped, nonmotile, Gram-negative, intracellular zoonotic bacillus Bartonella henselae (Okaro et al., 2021). The bacteria responsible for CSD were not discovered until decades after the condition was first characterized in 1931 (Opavsky, 1997). The prevalence of human infections caused by Bartonella species is rising globally. The bite or scratch of an infected animal can infect humans, resulting in chronic blood bacteremia (Jurja et al., 2022). CSD is likely the most prevalent cause of chronic lymphadenopathy in children and adolescents, typically affecting the lymph nodes that drain fluid from the inoculation site in the skin or conjunctiva (Waseem et al., 2023). Only 10%–20% of cases occur in adults (Nelson et al., 2016). This illness typically manifests as a self-limiting, benign condition with painful regional lymphadenopathy that lasts for 3 weeks or longer (Jurja et al., 2022). Furthermore, there are case reports of CSD that describe atypical clinical symptoms, including severe chronic systemic illness, osteomyelitis, arthritis, and involvement of the central nervous system (Maman et al., 2007). Cat erythrocytes and fleas contain B. henselae, which can contaminate saliva and eventually infect humans through bites and scratches (Chomel et al., 2006). Ctenocephalides felis, commonly known as cat fleas, are vectors that spread disease horizontally from cat to cat and can infect people through bites or scratches (Mosbacher et al., 2010). Dogs may be involved in human B. henselae infections, according to a recent Japanese report of a potential case of CSD brought on by contact with dogs (Iannino et al., 2018). Infection-infected cats are typically asymptomatic, although they can develop neurological disease and experience anterior uveitis, lymphadenitis, gingivitis, and stomatitis (Girma et al., 2019). In healthy cats, no significant clinical symptoms of CSD were observed. CSD is found in every part of North America and is widespread worldwide (Nelson et al., 2016). The disease is most common from August to October in northern temperate countries, typically in warm, humid climates. In the US, 22,000 new cases of CSD occur annually (Nawrocki et al., 2020). Cats are not particularly exposed through sharing food or water or through casual or sexual interactions (Tan et al., 2020). A history of cat exposure is crucial, but it is not always required to be diagnosed after coming into touch with an infected cat. The treatment of CSD varies according to the clinical manifestation of the disease. Antibiotics are not necessary for the majority of patients, particularly youngsters, who develop self-limiting lymphadenopathy over the course of two to eight weeks (Bruni et al., 2024). Although reports of additional clinical symptoms linked to this disease are growing, regional lymphadenopathy is the most prevalent clinical characteristic of CSD (Shin et al., 2014). When combined with the high prevalence of asymptomatic Bartonella infections in both humans and animals, this could make it challenging to establish the organism’s causative role and lead to an underdiagnosis of the disease. The current state of affairs demonstrates the growing significance of CSD as a new public health risk factor. The objective of this review is to present the literature on CSD, highlighting its public health impact on low-income countries and the scarcity of information regarding the disease. EtiologyBartonella belong to the α-2 subgroup of proteobacteria and the family Bartonellaceae. They are facultative intracellular Gram-negative (rod-shaped) bacteria that are not motile (Biswas and Rolain, 2010). As of 2023, more than 35 species and potential species of Bartonella are known to exist in mammals, the majority of which lack sufficient characterization (Luo et al., 2023). The bacterium that causes CSD most frequently is B. henselae (previously Rochalimaea henselae); however, other feline species, such as Bartonella (Bartonella clarridgeiae and Bartonella koehlerae), can also cause some instances (Regier et al., 2016). The Warthin–Starry silver impregnation staining method can be used to observe these organisms (Florin et al., 2008). This bacterium was recently shown to have Gram-negative staining properties that ranged from 0.2 to 0.3 microns in diameter and from 0.5 to 1.5 microns in length using the Brown–Hopps tissue Gram staining method (Diddi et al., 2013). HistoryRobert Debre, a pediatrician at the University of Paris in Paris, France, was the first to characterize CSD in 1931. The patient observed that a youngster with significant cat scratches on his ipsilateral hand had suppurative adenitis in the epitrochlear region. Although the findings of every bacteriological investigation were negative, he had a suspicion that the illness and interaction with cats were connected. He documented a number of like instances in the ensuing years and came up with the phrase “CSD” (Albert et al., 2021). Dr Franklin Hangar, a professor in the Department of Medicine at Columbia University in New York, NY, USA, was treated in 1946 for fluctuating epitrochlear and infraclavicular lymphadenitis, while his colleague, Dr. Harry Rose, obtained sterile pus from Hangar’s lymph nodes and performed skin tests on him. A surgeon who had previously had a similar condition after other bacteriological examinations had failed. Both tests were positive (Moriarty and Margileth, 1987). After some time, it was discovered that Hangar had a “very aggressive Siamese cat.” When researching tularemia, Hangar learned about Dr Lee Foshay, a microbiologist at the University of Cincinnati in Cincinnati, Ohio, who was also taking CSD into account as an entity (Harvey et al., 1991). In 1947, Debre met Foshay, who also provided him with some of the antigens used in the Hangar skin test. It was discovered that Dr. Pierre Mollaret and other medical professionals at the Pasteur Institute had also developed antigens for intradermal testing when Debre presented his first case report at the Société Médical des Hôpitaux in France in 1950. Although they did not fully comprehend the significance of cats, as Debre did, Mollaret and colleagues authored an essay that effectively established the disease as we know it today (Albert et al., 2021). In 1983, tiny Gram-negative pleomorphic bacilli were found in the lymph nodes of patients with CSD using silver impregnation staining, marking the first evidence of an etiologic agent (Wear et al., 1983). The organism was termed Afipia felis when it was grown. The significance of A. felis in CSD remains unknown, although recent serological, skin test, microbiological, and molecular investigations have strongly demonstrated that B. henselae (previously R. henselae) is the predominant causative agent of CSD (Opavsky, 1997). The four species of Bartonella are as follows: B. henselae and Bartonella quintana are most frequently linked to human illness, whereas Bartonella elizabethae seldom causes illness in persons with impaired immune systems. Bartonella vinsonii is not linked to human infection (Cheslock and Embers, 2019). Classic CSD typically affects immunocompetent patients and is most commonly associated with necrotizing granulomatous lesions caused by B. henselae (Shasha et al., 2014). In individuals with impaired immune systems, proliferative vascular lesions are more commonly linked to B. quintana, the causative agent of trench disease during World Wars I and II (Karem et al., 2000). It is crucial to understand that B. quintana rarely results in granulomatous lymphadenitis and that B. henselae can occasionally induce angiomatous lesions in immunocompromised patients (Okaro et al., 2017). B. quintana has also been linked to ectoparasites, including fleas and scabies, as well as contact between dogs and cats (Tsai et al., 2011). EpidemiologyThe prevalence of CSD is more common in warm, humid areas and is typically seasonal, peaking in the fall and winter (Windsor, 2001). This may be attributed to cat breeding habits or the purchase of pets during these seasons. There was a correlation between the highest incidence of B. henselae antibodies in the cat population and the highest prevalence of clinical illness (Nasirudeen and Thong, 1999). It has been demonstrated that cats less than 1 year old have higher amounts of B. henselae bacteremia and antibodies to the parasite, and one study discovered that wild cats had higher levels of both antibodies than domestic cats (Stepanić et al., 2024). Although B. henselae is believed to be transmitted from cats to people through the cat flea C. felis, it is transmitted between cats through this bug (Bouhsira et al., 2013). Human–human transmission has not been documented. Cats with CSD are found all over the world; reports of classic Bartonella infection have been published in the United States (Nelson et al., 2016), Europe (Razgūnaitė et al., 2021), Japan (Kikuchi et al., 2002), Australia (Flexman et al., 1995), and New Zealand (Kelly et al., 2004). According to seropidemiological research, B. henselae infections in domestic cats are found all over the world, and depending on the region, between 4% and 80% of cats have antibodies to the parasite (Stepanić et al., 2024). The duration of bacteremia in household cats can range from a few weeks to several years. Cats younger than 1 year old are more likely to develop bacteremia than older cats (Chomel et al., 1995). Significant regional differences in the prevalence of B. henselae types I (Houston I) and II (Marseille) have been observed in domestic cat populations. The majority of B. henselae isolates in East Asia are type I, although type II is the most prevalent strain in most European countries (Bai et al., 2015). In developing nations, there is an urgent need for more epidemiological research on a range of animal and arthropod species, as well as the public health implications of this zoonotic bacteria. Human cat-scratch disease, which is caused by B. henselae, is found all over the world. However, in the majority of nations, this illness is not a human sickness that requires reporting. The disease has been reported in France (Sanguinetti-Morelli et al., 2011), the US (Nelson et al., 2016), Switzerland (Mainardi et al., 1998), Spain (Alonso et al., 2021), Germany (Jendro et al., 1998), Italy (Brunetti et al., 2013), the UK (López-Rueda et al., 2024), Japan (Tsukahara, 2002), The Netherlands (Bergmans et al., 1997), Canada (Jurja et al., 2022), and Australia (Flexman et al., 1995). Only a few cases were reported in the UK after the late 1970s, which might have been caused by the discontinuation of the skin antigen test because of safety concerns (Opavsky, 1997). In temperate climes, the incidence of CSD cases is increased throughout the fall and winter months (Sanguinetti-Morelli et al., 2011). In the US, there are 22,000 occurrences of CSD, leading to over 2000 hospitalizations and costs of $12 million annually (Jackson et al., 1993). CSD is most common in the fall and winter in temperate climes; seasonal variations in disease prevalence are rarely observed in tropical regions (Windsor, 2001). However, in the majority of nations, human CSD cases are not often reported. As a result, there is insufficient evidence to pinpoint the precise incidence or prevalence of Bartonella infections. In 1992, there were between 22,000 and 24,000 cases of CSD in the US, with 2000 of those cases resulting in hospitalization (Reynolds et al., 2005). Unusual vascular proliferative lesions, known as bacillary angiomatosis and bacillary peliosis, are seen in people with weakened immune systems who have contracted B. henselae or B. quintana infections. These lesions are also associated with vascular proliferation in animals (Williams et al., 2002). The study demonstrated that among HIV-positive individuals who experienced fever, 68 out of 382 patients (18%) had evidence of Bartonella infection as determined by PCR testing, indirect immunofluorescent antibody (IFA) testing, or bacteriological culture (Santos et al., 2000). Public health importanceThe zoonotic nature of CSD makes it a public health concern because it can be transmitted from cats to people. CSD disproportionately affects children and contributes to a significant national illness burden (Reynolds et al., 2005). Comprehensive flea treatment for cats can help lower the risk of infection in people because CSD is a zoonotic infection that is sustained and spread among cats by fleas (Nelson et al., 2016). Handwashing after interacting with cats can also lower the danger because flea feces can burrow into injured skin (Jurja et al., 2022). Furthermore, limiting cat hunting activities may lower the risk of infection in humans because cats that hunt outdoors are far more likely to get B. henselae bacteremia (Stepanić et al., 2024). The target audience for educational initiatives should be cat owners, particularly those with immunocompromised conditions or those who have youngsters living with them (Nelson et al., 2016). To clarify the causes of the epidemiological variations identified in this study and risk factors for severe illness, further investigation is required. Humans possess antibodies to B. henselae are rather common, and many have never had cat-scratch illness. According to certain research, at least half of healthy children and adolescents have antibodies to this organism, with reported seroprevalence rates in the general population ranging from less than 1% to 25% or more (Nelson et al., 2016). Antibodies to certain organisms, especially those carried by rats, have been found in up to 10%–15% of persons, with greater rates among injectable drug users residing in impoverished areas. However, relatively few surveys have examined exposure to other Bartonella species (Smith et al., 2002). CSD primarily affects youngsters but can also sometimes affect adults (Busen and Scarborough, 1996). The symptoms are typically benign and self-limiting if the patient is in good health. The majority of healthy people, even those with neurologic involvement, fully recover and very little die (Girma et al., 2019). About 100 clinical instances of endocarditis were reported between 2006 and 2013, and it is believed that endocarditis, typically the most severe consequence of a Bartonella infection, accounts for ≤ 3% of all occurrences of infectious endocarditis in Europe (Charles et al., 2023). People with significantly compromised immune systems are more likely to suffer from serious illness, which can be lethal if treatment is not provided. CSD seldom recurs in healthy individuals, but reinfection is more common in those with weakened immune systems (Jurja et al., 2022). TransmissionCat fleas (C. felis) are the main vectors of B. henselae transmission. Most likely, flea feces are inoculated onto mucous membranes or torn skin, including the skin from flea bites (Duscher et al., 2018). This organism can live for 3 days in the feces of fleas and is excreted for at least 9 days following infection. Fleas also seem to spread several other species of Bartonella, and other arthropods such as flies (such as bat flies), keds, fleas, sand flies, ticks, and parasites found in bird nests have been proven to carry or may carry some organisms (Cheslock and Embers, 2019). Although sharing food or water containers and casual contact do not seem to be major exposure sources for cats, blood can spread B. henselae (e.g., transfusion and reuse of contaminated needles) (Pennisi et al., 2013). In a cat experiment, B. henselae was not transmitted during intercourse between bacteremic female cats and uninfected male cats (Stützer and Hartmann, 2012). Bacteremia in cats can last for weeks to months after infection, and some findings suggest that intermittent and variable bacteremia can last for 2 to 3 years (Chomel et al., 2003). Recent research cannot rule out the likelihood of reinfection. The CSD transmission process is shown in Figure 1. A highly sensitive PCR technique was used in one study to detect B. henselae DNA in the fetal tissue of many wild cats, but no evidence of transmission to kittens was identified in two investigations that injected cats with the parasite before or during pregnancy (Sander et al., 1999; Hansmann et al., 2005). Transplacental transmission of Bartonella appears to be conceivable but uncommon in rodent offspring, whereas studies of pregnant cows infected with B. bovis have not revealed any evidence of transfer to their calves. However, B. henselae has been recorded at least once in the viscera of aborted foals (Chastant-Maillard et al., 2015). Risk factorsRisk factors that make a cat more likely to experience a flea infestation and thus become infected with Bartonella include coming from a stray cat, coming from a shelter or animal welfare group, living in a household with many cats, going outdoors frequently, and living in a hot and humid area (Guptill et al., 2004). There are reports linking tick exposure to an increased risk of CSD in people (Wang et al., 2024). Likewise, exposure to ticks was found to be a risk factor for dogs’ seropositivity to subspecies of Bartonella vinsonii berkhoffi (Pappalardo et al., 1997). Further research is necessary to determine the precise function ticks play in the spread of Bartonella. Nonetheless, a number of recent studies have documented a high frequency of infections by Bartonella species in ticks from various global locations (Álvarez-Fernández et al., 2018).
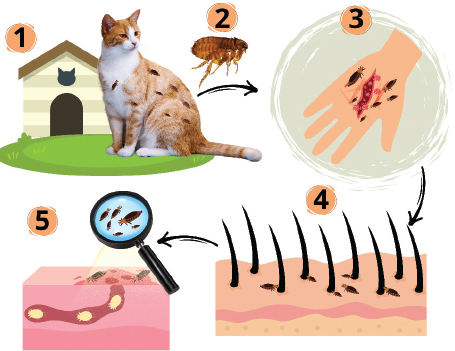
Fig. 1. Transmission process of cat-scratch disease (CSD) caused by B. henselae. The process begins when a domestic cat is infested with fleas, which act as the primary carriers of the bacteria (1). Fleas (C. felis) play a key role as vectors that transmit bacteria to cats (2). When an infected cat bites or scratches a human, it creates an entry point for the bacteria to enter the human body (3–4). Once inside, B. henselae spreads through the bloodstream (5), highlighting the systemic nature of the infection. The chance of developing CSD is increased by frequent contact with cats, particularly kittens, owning or being around a cat that has fleas (Sepúlveda-García et al., 2023). The danger of infection will increase in people with open wounds if an infected cat bites, scratches, or licks the wound (Jurja et al., 2022). Serious consequences of CSD are more likely to occur in people with compromised immune systems (James and Thozhuthumparambil, 2021). CSD is more common in children than in adults, particularly in those aged 5–9 years (Amin et al., 2022). Additional risk factors include not cleaning cat scratches or bites immediately and working with cats, such as veterinarians and animal groomers (Zangwill et al., 1993). Clinical signsBecause the Bartonella species appear to be asymptomatic, nothing is known about their significance as an animal infection. The inquiry is made more difficult by the high rate of infection in healthy animals, the uncertainty surrounding the diagnostic tests for this organism, and the potential for coinfection with other bacteria (Zangwill et al., 1993). Bacteremia is typically asymptomatic in cats that are naturally infected with B. henselae (Regnery and Tappero, 1995). In experimental trials, the majority of cats injected with this organism either showed no symptoms at all or only mild clinical signs, such as eosinophilia, reproductive abnormalities, mild transient anemia, mild nonspecific febrile illness, mild transient behavioral or neurologic symptoms, or inoculation site response (Jurja et al., 2022). A necropsy showed myocarditis in a very sick cat with a flea infection, which may have prevented the cat from mounting a strong immune response to the illness (Breitschwerdt, 2014). Demonstrating that Bartonella causes sickness in naturally afflicted cats is extremely difficult. In humans, B. henselae can spread and infect the liver, spleen, eyes, and central nervous system in certain people. Patients with localized disease typically have a self-limiting condition, whereas those with widespread disease may face potentially fatal outcomes (Lamps and Scott, 2004). The most prevalent clinical sign of CSD is chronic lymphadenopathy (Waseem et al., 2023). Common symptoms include red, aching, and warm lymph nodes (Jurja et al., 2022). Common but minor symptoms include fever and systemic symptoms such as headache, lethargy, malaise, and anorexia (Waseem et al., 2023). Only one lymph node is affected in most patients (around 50%–85%), with the most common lymph nodes being the axillary and epitrochlear, head and neck, and inguinal (Sulaiman et al., 2023). In immunocompetent patients, CSD is characterized by benign regional lymphadenopathy caused by B. henselae (Kunz et al., 2008). Papules and then pustules appear at the inoculation site 7–12 days after experiencing a cat scratch or bite. One to three weeks following inoculation, regional lymphadenopathy may develop and persist for a few weeks to months (Lin and Saccoccio, 2023). Patients with CSD encephalopathy usually fully recover without any problems in a year (Fan and Ali, 2020). Recently, B. henselae has been identified as a frequent cause of both protracted fever and fever of unknown origin in youngsters. Young people with Bartonella infections are also at risk of rheumatic symptoms (Al-Matar et al., 2002). Bacillary angiomatosis is one of the most prevalent clinical signs of Bartonella infection in people with immune system abnormalities (Sala et al., 2005). Patients with B. bacilliformis have chronic vascular proliferative lesions that are histologically and clinically identical to those caused by the bacteria (Rotundo et al., 2024). Bacillary angiomatosis lesions are more likely to occur in HIV-positive individuals with a CD4+ cell count 50/mm3. Histological analysis of cutaneous bacillary angiomatosis revealed a tumor-like growth pattern with epithelioid endothelial cells and significant capillary proliferation (Jung and Paauw, 1998). PathogenesisThe pathogenesis of CSD is poorly understood. The pathogenesis of CSD starts when a tiny wound from an infected cat’s bite or scratch allows the bacteria B. henselae to enter the human body. The bacteria will then proliferate locally in places like the skin and lymph nodes close to the wound (Jurja et al., 2022). After entering the body, the bacteria go to the lymphatic system, where they mostly impact the lymph nodes close to the infection entry site. As a result of this process, the primary symptom of CSD is lymphadenopathy or swelling of the lymph nodes (Hong et al., 2017). Additionally, these bacteria may trigger an inflammatory response in the surrounding tissue, leading to discomfort, swelling, and redness (Harms and Dehio, 2012). These signs may last for a few weeks. Bartonella henselae often only produces mild to moderate symptoms in healthy persons, but in patients receiving immunosuppressive therapy or those with compromised immune systems, such as those with HIV/AIDS, the infection can be more problematic (Mosepele et al., 2012). Rarely, the bacteria may move to the liver, eyes, or heart, where they may result in more severe adverse effects, such as endocarditis and eye conditions (Charles et al., 2023). T and B cells play crucial roles in the body’s immune response to this infection (Jin et al., 2023). The infection may become persistent depending on the immune status of the infected individual. PathologyHistological investigation of skin lesions and lymph nodes in immunocompetent people early in the clinical phase of CSD revealed lymphoid hyperplasia and arteriolar proliferation (Ridder et al., 2005). Stellate abscesses arise as granulomas with multinucleated giant cells that coalesce occasionally later due to necrosis and infiltration of neutrophils (Hansmann et al., 2005). The cooccurrence of granulomas and abscesses supports CSD; however, these findings can also be observed in biopsies of patients with tularemia, tuberculosis, and lymphogranuloma venereum (Guarner, 2012). The visibility of bacilli with Warthin–Starry silver staining is another histologic feature that aids in distinguishing CSD from other infections; nevertheless, the diagnosis is not ruled out if bacilli are not seen when using this method (Peng et al., 2020). Bartonella henselae cannot be distinguished from other Bartonella species using this staining method. If bacilli are seen, they are seen in blood vessel walls, red blood cells, and areas of necrosis. The histologic characteristics of bacillary angiomatosis in immunocompromised patients differ slightly; vascular proliferation and neutrophilic infiltration are more prevalent than granuloma development and stellate abscesses (Farouk et al., 2018). Immune responseFollowing infection, B. henselae will be identified by the body’s immune system as a foreign pathogen. The innate immune system, which includes cells such as neutrophils and macrophages, is activated as an initial reaction (Xi et al., 2024). Both the identification and consumption of bacteria and the generation of cytokines to initiate inflammatory responses are functions of these cells. Swollen lymph nodes surrounding the infection site are common symptoms of CSD that can result from this inflammatory process (Shin et al., 2014). The adaptive immune system, in addition to the innate immune response, is crucial in the fight against CSD. A more focused immune response against B. henselae is guided by T cells, particularly helper T cells (Harms and Dehio, 2012). In order to identify and eliminate microorganisms from the body, activated B cells generate antibodies (Jin et al., 2023). However, occasionally, B. henselae infections can last for a long time or develop into a chronic condition, indicating that the pathogen can evade the immune system’s total eradication (Bush et al., 2024). Although the body’s immune system fights infections, CSD can still pose problems for some people, particularly those with weakened immune systems (Rolain et al., 2004). The infection can spread to other organs in those with immunological problems, leading to more serious complications such as endocarditis or infections of other internal organs (Raybould et al., 2016). Therefore, proper treatment, such as the use of antibiotics, is extremely important to reduce the risk of further complications. Although the body’s defense mechanisms occasionally fall short, allowing the infection to continue or worsen, the immune response to CSD generally involves a complex interaction between innate and adaptive immunity to identify and defeat the infecting bacteria. DiagnosisThe heart tissue also contained the DNA of B. henselae and B. quintana (La Scola and Raoult, 1999). Culturing the Bartonella species is not advisable due to its challenging nature. Additionally, blood samples from the majority of cats with B. henselae-associated endocarditis showed positive results for the bacteria by DNA amplification but did not demonstrate development of the organism in bacteriological culture (Gouriet et al., 2007). A history of contact with cats and the presence of scratches or primary lesions on the skin, eyes, or mucous membranes, a positive result on the cat-scratch skin test, negative results from laboratory testing for other causes of lymphadenopathy, and distinctive histopathologic findings in lymph node biopsy specimens or at sites of systemic involvement are typically required for the diagnosis of CSD (Hansmann et al., 2005). The best initial test is an enzyme-linked immunosorbent assay or an indirect fluorescence assay. Serologic testing is more sensitive than culture, but it is less specific because many cats who do not exhibit any symptoms can test positive for the test because of prior exposure, which is frequently asymptomatic (Amin et al., 2022). CSD may be the cause of enlarged lymph nodes if there are scratches or wounds and a history of contact with cats. Physical examination can sometimes reveal splenomegaly or an enlarged spleen (Tirotta et al., 2021). Several Bartonella species can be found using the polymerase chain reaction (PCR) approach; however, compared with serology, PCR has a lower sensitivity but a very high specificity (Hansmann et al., 2005). For the diagnosis of Bartonella species infections, the PCR technique is a highly quick and precise way to identify the species (Johnson et al., 2003). PCR and serology can be used to detect Bartonella infection, but PCR is more specific. Culture can also be used to detect Bartonella, but PCR and serology are faster and more accurate (La Scola and Raoult, 1999). Differential diagnosisCSD is differentially diagnosed as lymphadenopathy, which encompasses other subacute or chronic lymphadenopathy causes (Ridder et al., 2002). This includes nontuberculous mycobacteria, fungi, Nocardia, and Actinomyces, as well as other granulomatous infections like Mycobacterium tuberculosis (Rolain et al., 2006). Malignancies and autoimmune disorders are examples of noninfectious illnesses that can exhibit comparable symptoms. Acute bacterial lymphadenitis is included in the differential diagnosis during the initial days of illness (Dhal et al., 2021). TreatmentTreatment strategies for Bartonella infections differ according to the patient’s immunological status and clinical signs. The research on the effectiveness of antibiotics in vitro and in vivo differs significantly. Numerous antimicrobial drugs, including macrolides, rifampin, β-lactams, third-generation cephalosporins, aminoglycosides, trimethoprim–sulfamethoxazole, and ciprofloxacin, have been reported to be effective against Bartonella species in vitro (Mazur-Melewska et al., 2015). Nevertheless, clinical experience has not verified this wide range of activity. Only aminoglycosides had bactericidal effects against Bartonella in vitro, whereas the majority of studied antibiotics exhibited bacteriostatic activity. The primary theories for why many antibiotics are unable to penetrate intracellular Bartonella sp. are related to their weak bacteriostatic activity and poor cell membrane penetration (Gadila and Embers, 2021). Antibiotics are not recommended for regional CSD because of its straightforward natural history. Immunocompetent patients with mild to severe infections are managed with reassurance, appropriate monitoring, and painkillers (Smith, 1997). In order to alleviate painful adenopathy, purulent nodes should be aspirated; however, due to the possibility of chronic sinus tract formation, incision and drainage are not advised. Because coalescing abscesses are frequently seen in many septate pockets, the needle must be moved to multiple sites during aspiration (Shin et al., 2014). Patients with severe lymphadenopathy can benefit from taking 10 mg/kg azithromycin on day 1 and 5 mg/kg daily on days 2–5. Additional antibiotic choices include trimethoprim–sulfamethoxazole (trimethoprim 8 mg/kg per day and sulfamethoxazole 40 mg/kg per day, in two divided doses), ciprofloxacin (20–30 mg/kg per day in two daily doses for two–three weeks), and rifampin (20 mg/kg per day in two divided doses for two to three weeks) (Rolain et al., 2004). Choosing the right treatment for B. henselae is increasingly challenging because the clinical spectrum of the disease it causes expands. Observational case studies provide a current understanding of the treatment of hepatosplenomegaly, neuroretinitis, endocarditis, encephalopathy, and bacillary angiomatosis, among other disease processes. Rifampin therapy should be administered for 10–14 days to treat children with hepatosplenic illness and protracted fever, according to limited data. Some specialists advise adding a second agent, such as gentamicin or azithromycin, due to the quick emergence of rifampin resistance (Biswas et al., 2007). PrognosisPatients with CSD who are immunocompetent have a good chance of full recovery. Significant morbidity happens in 5%–10% of instances, mainly as a result of disseminated multisystem disease or involvement of the central or peripheral nervous systems (Pinto et al., 2008). Patients who have only one episode of CSD are immune for the rest of their lives. ControlAuthorities advise against removing cats from houses due to their transient capacity to spread B. henselae (Chomel et al., 1995). Antibiotics may or may not be effective against B. henselae bacteremia in cats. There is no proof that routinely testing healthy cats for Bartonella via culture or serologic testing benefits their owners, according to the 2009 Guidelines for the Prevention of Opportunistic Infections in HIV-infected Adults and Adolescents (Pennisi et al., 2013). The danger of house cats contracting B. henselae or spreading it to other cats is decreased by flea treatment (Smolar et al., 2022). The majority of individuals are said to recover from the illness on their own in a few months. Eliminating fleas from cats and preventing severe injuries from cats are two ways to prevent CSD, which is particularly crucial for those with compromised immune systems (Smolar et al., 2022). The cat should ideally be an adult from a home free of fleas. Only cats that are seronegative can be adopted by potential owners thanks to serological testing (Stepanić et al., 2024). Moreover, trimming the cat claws is recommended. The best defense against B. henselae infection is common sense, which includes keeping cats clean and possibly altering cat owners’ behavior (Brunt et al., 2006). People should always take the required steps to control fleas, wash their hands after handling a pet, and wipe any cuts, bites, or scratches with soap and water as soon as possible (Foucault et al., 2006). To reduce cat bites and scratches, people should refrain from rough play with cats and kittens. ConclusionIn conclusion, CSD, caused by B. henselae, poses an increasing public health risk, particularly in children. Although often self-limiting, atypical symptoms can occur, thereby complicating diagnosis. Understanding its impact and transmission by pets is vital for effective public health strategies. AcknowledgmentsThe authors would like to thank the Universitas Airlangga and Badan Riset dan Inovasi Nasional. Author’s contributionsYP, ARK, HMR, and WT drafted the manuscript. SW, DAAK, BWKW, and IBM revise and edit the manuscripts. KHPR, KAF, SMY, and IFM participated in preparing and critical checking this manuscript. IF, ANMA, RZA, and MKJK edit the references. All authors have read and approved the final manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThis study was supported by Badan Riset dan Inovasi Nasional, Indonesia, which provided funding for this study by Penerima Program Riset dan Inovasi Untuk Indonesia Maju, year 2023, with grant number: 37/II.7/HK/2023. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAlbert, D.M., Salman, A.R., Winthrop, K.L. and Bartley, G.B. 2021. The Continuing Ophthalmic Challenge of Bartonella henselae. Ophthalmol. Sci. 1(3), 100048. Al-Matar, M.J., Petty, R.E., Cabral, D.A., Tucker, L.B., Peyvandi, B., Prendiville, J., Forbes, J., Cairns, R. and Rothstein, R. 2002. Rheumatic manifestations of Bartonella infection in 2 children. J. Rheumatol. 29(1), 184–186. Alonso, B.R., Alonso-Sardón, M., Almeida, H.M.R., Romero-Alegria, Á., Pardo-Lledias, J., Velasco-Tirado, V., López-Bernus, A., Pérez Arellano, J.L. and Belhassen-García, M. 2021. Epidemiological of cat scratch disease among inpatients in the Spanish health system (1997–2015). Eur. J. Clin. Microbiol. Infect. Dis. 40(4), 849–857. Álvarez-Fernández, A., Breitschwerdt, E.B. and Solano-Gallego, L. 2018. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 11(1), 624. Amin, O., Rostad, C.A., Gonzalez, M., Rostad, B.S., Caltharp, S., Quincer, E., Betke, B.A., Gottdenker, N.L., Wilson, J.J., Shane, A.L., Elmontser, M., Camacho-Gonzalez, A., Senior, T., Smith, O., Anderson, E.J. and Yildirim, I. 2022. Cat scratch disease: 9 years of experience at a pediatric center. Open Forum Infect. Dis. 9(9), ofac426. Bai, Y., Rizzo, M.F., Alvarez, D., Moran, D., Peruski, L.F. and Kosoy, M. 2015. Coexistence of Bartonella henselae and B. clarridgeiae in populations of cats and their fleas in Guatemala. J. Vector Ecol. 40(2), 327–332. Bergmans, A.M., de Jong, C.M., van Amerongen, G., Schot, C.S. and Schouls, L.M. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J. Clin. Microbiol. 35(9), 2256–2261. Biswas, S. and Rolain, J.M. 2010. Bartonella infection: treatment and drug resistance. Future Microbiol. 5(11), 1719–1731. Biswas, S., Raoult, D. and Rolain, J.M. 2007. Molecular mechanisms of resistance to antibiotics in Bartonella bacilliformis. J. Antimicrob. Chemother. 59(6), 1065–1070. Bouhsira, E., Franc, M., Boulouis, H.J., Jacquiet, P., Raymond-Letron, I. and Liénard, E. 2013. Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl. Environ. Microbiol. 79(23), 7439–7444. Breitschwerdt, E.B. 2014. Bartonellosis: one health perspectives for an emerging infectious disease. ILAR J. 55(1), 46–58. Brunetti, E., Fabbi, M., Ferraioli, G., Prati, P., Filice, C., Sassera, D., Valle, C.D., Bandi, C., Vicari, N. and Marone, P. 2013. Cat-scratch disease in Northern Italy: atypical clinical manifestations in humans and prevalence of Bartonella infection in cats. Eur. J. Clin. Microbiol. Infect. Dis. 32(4), 531–534. Bruni, L., Baldazzi, M., Greco, L., Vivacqua, D., Di Vincenzo, A.O., Corsini, I., Bruni, S., Minelli, R., Rossi, E., Paviglianiti, G., Napolitano, M., Lanari, M. and Lovato, L. 2024. Atypical clinical and sonographic manifestations of lymphadenopathy in a child with cat-scratch disease: a case report. J. Ultrasound 27(4), 935–939. Brunt, J., Guptill, L., Kordick, D.L., Kudrak, S., Lappin, M.R. and American Association of Feline Practitioners; Academy of Feline Medicine Advisory Panel. 2006. American Association of Feline Practitioners 2006 Panel report on diagnosis, treatment, and prevention of Bartonella spp. infections. J. Feline Med. Surg. 8(4), 213–226. Busen, N. and Scarborough, T. 1996. Diagnosis and management of cat-scratch disease in primary care. Internet J. Adv. Nurs. Pract. 1, 2. Bush, J.C., Robveille, C., Maggi, R.G. and Breitschwerdt, E.B. 2024. Neurobartonelloses: emerging from obscurity! Parasit Vectors 17(1), 416. Charles, K., Abraham, A., Bassi, R., Elsadek, R. and Cockey, G. 2023. A rare case of Bartonella henselae infective endocarditis causing an embolic cerebrovascular Accident. Cureus 15(7), e41364. Chastant-Maillard, S., Boulouis, H.J., Reynaud, K., Thoumire, S., Gandoin, C., Bouillin, C., Cordonnier, N. and Maillard, R. 2015. Lack of transplacental transmission of Bartonella bovis. Comp. Immunol. Microbiol. Infect. Dis. 38(1), 41–46. Cheslock, M.A. and Embers, M.E. 2019. Human bartonellosis: an underappreciated public health problem? Trop. Med. Infect. Dis. 4(2), 69. Chomel, B.B., Abbott, R.C., Kasten, R.W., Floyd-Hawkins, K.A., Kass, P.H., Glaser, C.A., Pedersen, N.C. and Koehler, J.E. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33(9), 2445–2450. Chomel, B.B., Boulouis, H.J., Maruyama, S. and Breitschwerdt, E.B. 2006. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 12(3), 389–394. Chomel, B.B., Kasten, R.W., Sykes, J.E., Boulouis, H.J. and Breitschwerdt, E.B. 2003. Clinical impact of persistent Bartonella bacteremia in humans and animals. Ann. N. Y. Acad. Sci. 990(1), 267–278. Dhal, U., Hicklen, R.S., Tarrand, J. and Kontoyiannis, D.P. 2021. Cat scratch disease as a mimicker of malignancy. Open Forum Infect. Dis. 8(11), ofab500. Diddi, K., Chaudhry, R., Sharma, N. and Dhawan, B. 2013. Strategy for identification & characterization of Bartonella henselae with conventional and molecular methods. Indian J. Med. Res. 137(2), 380–387. Duscher, G.G., Hodžić, A., Potkonjak, A., Leschnik, M.W. and Spergser, J. 2018. Bartonella henselae and Rickettsia felis detected in cat fleas (Ctenocephalides felis) derived from eastern Austrian cats. Vector Borne Zoonotic Dis. 18(5), 282–284. Fan, J. and Ali, H. 2020. Cat scratch disease causing encephalitis. Proc. Bayl. Univ. Med. Cent. 33(3), 440–441. Farouk, W.I., Hassan, N.H., Ismail, T.R., Daud, I.S. and Mohammed, F. 2018. Warthin-starry staining for the detection of Helicobacter pylori in gastric biopsies. Malays. J. Med. Sci. 25(4), 92–99. Flexman, J.P., Lavis, N.J., Kay, I.D., Watson, M., Metcalf, C. and Pearman, J.W. 1995. Bartonella henselae is a causative agent of cat scratch disease in Australia. J. Infect. 31(3), 241–245. Florin, T.A., Zaoutis, T.E. and Zaoutis, L.B. 2008. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics 121(5), e1413–25. Foucault, C., Brouqui, P. and Raoult, D. 2006. Bartonella quintana characteristics and clinical management. Emerg. Infect. Dis. 12(2), 217–223. Gadila, S.K.G. and Embers, M.E. 2021. Antibiotic susceptibility of Bartonella grown in different culture conditions. Pathogens 10(6), 718. Girma, G., Duguma, M. and Haile, G. 2019. A review on cat scratch disease and its zoonotic significance. Madridge J. Vet. Med. Res. 1(1), 1–7. Gouriet, F., Lepidi, H., Habib, G., Collart, F. and Raoult, D. 2007. From cat scratch disease to endocarditis, the possible natural history of Bartonella henselae infection. BMC Infect. Dis. 7(1), 30. Guarner, J. 2012. Detection of microorganisms in granulomas that have been formalin-fixed: review of the literature regarding use of molecular methods. Scientifica (Cairo) 2012(1), 494571. Guptill, L., Wu, C.C., HogenEsch, H., Slater, L.N., Glickman, N., Dunham, A., Syme, H. and Glickman, L. 2004. Prevalence, risk factors, and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States. J. Clin. Microbiol. 42(2), 652–659. Hansmann, Y., DeMartino, S., Piémont, Y., Meyer, N., Mariet, P., Heller, R., Christmann, D. and Jaulhac, B. 2005. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J. Clin. Microbiol. 43(8), 3800–3806. Harms, A. and Dehio, C. 2012. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 25(1), 42–78. Harvey, R.A., Misselbeck, W.J. and Uphold, R.E. 1991. Cat-scratch disease: an unusual cause of combative behavior. Am. J. Emerg. Med. 9(1), 52–53. Hong, J., Li, Y., Hua, X., Bai, Y., Wang, C., Zhu, C., Du, Y., Yang, Z. and Yuan, C. 2017. Lymphatic circulation disseminates Bartonella infection into bloodstream. J. Infect. Dis. 215(2), 303–311. Iannino, F., Salucci, S., Di Provvido, A., Paolini, A. and Ruggieri, E. 2018. Bartonella infections in humans dogs and cats. Vet. Ital. 54(1), 6372. Jackson, L.A., Perkins, B.A. and Wenger, J.D. 1993. Cat scratch disease in the United States: an analysis of three national databases. Am. J. Public Health 83(12), 1707–1711. James, S. and Thozhuthumparambil, K.P. 2021. Cat scratch disease sepsis in an immunocompromised patient. BMJ Case Rep. 14(7), e239932. Jaroš, F. 2021. The cohabitation of humans and urban cats in the anthropocene: the clash of welfare concepts. Animals (Basel) 11(3), 705. Jendro, M.C., Weber, G., Brabant, T., Zeidler, H. and Wollenhaupt, J. 1998. Reaktive Arthritis nach Katzenbiss: eine seltene Manifestation der Katzenkratzkrankheit--Kasuistik und Ubersicht [Reactive arthritis after cat bit: a rare manifestation of cat scratch disease--case report and overview]. Z. Rheumatol. 57(3), 159–163. Jin, X., Gou, Y., Xin, Y., Li, J., Sun, J., Li, T. and Feng, J. 2023. Advancements in understanding the molecular and immune mechanisms of Bartonella pathogenicity. Front. Microbiol. 14(1), 1196700. Johnson, G., Ayers, M., McClure, S.C., Richardson, S.E. and Tellier, R. 2003. Detection and identification of Bartonella species pathogenic for humans by PCR amplification targeting the riboflavin synthase gene (ribC). J. Clin. Microbiol. 41(3), 1069–1072. Jung, A.C. and Paauw, D.S. 1998. Diagnosing HIV-related disease: using the CD4 count as a guide. J. Gen. Intern. Med. 13(2), 131–136. Jurja, S., Stroe, A.Z., Pundiche, M.B., Axelerad, S.D., Mateescu, G., Micu, A.O., Popescu, R., Oltean, A. and Axelerad, A.D. 2022. The clinical profile of cat-scratch disease’s neuro-ophthalmological effects. Brain Sci. 12(2), 217. Karem, K.L., Paddock, C.D. and Regnery, R.L. 2000. Bartonella henselae, B. quintana, and B. bacilliformis: historical pathogens of emerging significance. Microbes Infect. 2(10), 1193–1205. Kelly, P.J., Meads, N., Theobald, A., Fournier, P.E. and Raoult, D. 2004. Rickettsia felis, Bartonella henselae, and B. clarridgeiae, New Zealand. Emerg. Infect. Dis. 10(5), 967–968. Kikuchi, E., Maruyama, S., Sakai, T., Tanaka, S., Yamaguchi, F., Hagiwara, T., Katsube, Y. and Mikami, T. 2002. Serological investigation of Bartonella henselae infections in clinically cat-scratch disease-suspected patients, patients with cardiovascular diseases, and healthy veterinary students in Japan. Microbiol. Immunol. 46(5), 313–316. Kunz, S., Oberle, K., Sander, A., Bogdan, C. and Schleicher, U. 2008. Lymphadenopathy in a novel mouse model of Bartonella-induced cat scratch disease results from lymphocyte immigration and proliferation and is regulated by interferon-alpha/beta. Am. J. Pathol. 172(4), 1005–1018. La Scola, B. and Raoult, D. 1999. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J. Clin. Microbiol. 37(6), 1899–1905. Lamps, L.W. and Scott, M.A. 2004. Cat-scratch disease: historic, clinical, and pathologic perspectives. Am. J. Clin. Pathol. 121(Suppl), S71–S80. Lin, S. and Saccoccio, F.M. 2023. Cat scratch disease: pediatric case series for varying presentations of Bartonella henselae. IDCases 33(1), e01875. López-Rueda, S., Valente-Acosta, B., Murillo-Zolezzi, A., Moreno-Sánchez, F., Hoyo-Ulloa, I. and Baquera-Heredia, J.J. 2024. A man in his forties with recurrent cat-scratch disease. Case Rep. Infect. Dis. 2024(1), 4411133. Luo, Y.Y., Yu, D., Zhang, H.Z., Liu, Z.X., Hong, R.D., Hong, M., Ai, Z.Q., Zhu, J.J. and Yin, J.X. 2023. Molecular detection of Bartonella species in wild small mammals in western Yunnan Province, China. Front. Vet. Sci. 10(1), 1301316. Mainardi, J.L., Figliolini, C., Goldstein, F.W., Blanche, P., Baret-Rigoulet, M., Galezowski, N., Fournier, P.E. and Raoult, D. 1998. Cat scratch disease due to Bartonella henselae serotype Marseille (Swiss cat) in a seronegative patient. J. Clin. Microbiol. 36(9), 2800. Maman, E., Bickels, J., Ephros, M., Paran, D., Comaneshter, D., Metzkor-Cotter, E., Avidor, B., Varon-Graidy, M., Wientroub, S. and Giladi, M. 2007. Musculoskeletal manifestations of cat scratch disease. Clin. Infect. Dis. 45(12), 1535–1540. Mazur-Melewska, K., Mania, A., Kemnitz, P., Figlerowicz, M. and Służewski, W. 2015. Cat-scratch disease: a wide spectrum of clinical pictures. Adv. Dermatol. Allergol. 32(3), 216–220. Moreira, A.J., Costa, S. and Casanova, C. 2024. Paths shared between people (Homo sapiens sapiens) and dogs (Canis familiaris): from aid therapy partners to friends and family members. Acta Sci. 46(1), e71036. Moriarty, R.A. and Margileth, A.M. 1987. Cat scratch disease. Infect. Dis. Clin. North Am. 1(3), 575–590. Mosbacher, M., Elliott, S.P., Shehab, Z., Pinnas, J.L., Klotz, J.H. and Klotz, S.A. 2010. Cat scratch disease and arthropod vectors: more to it than a scratch? J. Am. Board Fam. Med. 23(5), 685–686. Mosepele, M., Mazo, D. and Cohn, J. 2012. Bartonella infection in immunocompromised hosts: immunology of vascular infection and vasoproliferation. Clin. Dev. Immunol. 2012(1), 612809. Nasirudeen, A.M. and Thong, M.L. 1999. Prevalence of Bartonella henselae immunoglobulin G antibodies in Singaporean cats. Pediatr. Infect. Dis. J. 18(3), 276–278. Nawrocki, C.C., Max, R.J., Marzec, N.S. and Nelson, C.A. 2020. Atypical manifestations of cat-scratch disease, United States, 2005–2014. Emerg. Infect. Dis. 26(7), 1438–1446. Nelson, C.A., Saha, S. and Mead, P.S. 2016. Cat-scratch disease in the United States, 2005–2013. Emerg. Infect. Dis. 22(10), 1741–1746. Okaro, U., Addisu, A., Casanas, B. and Anderson, B. 2017. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin. Microbiol. Rev. 30(3), 709–746. Okaro, U., George, S. and Anderson, B. 2021. What is in a cat scratch? Growth of Bartonella henselae in a Biofilm. Microorganisms 9(4), 835. Opavsky, M.A. 1997. Cat scratch disease: the story continues. Can. J. Infect. Dis. 8(1), 43–49. Pappalardo, B.L., Correa, M.T., York, C.C., Peat, C.Y. and Breitschwerdt, E.B. 1997. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am. J. Vet. Res. 58(5), 467–471. Peng, J., Fan, Z., Zheng, H., Lu, J. and Zhan, Y. 2020. Combined application of immunohistochemistry and warthin-starry silver stain on the pathologic diagnosis of cat scratch disease. Appl. Immunohistochem. Mol. Morphol. 28(10), 781–785. Pennisi, M.G., Marsilio, F., Hartmann, K., Lloret, A., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Frymus, T., Gruffydd-Jones, T., Hosie, M.J., Lutz, H., Möstl, K., Radford, A.D., Thiry, E., Truyen, U. and Horzinek, M.C. 2013. Bartonella species infection in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 15(7), 563–569. Pinto, V.L., Curi, A.L., Pinto, A.S., Nunes, E.P., Teixeira, M.L.B., Rozental, T., Favacho, A.R., de Lemos, E.R.S. and Neves Bóia, M.N. 2008. Cat scratch disease complicated with aseptic meningitis and neuroretinitis. Braz. J. Infect. Dis. 12(2), 158–160. Raybould, J.E., Raybould, A.L., Morales, M.K., Zaheer, M., Lipkowitz, M.S., Timpone, J.G. and Kumar, P.N. 2016. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect. Dis. Clin. Pract. 24(5), 254–260. Razgūnaitė, M., Lipatova, I., Paulauskas, A., Karvelienė, B., Riškevičienė, V. and Radzijevskaja, J. 2021. Bartonella infections in cats and cat fleas in lithuania. Pathogens 10(9), 1209. Regier, Y., O’ Rourke, F. and Kempf, V.A. 2016. Bartonella spp. - a chance to establish One Health concepts in veterinary and human medicine. Parasit. Vectors 9(1), 261. Regnery, R. and Tappero, J. 1995. Unraveling mysteries associated with cat-scratch disease, bacillary angiomatosis, and related syndromes. Emerg. Infect. Dis. 1(1), 16–21. Reynolds, M.G., Holman, R.C., Curns, A.T., O’Reilly, M., McQuiston, J.H. and Steiner, C.A. 2005. Epidemiology of cat-scratch disease hospitalizations among children in the United States. Pediatr. Infect. Dis. J. 24(8), 700–704. Ridder, G.J., Boedeker, C.C., Technau-Ihling, K. and Sander, A. 2005. Cat-scratch disease: Otolaryngologic manifestations and management. Otolaryngol. Head. Neck. Surg. 132(3), 353–358. Ridder, G.J., Boedeker, C.C., Technau-Ihling, K., Grunow, R. and Sander, A. 2002. Role of cat-scratch disease in lymphadenopathy in the head and neck. Clin. Infect. Dis. 35(6), 643–649. Rolain, J.M., Brouqui, P., Koehler, J.E., Maguina, C., Dolan, M.J. and Raoult, D. 2004. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents Chemother. 48(6), 1921–1933. Rolain, J.M., Lepidi, H., Zanaret, M., Triglia, J.M., Michel, G., Thomas, P.A., Texereau, M., Stein, A., Romaru, A., Eb, F. and Raoult, D. 2006. Lymph node biopsy specimens and diagnosis of cat-scratch disease. Emerg. Infect. Dis. 12(9), 1338–1344. Rotundo, S., Tassone, M.T., Marascio, N., Morrone, H.L., Gigliotti, S., Quirino, A., Russo, A., Matera, G., Trecarichi, E.M. and Torti, C. 2024. A systematic review on antibiotic therapy of cutaneous bacillary angiomatosis not related to major immunocompromising conditions: from pathogenesis to treatment. BMC Infect. Dis. 24(1), 380. Sala, M., Font, B., Sanfeliu, I., Quesada, M., Ponts, I. and Segura, F. 2005. Bacillary angiomatosis caused by Bartonella quintana. Ann. N. Y. Acad. Sci. 1063(1), 302–307. Sander, A., Posselt, M., Böhm, N., Ruess, M. and Altwegg, M. 1999. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. J. Clin. Microbiol. 37(4), 993–997. Sanguinetti-Morelli, D., Angelakis, E., Richet, H., Davoust, B., Rolain, J.M. and Raoult, D. 2011. Seasonality of cat-scratch disease, France, 1999–2009. Emerg. Infect. Dis. 17(4), 705–707. Santos, R., Cardoso, O., Rodrigues, P., Cardoso, J., Machado, J., Afonso, A., Bacellar, F., Marston, E. and Proença, R. 2000. Bacillary angiomatosis by Bartonella quintana in an HIV-infected patient. J. Am. Acad. Dermatol. 42(2 Pt 1), 299–301. Sepúlveda-García, P., Alabi, A., Álvarez, K., Rojas, L., Mella, A., Gonçalves, L.R., André, M.R., Machado, R.Z., Müller, A. and Monti, G. 2023. Bartonella spp. in households with cats: Risk factors for infection in cats and human exposure. One Health 16(1), 100545. Shasha, D., Gilon, D., Vernea, F., Moses, A.E. and Strahilevitz, J. 2014. Visceral cat scratch disease with endocarditis in an immunocompetent adult: a case report and review of the literature. Vector Borne Zoonotic Dis. 14(3), 175–181. Shin, O.R., Kim, Y.R., Ban, T.H., Lim, T., Han, T.H., Kim, S.Y. and Seo, K.J. 2014. A case report of seronegative cat scratch disease, emphasizing the histopathologic point of view. Diagn. Pathol. 9(1), 62. Smith, D.L. 1997. Cat-scratch disease and related clinical syndromes. Am. Fam. Physician 55(5), 1783–1789. Smith, H.M., Reporter, R., Rood, M.P., Linscott, A.J., Mascola, L.M., Hogrefe, W. and Purcell, R.H. 2002. Prevalence study of antibody to ratborne pathogens and other agents among patients using a free clinic in downtown Los Angeles. J. Infect. Dis. 186(11), 1673–1676. Smolar, A.L.O., Breitschwerdt, E.B., Phillips, P.H., Newman, N.J. and Biousse, V. 2022. Cat scratch disease: What to do with the cat? Am. J. Ophthalmol. Case Rep. 28(1), 101702. Stepanić, M., Duvnjak, S., Reil, I., Hađina, S., Kempf, V.A.J., Špičić, S., Mihaljević, Ž. and Beck, R. 2024. Epidemiology of Bartonella henselae infection in pet and stray cats in Croatia with risk factors analysis. Parasit. Vectors 17(1), 48. Stützer, B. and Hartmann, K. 2012. Chronic Bartonellosis in cats: what are the potential implications? J. Feline Med. Surg. 14(9), 612–621. Sulaiman, Z.I., Samra, H. and Askar, G. 2023. Cat scratch disease: an unusual case of right inguinal lymphadenitis due to Bartonella henselae. Cureus 15(8), e44280. Tan, S.M.L., Stellato, A.C. and Niel, L. 2020. Uncontrolled outdoor access for cats: an assessment of risks and benefits. Animals (Basel) 10(2), 258. Tirotta, D., Mazzeo, V. and Nizzoli, M. 2021. Hepatosplenic cat scratch disease: description of two cases undergoing contrast-enhanced ultrasound for diagnosis and follow-up and systematic literature review. SN Compr. Clin. Med. 3(10), 2154–2166. Tsai, Y.L., Lin, C.C., Chomel, B.B., Chuang, S.T., Tsai, K.H., Wu, W.J., Huang, C.G., Yu, J.C., Sung, M.H., Kass, P.H. and Chang, C.C. 2011. Bartonella infection in shelter cats and dogs and their ectoparasites. Vector Borne Zoonotic Dis. 11(8), 1023–1030. Tsukahara, M. 2002. Cat scratch disease in Japan. J. Infect. Chemother. 8(4), 321–325. Wang, Y., Li, R., Yin, T., He, Z., Lu, Z., Shao, Z. and Long, Y. 2024. Prevalence of tick infection with Bartonella in China: a review and meta-analysis. Acta Parasitol. 69(4), 2083–2095. Waseem, R., Seher, M., Ghazal, S., Shah, H.H. and Habiba, U. 2023. Cat scratch disease in a 23-year-old male-case report. Front. Public Health 10(1), 1046666. Wear, D.J., Margileth, A.M., Hadfield, T.L., Fischer, G.W., Schlagel, C.J. and King, F.M. 1983. Cat scratch disease: a bacterial infection. Science 221(4618), 1403–1405. Williams, A., Sheldon, C.D. and Riordan, T. 2002. Cat scratch disease. BMJ 324(7347), 1199–1200. Windsor, J.J. 2001. Cat-scratch disease: epidemiology, aetiology and treatment. Br. J. Biomed. Sci. 58(2), 101–110. Xi, Y., Li, X., Liu, L., Xiu, F., Yi, X., Chen, H. and You, X. 2024. Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella. Virulence 15(1), 2322961. Zangwill, K.M., Hamilton, D.H., Perkins, B.A., Regnery, R.L., Plikaytis, B.D., Hadler, J.L., Cartter, M.L. and Wenger, J.D. 1993. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N. Engl. J. Med. 329(1), 8–13. | ||
| How to Cite this Article |
| Pubmed Style Puspitasari Y, Khairullah AR, Raharjo HM, Fauziah I, Tyasningsih W, Kurniasih DAA, Kusala MKJ, Moses IB, Wardhani BWK, Fauzia KA, Riwu KHP, Ahmad RZ, Yanestria SM, Wibowo S, Ansori ANM, Ma'ruf IF. Uncovering the truth about cat-scratch disease. Open Vet. J.. 2025; 15(5): 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 Web Style Puspitasari Y, Khairullah AR, Raharjo HM, Fauziah I, Tyasningsih W, Kurniasih DAA, Kusala MKJ, Moses IB, Wardhani BWK, Fauzia KA, Riwu KHP, Ahmad RZ, Yanestria SM, Wibowo S, Ansori ANM, Ma'ruf IF. Uncovering the truth about cat-scratch disease. https://www.openveterinaryjournal.com/?mno=230684 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.5 AMA (American Medical Association) Style Puspitasari Y, Khairullah AR, Raharjo HM, Fauziah I, Tyasningsih W, Kurniasih DAA, Kusala MKJ, Moses IB, Wardhani BWK, Fauzia KA, Riwu KHP, Ahmad RZ, Yanestria SM, Wibowo S, Ansori ANM, Ma'ruf IF. Uncovering the truth about cat-scratch disease. Open Vet. J.. 2025; 15(5): 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 Vancouver/ICMJE Style Puspitasari Y, Khairullah AR, Raharjo HM, Fauziah I, Tyasningsih W, Kurniasih DAA, Kusala MKJ, Moses IB, Wardhani BWK, Fauzia KA, Riwu KHP, Ahmad RZ, Yanestria SM, Wibowo S, Ansori ANM, Ma'ruf IF. Uncovering the truth about cat-scratch disease. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 Harvard Style Puspitasari, Y., Khairullah, . A. R., Raharjo, . H. M., Fauziah, . I., Tyasningsih, . W., Kurniasih, . D. A. A., Kusala, . M. K. J., Moses, . I. B., Wardhani, . B. W. K., Fauzia, . K. A., Riwu, . K. H. P., Ahmad, . R. Z., Yanestria, . S. M., Wibowo, . S., Ansori, . A. N. M. & Ma'ruf, . I. F. (2025) Uncovering the truth about cat-scratch disease. Open Vet. J., 15 (5), 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 Turabian Style Puspitasari, Yulianna, Aswin Rafif Khairullah, Hartanto Mulyo Raharjo, Ima Fauziah, Wiwiek Tyasningsih, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Syahputra Wibowo, Arif Nur Muhammad Ansori, and Ilma Fauziah Ma'ruf. 2025. Uncovering the truth about cat-scratch disease. Open Veterinary Journal, 15 (5), 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 Chicago Style Puspitasari, Yulianna, Aswin Rafif Khairullah, Hartanto Mulyo Raharjo, Ima Fauziah, Wiwiek Tyasningsih, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Syahputra Wibowo, Arif Nur Muhammad Ansori, and Ilma Fauziah Ma'ruf. "Uncovering the truth about cat-scratch disease." Open Veterinary Journal 15 (2025), 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 MLA (The Modern Language Association) Style Puspitasari, Yulianna, Aswin Rafif Khairullah, Hartanto Mulyo Raharjo, Ima Fauziah, Wiwiek Tyasningsih, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, Ikechukwu Benjamin Moses, Bantari Wisynu Kusuma Wardhani, Kartika Afrida Fauzia, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Syahputra Wibowo, Arif Nur Muhammad Ansori, and Ilma Fauziah Ma'ruf. "Uncovering the truth about cat-scratch disease." Open Veterinary Journal 15.5 (2025), 1895-1906. Print. doi:10.5455/OVJ.2025.v15.i5.5 APA (American Psychological Association) Style Puspitasari, Y., Khairullah, . A. R., Raharjo, . H. M., Fauziah, . I., Tyasningsih, . W., Kurniasih, . D. A. A., Kusala, . M. K. J., Moses, . I. B., Wardhani, . B. W. K., Fauzia, . K. A., Riwu, . K. H. P., Ahmad, . R. Z., Yanestria, . S. M., Wibowo, . S., Ansori, . A. N. M. & Ma'ruf, . I. F. (2025) Uncovering the truth about cat-scratch disease. Open Veterinary Journal, 15 (5), 1895-1906. doi:10.5455/OVJ.2025.v15.i5.5 |