| Research Article | ||
Open Vet. J.. 2025; 15(4): 1624-1636 Open Veterinary Journal, (2025), Vol. 15(4): 1624-1636 Research Article Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology cultureSuzana Hađina1*, Magdalena Kolenc2, Renata Ivanek3, Maja Ferenčaković4, Josipa Habuš1, Vladimir Stevanović1, Matko Perharić1 and Zrinka Štritof11Department of Microbiology and Infectious Diseases With Clinic, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia 2Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia 3Department of Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 4Department of Animal Science, Faculty of Agriculture, University of Zagreb, Zagreb, Croatia *Corresponding Author: Suzana Hađina. Department of Microbiology and Infectious Diseases With Clinic, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia. Email: suzana.hadina [at] vef.unizg.hr Submitted: 19/11/2024 Accepted: 25/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
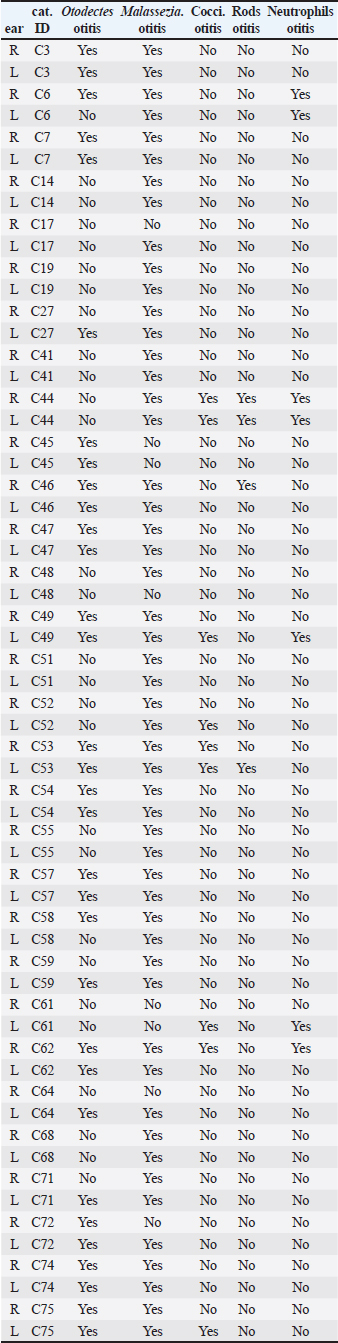
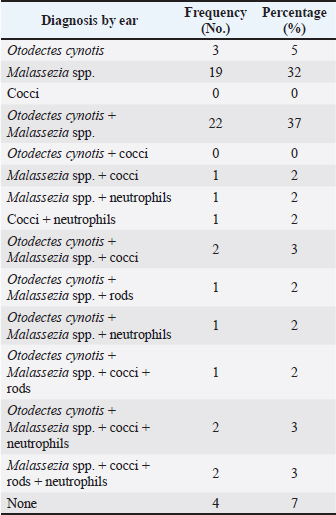
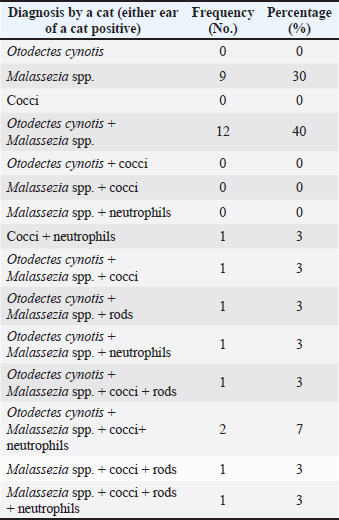
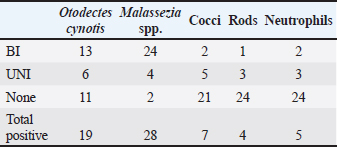
ABSTRACTBackground: Free-roaming cats are a potential source of several infectious and parasitic diseases that can be easily transmitted to outdoor pet cats. Aim: The purpose of this study was to determine the prevalence and pattern of co-occurrence of causative agents of otitis externa and to identify associated risk factors in free-roaming cats living in the continental region of Croatia. In addition, we investigated the presence of Malassezia yeasts in the external ear canals of cats using mycology culture. Methods: Clinical assessment of otitis externa involved auricular and otoscopic examinations. Samples of cerumen and ear swabs were collected and analyzed via cytological examination and mycological culture. Results: The frequency of otitis externa in the population of free-roaming cats was 40.5% (30/74) based on the presence of Otodectes cynotis, Malassezia yeasts, cocci, rods, and/or neutrophils. Results revealed multiple diagnoses for the same ears and unevenly affected ears of the same cats. O. cynotis was detected in 25.7% (19/74) of the cats and was the primary cause of otitis in 63.3% (19/30) infected cats. Co-diagnosis of O. cynotis and Malassezia yeasts was common at both the ear (39.3%, 22/56 infected ears) and cat (40.0%, 12/30 infected cats) levels, while Malassezia was causing otitis in 30% (9/30) infected cats. Three or more diagnoses co-occurred in 15% of infected ears and 27% of infected cats. The clinical signs of otitis externa were absent in 8.9% (5/56) of infected ears, all affected by Malassezia alone or with O. cynotis. Malassezia yeasts were cultured in 83.3% (25/30) of the otitis cases, with heavy growth in 70% and Malassezia pachydermatis as the predominant species in all cases. Conclusion: The high prevalence of otitis externa caused by O. cynotis and Malassezia yeasts in free-roaming cats in Croatia provides important insights into the health status of this cat population. Although otitis externa and its causative agents are frequently found in free-roaming cats, the actual prevalence of otitis externa and all associated risk factors have not been thoroughly assessed. Keywords: Free-roaming cats, Otitis externa, Clinical examination, Cytology, Mycology culture. IntroductionFeline otitis is a common health disorder associated with the presence of primary, secondary, and perpetuating factors (Brame and Cain, 2021). Reports indicate that otitis externa affects a significant proportion of stray, free-roaming, and feral cats, with prevalence rates ranging from 0.9% to 55.1% (Perego et al., 2014; Bollez et al., 2018; Tyler et al., 2019). The diagnosis of feline otitis is based on a history of disease and clinical examination followed by cytology, and sometimes cultural methods (Brame and Cain, 2021). In the populations of community cats, the clinician must rely only on clinical examination, otoscopy, and cytology to diagnose the disease since there is no available anamnesis. Moreover, unlike dogs in which the OTIS scoring system is used (Nuttall and Bensignor, 2014), no standardized grading system for otitis has been developed for cats. This makes it challenging to objectively include and interpret the signs of otitis, especially in cases of mild otitis or subclinical infection where most of the clinical signs may be absent. Cytology represents the second step in the diagnosis of otitis, but it is becoming increasingly complex because there is no unique interpretation of Malassezia and bacterial cell counts evaluated in ear swab samples from dogs, much less cats (Millward, 2018). Various cytologic interpretation criteria for Malassezia yeasts, coccoid, and rod-shaped bacteria in healthy cats and cats with otitis have been provided by previous studies using high power or immersion oil field magnification (HPF and OIF, respectively) (Ginel et al., 2002; Tater et al., 2003; Perego et al., 2014; Pressanti et al., 2014; Tyler et al., 2019). Additionally, Malassezia otitis is common in cats where lipophilic Malassezia pachydermatis is not the only species involved in its pathogenesis (Crespo et al., 2002; Shokri et al., 2010). Despite this fact, conventional cultural identification of all Malassezia yeasts is not routinely performed because the Sabouraud dextrose culture media (SDA) used in veterinary diagnostic laboratories supports the growth of only M. pachydermatis. Results from previous studies reported that a comparison between ear cytology and Malassezia culture methods for the diagnosis of otitis externa yielded low sensitivity and good specificity (Cafarchia et al., 2005). On the other hand, the diagnostic value of culture-based enumeration of Malassezia yeasts in canine dermatitis has been recognized for a long time (Bond et al., 1994), including a study proposing a quantitative polymerase chain reaction method to evaluate effective otitis treatment in dogs (Puig et al., 2019). The aim of this study was to determine the prevalence and pattern of co-occurrence of causative agents of otitis externa and to identify associated risk factors in free-roaming cats living in the continental region of Croatia based on observed clinical assessment and cytology. We also sought to determine the presence of Malassezia yeasts in the external ear canal using mycology culture. Part of this data was previously presented as an abstract (Kolenc et al., 2019). Materials and MethodsSample size and study populationNo reports exist on the prevalence of otitis externa in free-roaming cats in Croatia. The reported prevalence in Italy, Belgium, and the United Kingdom varies from 0.9% to 55.1% (Perego et al., 2014; Bollez et al., 2018; Tyler et al., 2019). Based on this reported range and assuming the most conservative prior prevalence of 50%, the required sample size is 67 cats for the desired margin of error of at least 12% (at 95% confidence interval) (Dohoo et al., 2009). The study population comprised free-roaming cats that inhabited the city of Zagreb and the suburban area, captured as part of the trap-neuter-return project (TNP) in the spring, summer, and fall of 2019. We enrolled 75, of which one cat was not examined, resulting in a sample size of 74 for the study. Collection of samplesThe 74 enrolled cats were sampled by collecting cerumen samples and ear swabs. The location, breed, sex, and age of the animals, as well as the pregnancy data for intact females, were recorded. Based on the age estimation, the animals were divided into two groups: less than 1 year old (G1:<1 year) and older cats (G2: >1 year). Clinical examination of the external ear canal and cytological evaluation of swab impressionsPinna was assessed for the presence of alopecia, erythema, crust, ulceration, or swelling (aural hematoma) (Nuttall and Bensignor, 2014; Bollez et al., 2018; Englar, 2019). An otoscopy was performed to detect possible obstructions of the external ear canal with cerumen or exudate, foreign bodies, polyps, or neoplasia. The presence of cerumen was described according to its (i) color (light brown and dark brown), (ii) consistency (crumbly, waxy, greasy, and gelatinous; in the statistical analysis the presence or absence of crumbly appearance was captured in a variable ”earwax.discharge.crumbly’ with levels yes versus no), and (iii) amount as small, moderate, or large (which was for statistical analysis coded in a variable “earwax.discharge.crumbly” with levels 1–3, respectively) depending on the visualization of the external ear canal and the tympanic membrane. A mineral oil technique was used to examine cerumen samples for the presence of ear mites at low magnification. Swab impressions from the external ear canal were stained with Diff-Quik (BioGnost, Croatia), and counting of cells (Malassezia yeast cells, coccoid, rod-shaped bacteria, and neutrophils) was carried out in 10 different fields under OIF (Angus, 2004) and interpreted based on the counts (neutrophils) or the average of counts in the 10 fields (Malassezia, cocci, and rods). The diagnosis of otitis externa at the ear level was made on the basis of cytological evaluation as follows: Otodectes positive if present ≥1 egg, juvenile or adult mite (Sotiraki et al., 2001; Perego et al., 2014) or neutrophil positive if ≥1 neutrophil is present. Additionally, cocci positive if present >5 coccoid bacteria, Malassezia positive if present >1 Malassezia yeast, and rods positive if present >1 rod-shaped bacterium per OIF (Coyner, 2020). Culture of Malassezia yeastsA swab was taken from each ear, plated onto modified Dixon agar, and incubated at 32°C for 2 weeks. The identification of Malassezia yeasts was based on the appearance of the colonies, which were yellowish to creamy in color and had a characteristic budding cell shape when viewed under a microscope. Lipid dependence was determined by observing SDA growth at 37°C for 72 hours (Gueho-Kellermann et al., 2010). The number of grown colonies on plates was expressed as colony-forming units (CFUs), and growth was classified as: scarce (<10 CFU), moderate (10–50 CFU), and heavy (>50 CFU) using a semi-quantitative method previously described (Crespo et al., 2002). Statistical analysesOtitis externa diagnoses at the ear level based on the five cytological evaluations described in the “Clinical examination of the external ear canal and cytological evaluation of swab impressions” section (i.e., the presence of Otodectes, Malassezia, cocci, rods, and neutrophils above a specified threshold) were the binary outcomes of interest. These outcomes were interpreted individually and combined. At the individual level, Otodectes, cocci, and Malassezia were statistically analyzed, although a small number of positive rod and neutrophil counts precluded their consideration as outcomes in statistical analysis. When combined, the five diagnoses were interpreted in parallel, meaning that an ear was considered positive for a “general otitis externa” outcome if it was positive for any one or more of the five diagnoses and was negative otherwise. The goals of statistical analyses were to (1) determine patterns of individual outcome co-occurrence and (2) identify explanatory variables associated with the occurrence of individual and combined outcomes. The considered explanatory variables (with corresponding levels) included: sex [female (F; baseline)], male (M), spayed female (SF), and neutered male (NM); pregnancy status [no (baseline) and yes]; age [G1:<1 year (baseline) and G2:>1 year]; sampling period [spring (baseline), summer, and autumn], and clinical signs in terms of the presence of a wound [“wound”: no (baseline) and yes]; stenosis [“stenosis”: no (baseline) and yes]; invisibility of the tympanic membrane [“TM.invisible” no (baseline) and yes]; the color of the earwax discharge [“earwax.discharge”:light/brown (baseline) and dark.brown]; the amount of earwax discharge [“earwax.discharge.amount”:1 (baseline), 2, and 3]; and the crumbly consistency of the earwax discharge [“earwax.discharge.crumbly”: no (baseline) and yes]. Additionally, binary variables for the presence/absence of Otodectes cynotis, Malassezia, cocci, rods, and neutrophils were considered explanatory variables for individual outcomes. The binary individual and combined outcomes of interest and explanatory variables were subjected to descriptive analysis. We cross-tabulated the five individual diagnoses across both ears of a cat and across cats to determine the frequency of the co-occurrence of multiple diagnoses at the ear and cat levels. Additionally, for each individual outcome of interest, we interpreted whether the ear infection in a cat was unilateral (UNI) or bilateral (BI). These results revealed multiple diagnoses for the same ears and unevenly affected ears of the same cats. Therefore, subsequent univariable and multivariable statistical analyses of the associations of individual and combined outcomes with explanatory variables were conducted at the ear level. In the univariable analysis, categorical explanatory variables were tested using the chi-square test or Fisher’s exact test (as applicable), while a single continuous variable (weight) was tested using the Mann–Whitney U test (Wilcoxon test). Predictor variables displaying significant associations (p < 0.05) with outcomes at the univariable level were tested in multivariable logistic regression models. The final logistic regression model was selected through a stepwise backward elimination process using the likelihood ratio test and the Akaike information criterion. Two-way interactions between variables included in the final multivariable model were also assessed, but none were identified. The association between the outcome and a statistically significant explanatory variable was expressed as odds ratio (OR) and the associated 95% confidence interval (95% CI) based on the Wald test or Fisher’s exact test (as applicable). All statistical analyses were performed using R software (R Core Team, 2021) and JMP® (Version 16.0. SAS Institute Inc., Cary, NC, 1989–2021). p-values were considered statistically significant at 0.05. Correction for multiple comparisons in the univariable analyses was not conducted due to the exploratory nature of the investigation. Because the analyses of associations were conducted at the ear level, the p-values for explanatory variables that vary at the cat level (i.e., season, sex, age, pregnancy, and weight) might be underestimated due to the unaccounted repeated observations of the same cat level observation twice (once for each ear). However, considering the smaller number of explanatory variables that vary at the cat versus ear level and that preliminary analysis indicated p-values for most cat-level variables were >0.05 in univariable analyses and there was only one instance of a cat-level variable (season) in final multivariable models, we considered this to be a reasonable approach that allowed streamlined statistical analyses and aided interpretation while having minimal effects on conclusions. Ethical approvalThe sampling was approved by the Veterinary Ethics Committee of the Faculty of Veterinary Medicine, University of Zagreb, Croatia (602-04/18-01/5, 251-61-41/258-19-4-S.V.). ResultsFree-roaming cat populationA total of 74 free-roaming cats were examined. All cats were of the domestic short-hair breed. Twenty-six (26/74; 35.1%) cats were male (intact (24/26; 92.3%) or neutered (2/26; 7.7%), and 48/74 (64.9%) were female (intact (43/48; 89.6%) or spayed (5/48; 10.4%). Of the 43 intact cats, 42 were checked for pregnancy, and six (6/42; 14%) were pregnant. Most cats were under 1 year of age [55/73; 75.3%; the age of one animal (1/74; 1.4%) was not determined]. Thirty-five animals (35/74; 47.3%) were sampled in spring, 17/74 (23.0%) in summer, and 22/74 (29.7%) in autumn. Otitis externaOf the 74 cats subjected to statistical analysis in this study, 30 were positive for at least one of the five otitis diagnoses: O. cynotis, Malassezia, cocci, rods, and/or neutrophils (Supplementary Table S1). This analysis revealed that cats commonly had multiple etiologies detected at the individual ear (Tables 1 and Supplementary Table S1) and cat (Tables 2 and Supplementary Table S2) levels. Co-diagnosis of O. cynotis and Malassezia yeasts was particularly common at both the ear (39.3%, 22/56 infected ears) and cat (40.0%, 12/30 infected cats) levels. Interestingly, three or more diagnoses co-occurred in 15% of infected ears (Table 1) and 27% of infected cats (Table 2). Importantly, no clinical signs of inflammation of the external ear canal were observed in 8.9% (5/56) of infected ears, all of which were infected with O. cynotis and Malassezia or Malassezia alone (Supplementary Table S1). Supplementary Table S1. Diagnosis of otitis externa by ear for the five otitis externa diagnoses individually.
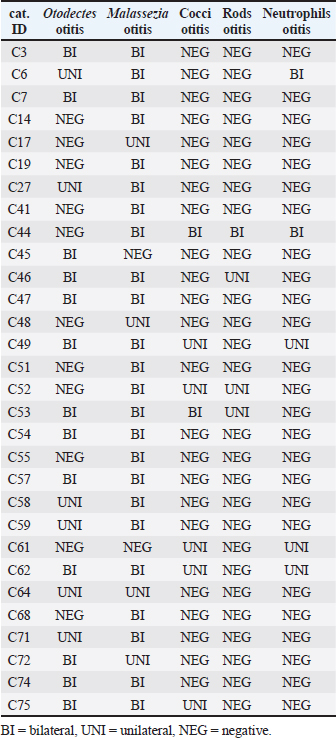
For each diagnosis, we interpreted whether the infection in a cat was absent (none), UNI, or BI (Supplementary Table S2). When diagnosed with otitis externa caused by O. cynotis and Malassezia spp., the infection was BI 68% (13/19) and 86% (24/28) of the time, respectively (Table 3). These findings support the need for treatment of both ears for O. cynotis. For cocci, when present, the infection was more likely (71%) UNI, supporting the UNI application of antibiotic treatment; however, because of the small sample size, more research is needed to support the generalizability of this finding. Table 1. Frequency of occurrence of individual and combinations of otitis externa diagnoses by ear among the 30 cats diagnosed with otitis externa.
Table 2. Frequency of occurrence of individual and combined otitis externa diagnoses among the 30 cats diagnosed with otitis externa.
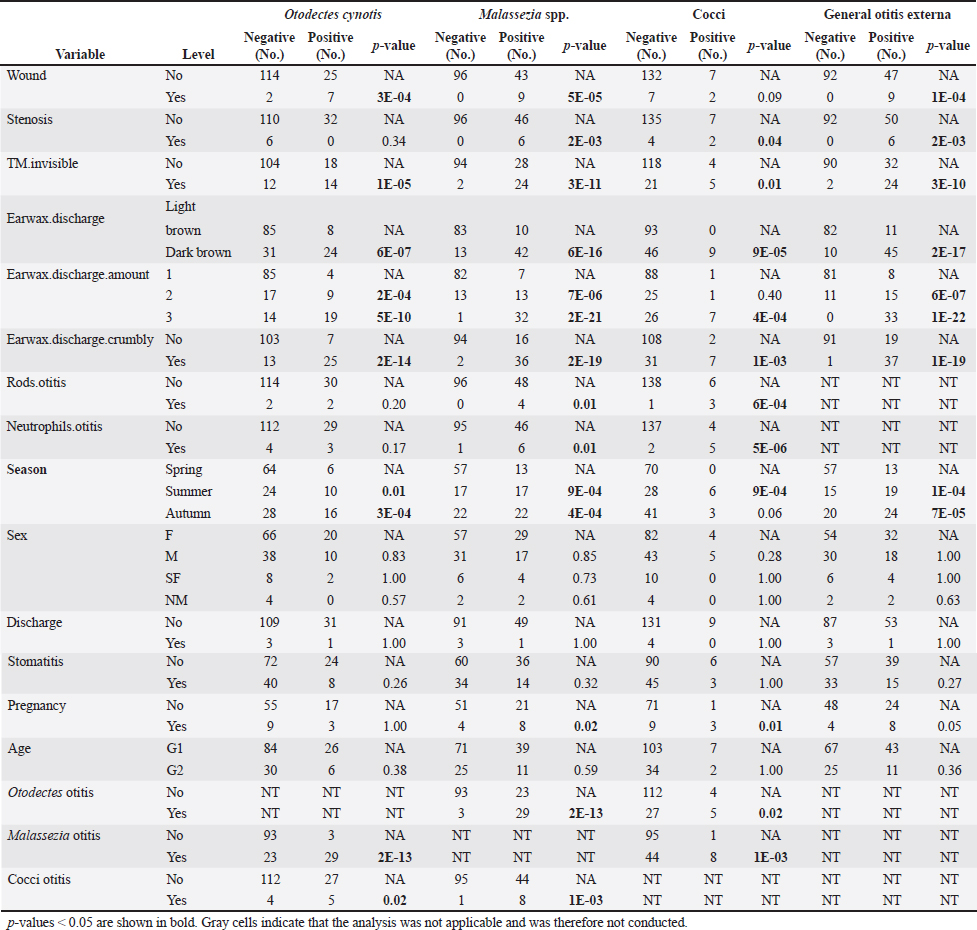
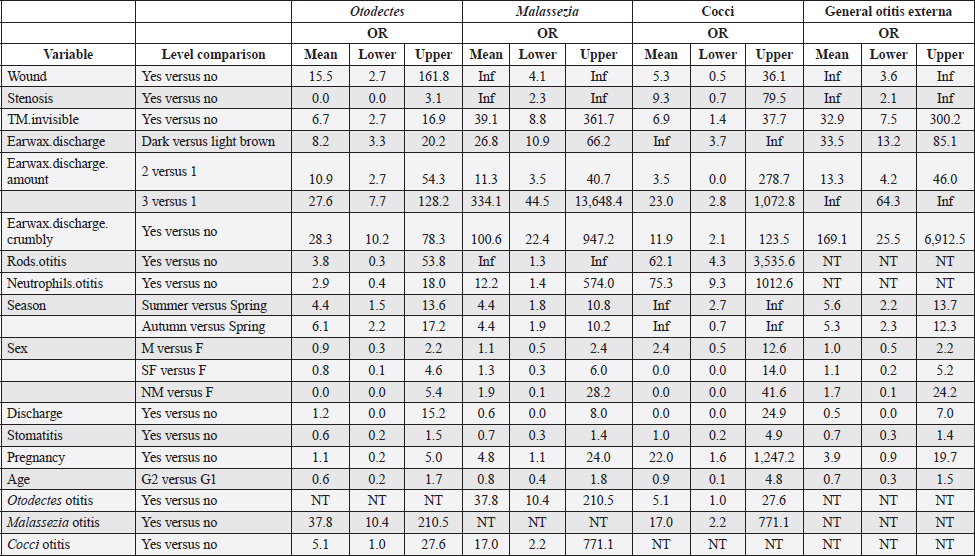
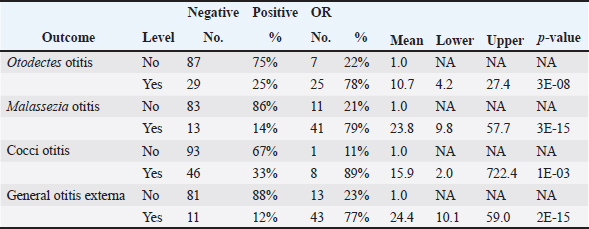
Table 4 presents the results of univariable analyses of associations between explanatory variables and individual diagnoses of otitis based on the presence of O. cynotis, Malassezia yeasts, or cocci, as well as general otitis externa (i.e., positive for any or more of the five diagnoses). Supplementary Table S3 shows the ORs and associated 95% CIs for the tested associations in these univariable analyses. The only statistically significantly associated cat-level explanatory variables of ear infection were pregnancy, season, and body weight. Specifically, ear infections were more likely in pregnant cats than in non-pregnant cats for Malassezia and cocci, but not for O. cynotis (Tables 4 and Supplementary Table S3). In addition, ear infection was more likely in summer and autumn compared with spring for all three individual diagnoses as well as the combined diagnosis, except that the association did not reach the statistical significance threshold for the autumn and cocci relationship (p-value=0.06; Tables 4 and Supplementary Table S3). Cat weight was associated with Otodectes otitis diagnosis, with a significantly (p-value=4.8E-03) lower weight in Otodectes-positive cats (mean=2.94, median=2.98, and range: 1.40–5.35 kg) compared with Otodectes-negative cats (mean=3.40, median=3.25, and range: 1.60–5.35 kg). The presence of the evaluated clinical predictors (wound, stenosis, tympanic membrane invisibility, dark brown color of discharge, and crumbly discharge appearance) statistically significantly increased the odds of the three individual diagnoses and the combined diagnosis, except for no detected association of stenosis with Otodectes and wound with cocci (Tables 4 and Supplementary Table S3). The presence of rods and neutrophils was not associated with the diagnosis of Otodectes, but rods and neutrophils increased the odds of diagnosis of Malassezia- and cocci-based otitis. Finally, Otodectes, Malassezia, and cocci were statistically significantly associated with each other, where otitis diagnosed based on the presence of one of the pathogens increased the odds of a positive diagnosis for the other two pathogens; the association between Otodectes and Malassezia was particularly strong (OR mean 37.8, 95%CI: 10.4–210.5, p-value 10-13) (Tables 4 and Supplementary Table S3), which is unsurprising considering their common co-occurrence (Tables 1 and Supplementary Table S1). Supplementary Table S2. UNI versus BI positive diagnoses in 30 cats with diagnosed otitis externa.
Table 3. Frequency (No.) of UNI versus BI otitis externa by diagnosis.
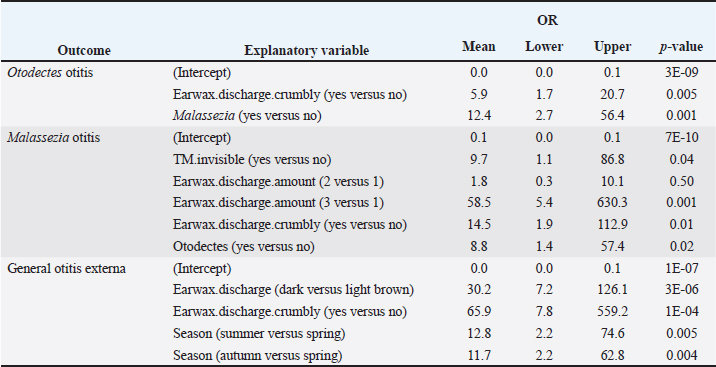
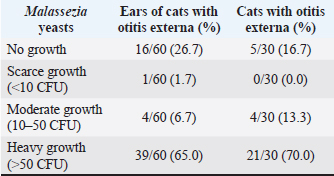
No multivariable model was constructed for cocci. However, the final multivariable logistic regression models for O. cynotis, Malassezia yeasts, and general otitis externa differed (Tables 5 and Supplementary Table S3), which is unsurprising considering the differences in the patterns of diagnoses across cats and individual cats’ ears (Supplementary Tables S1 and S2). After controlling for other factors, only a few clinical explanatory variables were associated with the two causative agents, albite, with very wide 95% CIs. Specifically, the odds of otitis externa caused by the primary factor O. cynotis were higher in the presence of crumbly earwax discharge (OR: 5.9, 95% CI: 1.7–20.7, and p-value: 0.005) and co-occurrence with the secondary (opportunistic) factor, Malassezia yeast (OR: 12.4, 95% CI: 2.7–56.4, and p-value: 0.001) (Tables 5 and Supplementary Table S3). The odds of Malassezia yeast diagnosis increased in the presence of an invisible tympanic membrane (OR: 9.7, 95% CI: 1.1–86.8, and p-value: 0.04), large (but not medium) amount of earwax discharge (OR: 58.5, 95% CI: 5.4–630.3, and p-value: 0.001), crumbly discharge (OR: 14.5, 95% CI: 1.9–112.9, and p-value: 0.01), and co-occurrence with the primary agent, O. cynotis (OR: 8.8, 95% CI: 1.4–57.4, and p-value: 0.01) (Tables 5 and Supplementary Table S3). Finally, the odds of general otitis externa were higher in the presence of dark brown discharge (OR: 30.2, 95% CI: 7.2–126.1, and p-value: 3*10-6) and crumbly discharge (OR: 65.9, 95% CI: 7.8–559.2, and p-value: 10–4). The odds of general otitis were also higher in summer and autumn than in spring (OR: 12.8, 95% CI: 2.2–74.6, and p-value: 0.005; 11.7, 95% CI: 2.2–62.8, and p-value: 0.004, respectively) (Tables 5 and Supplementary Table S3). Culture of Malassezia yeastsDuring the examination of cats, Malassezia yeasts were cultured from the external ear canal of 31 of 74 (41.9%) cats, of which 74.2% (23/31) bilaterally and 25.8% (8/31) unilaterally. Interestingly, 22.6% (7/31) culture-positive cats were negative on cytological investigation for Mallasezia yeast, and 14.3% (4/28) were positive on cytology but had negative culture results. Malassezia yeasts were isolated from 83.3% (25/30; Table 6) of cats diagnosed with otitis based on the cytological investigation (i.e., presence of Otodectes, Malassezia, cocci, rods, and/or neutrophils). Out of the 30 cats with otitis, 4 (13.3%) exhibited moderate Malassezia growth, while 21 (70%) showed heavy Malassezia growth in at least one ear (Table 6). Five (16.7%) of the 30 cats diagnosed with otitis based on any etiology showed no Malassezia growth. Malassezia pachydermatis was the predominant species isolated from all positive cats. Of the 30 cats diagnosed with otitis, 28 (93.3%) had these yeasts present based on the cytological evaluation. Supplementary Table S4 shows the strong univariable associations between the result of Malassezia yeast culture and Malassezia otitis diagnoses at the ear level: OR: 23.8, 95%CI: 9.8–57.7, and p-value: 3*10–15. Similarly, analysis at the cat level indicated a strong association between culture and the diagnosis of Malassezia otitis: OR: 33.4, 95%CI: 8.8–126.6, and p-value: 2*10–9 (not shown here). Table 4. Univariable analysis of the associations between explanatory variables and individual and combined diagnoses.
DiscussionFree-roaming cat populations are a potential source of several infectious and parasitic diseases that can be easily transmitted to outdoor pet cats (Gerhold and Jessup, 2013). In this study, general otitis externa (diagnosed based on the presence of O. cynotis, Malassezia yeasts, cocci, rods, and/or neutrophils) was found in 40.5% (30/74) of the cats. This prevalence is lower than the previously reported rate of 55.1% in stray cats in Italy (Perego et al., 2014) but significantly higher than the 2% reported in Belgium (Bollez et al., 2018). Cross-tabulation of the five individual diagnoses across cats and ears of individual cats revealed that the five evaluated diagnoses often but not always co-occur in an individual ear (Tables 1 and Supplementary Table S1) or cat (Tables 2 and Supplementary Table S2). This suggests the multicausal etiology of otitis externa in cats and underscores the importance of comprehensive cytological evaluations, such as those performed here, to reduce the risk of false-negative diagnoses. The higher prevalence of general otitis externa at the ear level was observed in summer and autumn compared with spring, thereby contributing to the fact that otitis is more common in warmer seasons (Topală et al., 2007). In this study, neither sex nor age was a significant predisposing factor for the development of otitis, consistent with previous reports in stray cats (Perego et al., 2014). Although not statistically significant, it has been observed that male cats and cats younger than 1 year are more likely to develop otitis. A previous study noted a higher incidence of otitis in 35.3% of cats up to 1 year old (Topală et al., 2007). One of the reasons is that younger cats are more susceptible to developing otitis from ear mites due to maternal transmission and social interactions (Fanelli et al., 2020). Univariable statistical analyses indicated that pregnancy increased the likelihood of developing Malassezia and cocci otitis; however, after controlling for other factors in multivariable analysis, pregnancy was no longer a significant predictor (possibly due to the small sample size of the subset of intact cats checked for pregnancy to which this analysis was applicable). Nevertheless, hormonal fluctuations can disrupt the balance of the skin microbiome during pregnancy, creating favorable conditions for the proliferation of Malassezia yeasts and dysbiosis. In this study, ear mites were found in 25.7% (19/74) of the examined cats. This is similar to previous findings from studies on stray and feral cats (Perego et al., 2014; Fanelli et al., 2020). They were the primary cause of otitis in 63.3% (19/30) of the cats in our study, which is slightly higher than the 53.3% prevalence reported in Italy (Perego et al., 2014) and considerably higher than the 2% reported in Belgium (Bollez et al., 2018). Studies have found that the prevalence of O. cynotis in cats can vary greatly, with reported rates ranging from 0.9% to 47.1% (Sotiraki et al., 2001; Akucewich et al., 2002; Tyler et al., 2019; Fanelli et al., 2020; Hiblu et al., 2021). All observed variations may be attributed to differences in environmental conditions, geographic locations, and acaricide treatments (Perego et al., 2014; Bollez et al., 2018; Fanelli et al., 2020). The primary factor of otitis in cats is O. cynotis, which often leads to an imbalanced microenvironment in the external ear canal that can promote excessive growth of resident bacteria or yeasts in the ear and otitis manifestation (Tielemans et al., 2021). The majority of otitis externa in this study was caused by O. cynotis together with Malassezia spp. (40%, 12/30), followed by Malassezia spp. alone (30%, 9/30) at the cat level, and the findings were similar at the ear level [37% (22/60) and 32% (19/60), respectively] of cats with otitis (Tables 1 and 2). These prevalences are significantly higher than the observed prevalences of 8.7% and 5.8% in Italian stray cats (Perego et al., 2014). In another study, 70.1% of pet cats had otitis caused by O. cynotis and M. pachydermatis, which is a significantly higher percentage. However, our results are consistent with the reported 28.3% prevalence of Malassezia infections (Nardoni, 2014). The proliferation of coccoid bacteria was detected in 23.3% (7/30) and rod-shaped bacteria in 13.3% (4/30) of cats with otitis. These findings are consistent with previously reported rates of 21.4% and 16%, respectively (Perego et al., 2014). Although bacterial cultures were not performed, these findings suggest that coccoid bacteria are more prevalent in causing otitis in cats, as previously reported (Roy et al., 2011; Nardoni, 2014). Finally, neutrophils were detected in 16.7% (5/30) of the cats with otitis, but never alone (Table 2). Supplementary Table S3. ORs and 95% confidence intervals for univariable associations of explanatory variables with individual and combined otitis externa diagnoses. TN indicate that the analysis was not applicable and thus not conducted.
Table 5. The final multivariable logistic regression models for the primary agent of otitis (O. cynotis), the secondary agent (Malassezia yeast), and general otitis externa (defined as an ear positive for O. cynotis, Malassezia yeasts, cocci, rods, and/or neutrophils). A multivariable model could not be built for the cocci-based diagnosis.
Table 6. Semiquantitative evaluation of the growth of Malassezia yeasts cultured in 30 cats with otitis externa at the ear and cat levels. The cat level category was assigned based on the ear with the highest observed Malassezia growth.
Using the cultural method, Malassezia yeasts were isolated in 83.3% (25/30) of otitis cases. This is a lower percentage compared to 95% positive Persian and domestic short-haired cats (Shokri et al., 2010) but higher than that previously reported for pet cats (Crespo et al., 2002; Cafarchia et al., 2005). The heavy growth of Malassezia yeasts was found in 70% of cats with otitis, indicating that they play a significant role as secondary infectious agents. Among the positive cats, M. pachydermatis was the most frequently detected species, which is consistent with the findings of previous culture-based studies (Cafarchia et al., 2005; Shokri et al., 2010; Nardoni, 2014). This result is in contrast with the lower recovery rates observed in other studies (Crespo et al., 2002; Dizotti and Coutinho, 2007). Supplementary Table S4. Association between Malassezia yeasts culture and evaluated individual and combined otitis diagnoses outcomes.
In clinical practice, the presence of a small amount of cerumen in the external ear canal of healthy cats is considered normal (Brame and Cain, 2021). However, increased cerumen content could indicate otitis externa, as confirmed by the results of this study. The presence of moderate or large amounts of cerumen increased the odds of otitis diagnosis caused by the primary agent O. cynotis and the secondary agent Malassezia yeasts, as well as general otitis, by at least 10-fold (ORs > 10), while a similar impact on cocci was seen only in the presence of a large amount of cerumen. Diagnosing otitis externa requires a combination of patient history, clinical signs, and cytology. This study demonstrated associations between individual clinical signs and cytology (Table 4 and Supplementary Table S3). However, after controlling for the effect of other factors in multivariable analysis, the crumbly appearance of discharge was the only clinical sign that remained statistically significantly associated with otitis (individual and combined diagnoses; ORs > 10). The discharge amount was predictive of Malassezia otitis (OR > 1.8), and the dark color of the discharge was predictive of general otitis externa (OR=30.2). Different clinical signs associated with individual diagnoses are expected, considering different patterns of diagnosis co-occurrence (Tables 1, 2, and Supplementary Tables S1 and S2) and different interactions of pathogens with the ear tissues. Importantly, no clinical signs of inflammation of the external ear canal were observed in 8.9% (5/56) of infected ears detected in this study, and in all instances, the ears were infected with O. cynotis accompanied by the secondary agent, Malassezia yeasts, or solely with Malassezia yeasts alone. In a previous study, O. cynotis was found in 10.8% of cats without concurrent clinical signs of otitis externa (Akucewich et al., 2002). As expected, a strong but not perfect association between cytology evaluation and mycology culture (Supplementary Table S4), indicating that culturing is not particularly suitable for screening otitis externa. However, a culture-based approach is recommended because of increasing reports of treatment failures in Malassezia infections and their resistance to antifungal agents (Angileri et al., 2019; Bond et al., 2020). The authors acknowledge the lack of unique defined thresholds for the diagnosis of otitis when semiquantitative or quantitative cytology is used. In addition, the evaluation of cell counts under HPF or OIF could also lead to differences that could affect the comparison of the results discussed and lead to biased critical opinions among the scientific reader. These facts present known challenges in interpreting the final results. While our research is the first of its kind to investigate the prevalence, risk factors, and pattern of occurrence of otitis externa in free-roaming cats in Croatia, its design has several limitations. We enrolled a sample of free-roaming cats captured as part of the TNP, which may have introduced selection bias, as captured cats may be different from those not captured. Although the confounding bias was controlled through multivariable analyses, residual confounders for which data were not collected may have been present. The relatively small sample size may have resulted in insufficient statistical power to detect additional risk factors for otitis externa. ConclusionA comprehensive understanding of the etiology of feline otitis and its risk factors, along with further data on this condition, is crucial for advancing our knowledge, particularly concerning free-roaming cat populations. Although the study design has certain limitations and the diagnostic criteria present known weaknesses, the findings offer valuable insights into the prevalence of otitis externa among free-roaming cats in a specific geographic region. AcknowledgmentsThe authors thank Professor Ljiljana Pinter for her valuable assistance and for providing laboratory space and equipment for this study. Special thanks to the non-profit organization “Prava šapa” for providing samples from the TNP project and to Dragan Petrović (DVM) (Small Animal Veterinary Clinic “Nera” and his staff for providing time, space, and equipment. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Authors contributionSH designed the study and wrote the first draft of the manuscript and edited and revised the manuscript. MK performed sampling, laboratory analyses, and culturing, and assisted in writing the first draft. ZŠ was involved in designing the study, and performed double-blinded cell counting, and sample preservation. RI and MF performed statistical analysis, interpreted results, and wrote data analysis. ZŠ, JH, MP, RI, and VS helped in writing the manuscript, critically revised it, and edited it. All authors have read and approved the final manuscript. Data availabilityThe data supporting the findings of this study are presented in the manuscript and supplementary tables. Further details can be obtained from the corresponding author upon reasonable request. ReferencesAkucewich, L.H., Philman, K., Clark, A., Gillespie, J., Kunkle, G., Nicklin, C.F. and Greiner, E.C. 2002. Prevalence of ectoparasites in a population of feral cats from north central Florida during the summer. Vet. Parasitol. 109, 129–139. Angileri, M., Pasquetti, M., De Lucia, M. and Peano, A. 2019. Azole resistance of Malassezia pachydermatis causing treatment failure in a dog. Med. Mycol. Case Rep. 23, 58–61. Angus, J.C. 2004. Otic cytology in health and disease. Vet. Clin. North Am. Small Anim. Pract. 34, 411–424. Bollez, A., de Rooster, H., Furcas, A. and Vandenabeele, S. 2018. Prevalence of external ear disorders in Belgian stray cats. J. Feline Med. Surg. 20, 149–154. Bond, R., Collin, N.S. and Lloyd, D.H. 1994. Use of contact plates for the quantitative culture of Malassezia pachydermatis from canine skin. J. Small Anim. Pract. 35, 68–72. Bond, R., Morris, D.O., Guillot, J., Bensignor, E.J., Robson, D., Mason, K.V., Kano, R. and Hill, P.B. 2020. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 31, 28–74. Brame, B. and Cain, C. 2021. Chronic otitis in cats: clinical management of primary, predisposing and perpetuating factors. J. Feline Med. Surg. 23, 433–446. Cafarchia, C., Gallo, S., Capelli, G. and Otranto, D. 2005. Occurrence and population size of Malassezia spp. in the external ear canal of dogs and cats both healthy and with otitis. Mycopathologia 160, 143–149. Coyner, K.S. 2020. Dermatology diagnostics. In Clinical atlas of canine and feline dermatology. Ed., Coyner, K.S. Hooboken, NJ: Wiley-Blackwell, pp: 1–22. Crespo, M.J., Abarca, M.L. and Cabanes, F.J. 2002. Occurrence of Malassezia spp. in the external ear canals of dogs and cats with and without otitis externa. Med. Mycol. 40, 115–121. Dizotti, C.E. and Coutinho, S.D. 2007. Isolation of Malassezia pachydermatis and M. sympodialis from the external ear canal of cats with and without otitis externa. Acta Vet. Hung. 55, 471–477. Dohoo, I.R., Martin, S.W. and Stryhn, H. 2009. Veterinary epidemiologic research. Charlottetown, Canada: AVC Inc. Englar, R.E. 2019. Abnormal appearances of the external ear. In Common clinical presentations in dogs and cats. Ed., Englar R.E. Hooboken, NJ: Wiley-Blackwell, pp: 411–427. Fanelli, A., Doménech, G., Alonso, F., Martínez-Carrasco, F., Tizzani, P. and Martínez-Carrasco, C. 2020. Otodectes cynotis in urban and peri-urban semi-arid areas: a widespread parasite in the cat population. J. Parasit. Dis. 44, 481–485. Gerhold, R.W. and Jessup, D.A. 2013. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health. 60, 189–195. Ginel, P.J., Lucena, R., Rodriguez, J.C. and Ortega, J. 2002. A semiquantitative cytological evaluation of normal and pathological samples from the external ear canal of dogs and cats. Vet. Dermatol. 13, 151–156. Gueho-Kellermann, E., Boekhout, T. and Begerow, D. 2010. Biodiversity, phylogeny and ultrastructure. In Malassezia and the skin. Science and clinical practice. Eds., Boekhout, T.,Mayser, P., Guého-Kellermann, E. and Velegraki, A. Berlin, Germany: Springer Verlag, pp: 17–63. Hiblu, M.A., Ellraiss, O.M., Karim, E.S., Elmishri, R.A., Duro, E.M., Altaeb, A.A. and Bennour, E.M. 2021. Otodectic and bacterial etiology of feline otitis externa in Tripoli, Libya. Open Vet. J. 10, 377–383. Kolenc, M., Petrović, D., Pinter, L., Štritof, Z., Habuš, J., Stevanović, V., Perharić, M. and Hađina, S. 2019. The evidence of otitis externa in free-roaming cats based on the otoscopic examination and cytological evaluation. In Book of abstracts: 8th International Congress “Veterinary Science and Profession”, Zagreb, Croatia: Faculty of Veterinary Medicine, University of Zagreb, p 139. Millward, L.M. 2018. Cytology. In Field manual for small animal medicine. Eds., Polak, K. and Kommedal, A.T. Hooboken, NJ: Wiley-Blackwell, pp: 441–456. Nardoni, S. 2014. Malassezia, mites and bacteria in the external ear canal of dogs and cats with otitis externa. Slov. Vet. Res. 51, 113–118. Nuttall, T. and Bensignor, E. 2014. A pilot study to develop an objective clinical score for canine otitis externa. Vet. Derm. 25, 530–537. Perego, R., Proverbio, D., Bagnagatti De Giorgi, G., Della Pepa, A. and Spada, E. 2014. Prevalence of otitis externa in stray cats in northern Italy. J. Feline Med. Surg. 16, 483–490. Pressanti, C., Drouet, C. and Cadiergues, M.C. 2014. Comparative study of aural microflora in healthy cats, allergic cats and cats with systemic disease. J. Feline Med. Surg. 16, 992–996. Puig, L., Castellã, G. and Cabaães, F.J. 2019. Quantification of Malassezia pachydermatis by real-time PCR in swabs from the external ear canal of dogs. J. Vet. Diagn. Invest. 31, 440–447. R Core Team (2021). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available via https://www.R-project.org/ Roy, J., Bédard, C. and Moreau, M. 2011. Treatment of feline otitis externa due to Otodectes cynotis and complicated by secondary bacterial and fungal infections with Oridermyl auricular ointment. Can. Vet. J. 52, 277–282. Shokri, H., Khosravi, A., Rad, M. and Jamshidi, S. 2010. Occurrence of Malassezia species in Persian and domestic short hair cats with and without otitis externa. J. Vet. Med. Sci. 72, 293–296. Sotiraki, S.T., Koutinas, A.F., Leontides, L.S., Adamama-Moraitou, K.K. and Himonas, C.A. 2001. Factors affecting the frequency of ear canal and face infestation by Otodectes cynotis in the cat. Vet. Parasitol. 96, 309–315. Tater, K.C., Scott, D.W., Miller, W.H. Jr and Erb, H.N. 2003. The cytology of the external ear canal in the normal dog and cat. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 50, 370–374. Tielemans, E., Prullage, J., Tomoko, O., Liebenberg, J., Capãri, B., Sotiraki, S., Kostopoulou, D., Ligda, P., Ulrich, M. and Knaus, M. 2021. Efficacy of a novel topical combination of esafoxolaner, eprinomectin and praziquantel against ear mite (Otodectes cynotis) infestations in cats. Parasite 28, 26. Topală, R., Burtan, I., Fãntãnaru, M. and Liviu, B. 2007. Epidemiological studies of otitis externa at carnivores. Lucr. St. Med. Vet. 40, 647–651. Tyler, S., Swales, N., Foster, A.P., Knowles, T.G. and Barnard, N. 2019. Otoscopy and aural cytological findings in a population of rescue cats and cases in a referral small animal hospital in England and Wales. J. Feline Med. Surg. 18, 161–167. | ||
| How to Cite this Article |
| Pubmed Style Hađina S, Kolenc M, Ivanek R, Ferenčaković M, Habuš J, Stevanović V, Perharić M, Štritof Z. Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Vet. J.. 2025; 15(4): 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 Web Style Hađina S, Kolenc M, Ivanek R, Ferenčaković M, Habuš J, Stevanović V, Perharić M, Štritof Z. Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. https://www.openveterinaryjournal.com/?mno=229396 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.14 AMA (American Medical Association) Style Hađina S, Kolenc M, Ivanek R, Ferenčaković M, Habuš J, Stevanović V, Perharić M, Štritof Z. Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Vet. J.. 2025; 15(4): 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 Vancouver/ICMJE Style Hađina S, Kolenc M, Ivanek R, Ferenčaković M, Habuš J, Stevanović V, Perharić M, Štritof Z. Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 Harvard Style Hađina, S., Kolenc, . M., Ivanek, . R., Ferenčaković, . M., Habuš, . J., Stevanović, . V., Perharić, . M. & Štritof, . Z. (2025) Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Vet. J., 15 (4), 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 Turabian Style Hađina, Suzana, Magdalena Kolenc, Renata Ivanek, Maja Ferenčaković, Josipa Habuš, Vladimir Stevanović, Matko Perharić, and Zrinka Štritof. 2025. Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Veterinary Journal, 15 (4), 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 Chicago Style Hađina, Suzana, Magdalena Kolenc, Renata Ivanek, Maja Ferenčaković, Josipa Habuš, Vladimir Stevanović, Matko Perharić, and Zrinka Štritof. "Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture." Open Veterinary Journal 15 (2025), 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 MLA (The Modern Language Association) Style Hađina, Suzana, Magdalena Kolenc, Renata Ivanek, Maja Ferenčaković, Josipa Habuš, Vladimir Stevanović, Matko Perharić, and Zrinka Štritof. "Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture." Open Veterinary Journal 15.4 (2025), 1624-1636. Print. doi:10.5455/OVJ.2025.v15.i4.14 APA (American Psychological Association) Style Hađina, S., Kolenc, . M., Ivanek, . R., Ferenčaković, . M., Habuš, . J., Stevanović, . V., Perharić, . M. & Štritof, . Z. (2025) Otitis externa in free-roaming cat population: Clinical examination, cytology, and mycology culture. Open Veterinary Journal, 15 (4), 1624-1636. doi:10.5455/OVJ.2025.v15.i4.14 |