| Research Article | ||
Open Vet J. 2025; 15(4): 1565-1575 Open Veterinary Journal, (2025), Vol. 15(4): 1565-1575 Research Article Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh musclesIbrahim Albokhadaim*Department of Biomedical Sciences, College of Veterinary Medicine, King Faisal University, Al-Hassa, Saudi Arabia *Corresponding Author: Ibrahim Albokhadaim. Department of Biomedical Sciences, College of Veterinary Medicine, King Faisal University, Al-Hassa, Saudi Arabia. Email: ialbokhadaim [at] kfu.edu.sa Submitted: 18/11/2024 Accepted: 03/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
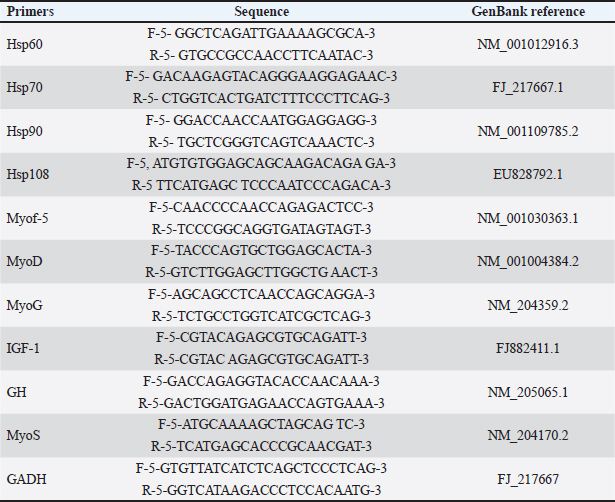
AbstractBackground: Native Saudi chickens have the genetic capacity to develop well and produce eggs in hot environments, allowing them to withstand heat stress. Thermal alteration is one of the most significant strategies to genetically improve muscle growth and growth performance during embryogenesis. Aim: This study assessed the impact of temperature on the hatchability, body weight (BW), and myofiber diameter of the pectoral and thigh muscles of native Saudi chickens. Methods: In this study, 300 viable eggs were equally divided and randomly divided into three groups. The control group was maintained at 37.8°C with 56% relative humidity (RH). The other two groups thermomodulated (TM1 and TM2) were subjected to temperature manipulation and kept at 39°C for 18 hours daily at 65% RH during the embryonic days (ED 4–7 and ED 7–10, respectively. Changes were identified by analyzing paraffin slices of the thigh and pectoral muscles. Results: The hatchability rate was somewhat higher in the TM1 and TM2 groups than in the control group, but there was no appreciable shift in the embryonic BW. The TM2 group had a significantly higher BW on post-hatch days 10 and 15 than the TM1 and control groups. Furthermore, compared with the TM1 and control groups, the TM2 group’s pectoral and thigh myofiber diameters were considerably more significant. Thus, without negatively impacting the hatchability rate or embryonic BW, the morphometric outcomes of the TM2 pectoral and thigh muscles during ED 7–10 increased the sensitivity of the pectoral and thigh muscles to heat stress. Gene expression analysis revealed that heat shock proteins were more highly expressed in the TM1 thigh muscle than in the control group, with heat shock proteins (Hsp)70 showing a higher expression than Hsp60, Hsp90, and Hsp108. In contrast, there was no appreciable change in the mRNA expression of Hsp60, Hsp70, and Hsp108 in the thigh muscle when TM1 was heated in the ED18 group. Conclusion: Thus, commercial breeders can use thermal manipulation to improve the muscle growth of local chickens in the Kingdom of Saudi Arabia. Keywords: Embryogenesis, Fiber diameter, Pectoral muscle, Thigh muscle, Thermal manipulation. IntroductionBecause of their tasty meat and eggs, resistance to various infectious diseases, and tolerance to extreme heat, native Saudi chickens are important socioeconomic elements to smallholder communities (Fathi et al., 2017). Acute heat stress exposure in hens results in a decline in growth performance, affecting the quantity and quality of meat produced, leading to a high death rate and significant financial losses (Nawaz et al., 2021). According to Yacoub and Fathi (2013), native Saudi chickens have the genetic capacity to develop well and produce eggs in hot environments, allowing them to withstand heat stress. Thermal change is one of the most important methods for genetically enhancing muscle growth and growth performance during embryogenesis. This low-cost approach aims to counteract the detrimental effects of heat stress in hot regions, such as Saudi Arabia (Loyau et al., 2013; Dasari et al., 2021; Ali et al., 2022; Dalab et al., 2022). Early embryogenesis is when poultry myofiber is first formed, and before a bird hatches, the total number of muscle fibers is nearly set (Wang et al., 2022). Early embryogenesis, which continues through adulthood until the ultimate mature size and strength obtained, is intimately linked to molecular and cellular mechanisms (myoblasts proliferation and myocytes differentiation) that govern the histogenesis of embryonic pectoral and thigh muscle fibers (Wang et al., 2022). The growth of posthatch muscle and the final quantity and quality of meat depends on muscle development during the embryonic stage (Wang et al., 2022). The hallmark of post-hatch muscle growth is an increase in myofiber size, which is associated with an increase in cytoplasmic volume but not with an increase in myofiber quantity (Mozdziak et al., 1994, 2000). Heat shock proteins (HSPs) are ubiquitous proteins present within the cellular or extracellular milieu. HSPs are primarily responsible for preserving the integrity and structure of cellular membranes under heat stress. Moreover, HSPs regulate some subsets of processes such as protein folding, degradation, aggregation, assembly, transportation of newborn proteins, and stabilization events within cellular compartments (Sharma et al., 2020). HSPs are mainly expressed under various stress conditions, such as heat, and their expression levels vary based on environmental conditions (Alagar Boopathy et al., 2022). To the best of our knowledge, no previous research has examined how heat modification timing affects hatchability and muscle fiber diameter in native Saudi chicken embryogenesis. The genetic mechanisms behind embryonic thermal manipulation and the gene expression of HSPs (Hsp60, Hsp70, Hsp90, and Hsp70) in the pectoral and thigh muscles are also revealed by morphometric examination of the myofiber diameter. Therefore, our research aimed to examine how early embryonic heat manipulation affects the fibers of the pectoral and thigh muscles to enhance body growth performance through modification of the myofiber diameter. To provide further insight into the molecular processes that underlie heat stress during embryogenesis, this study assessed the expression profile of HSP candidate genes in the pectoral and thigh muscles of native Saudi chicken. Thus, the findings of this study may help commercial breeders produce and enhance the growth efficiency of native Saudi chicken. Materials and MethodsPlan and incubation managementAll protocols regarding samples, experimental incubation, hatching management, and the conditions were approved by the King Faisal University Animal Care and Use Committee (KFU-ACUC). Three hundred viable eggs were provided from King Faisal University’s agricultural research station farms (from a 105-week-old flock) at Al-Hassa, Saudi Arabia. Broken, unsuitable, large, or atypical eggs (48 g < eggs < 55 g) were excluded. Next, the semi-commercial incubators (OVA-Easy 380 Advance Series II, Brinsea, Sandford, UK) incubated the fit eggs. Eggs were divided into three groups for incubation and treatment. The control group was incubated at 37.8°C with 56% relative humidity (RH). On the other hand, two groups were thermomodulated (TM) at particular embryonic days (ED) during extended EDs (21 days). The TM1 and TM2 groups were exposed to 39°C for 18 hours with 65% RH daily at EDs 4–7 and ED 7–10 during the extended EDs, respectively. Hatchability and hatchling managementThe hatchability percentage was measured in each group, and the results were compared between groups. After hatching, each hatchling was taken to the agriculture research station where the field study was conducted. With access to food and water on demand, the chicks were kept in a room with an initial temperature of (31°C ± 1°C) that was dropped by an average of 0.2°C–0.3°C every day. Morpho-metrical measurementMorphological measurements were carried out using an Image Processing and Analysis (ImageJ 1.52a) analyzer (Wayne Rasband, National Institutes of Health, USA, (http://imagej.nih.gov/ij). Morphometrical measurements were performed pre- and post-hatching. Five embryos from each group were collected, and the weights of the thigh and pectoral muscles were measured at ED7, ED10, and ED18 for each embryo. Moreover, five post-hatching post-hatchings from each group were humanly euthanized. Five pectoral and five thigh muscle samples were obtained from each group of chicks on days 1, 10, and 15. Histo-metrical examinationAt ED 18 and posthatching day 10, pectoral and thigh muscle samples measuring about 1 cm were taken from five chicks in each group. Tissue samples were treated with 4% paraformaldehyde overnight. Following these procedures, all of the trimmed samples were prepared for morphometric analysis: Using a tissue processor (Histokintte, Leica, Germany), the specimens were fixated, then dehydrated in increasing grades of alcohol, cleaned with xylene, and infiltrated by melted paraffin wax. Then, they were embedded in molten paraffin wax (Histokintte, Leica, Germany) at 56°C–59°C in a paraffin dispenser, and the plate was chilled to 6°C to solidify for further steps. Using a Leica rotary microtome, tissue samples were sectioned to a thickness of 5 μm. Afterwards, the sectioned tissues floated in a 41°C warm water bath for optimal tissue stretching. Finally, the tissues were placed on Marenfeld microscope slides and heated on a hot plate to complete drying. The tissue sections were stained with hematoxylin and eosin stain, following the protocol described by Bancroft and Stevens (1990). The dyed slides were inspected using a light microscope (Leit, Germany) after being mounted on coverslips with dibutyl phthalate polystyrene xylene. RNA isolation and semi-quantitative real-time reverse transcription polymerase chain reaction (RT-PCR)analysisRNA isolation The mRNA expression levels of HSPs during embryogenesis and the subsequent heat challenge (TC) at 35 days post-hatching were examined and assessed using semi-quantitative real-time RT-PCR. We used the PureZOL TM RNA isolation method (BIO-RAD, Catalog #732-6890, Hercules, CA). Total RNA was extracted from the homogenized pectoral and thigh muscles using a Bead Ruptor (24 Bead Mill Homogenizer, OMNI, USA). DNA contamination was removed using the Ambion DNase I kit. The RNA samples were tested for concentration and purity (260:280 nm absorbency) using a SynergyTM Mx Monochromator-Based Multi-Mode Microplate Reader (Bio-Tek. USA). Finally, using the iScriptc DNA synthesis kit (BIO-RAD, Catalog #170-8890, Hercules, CA) in a reaction mixture, reverse transcription was carried out on 2 μg of RNA to obtain cDNA. Primers synthesis For semi-quantitative real-time RT-PCR analysis, primer sets for the following genes were synthesized: HSPs60, HSPs70, HSPs90, HSPs108, Myof-5, MyoD, MyoG, MyoS, IGF-1, GH, and GADH. The primers and reference sequences for each gene are provided in Table 1. Relative quantification protocol A quantitative CFX96 TouchTM Real-Time qPCR analysis (BIO-RAD, Hercules, CA) was performed using a GoTaq® qPCR Master Mix kit (Promega, Madison, CA, USA). The reaction mixture was made up of 10 μl of the GoTaq® qPCR Master Mix, 2 μl of each of the forward and reverse primers pm/μl (Table 1), 2 μl of DNA sample, and 4 μl of nuclease-free water. The total volume of the reaction mixture was 20 μl. The cycling conditions were 22 min at 95°C, 40 cycles of 115 sat 95°C, 330 sat 60°C, and 220 sof final melting at 95°C. Duplicates from each DNA sample were analyzed, fluorescence emission was detected, and relative quantification was automatically calculated using the internal GADH housekeeping control to normalize the other transcripts’ threshold cycle values. Table 1. Primers for semiquantitative real-time RT-PCR analysis.
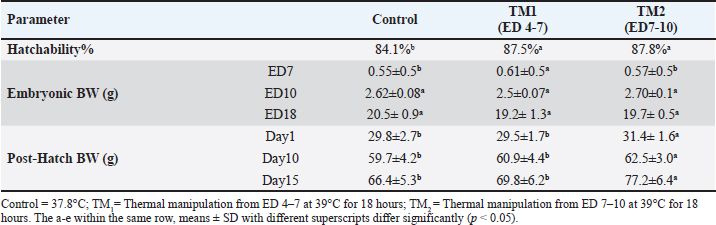
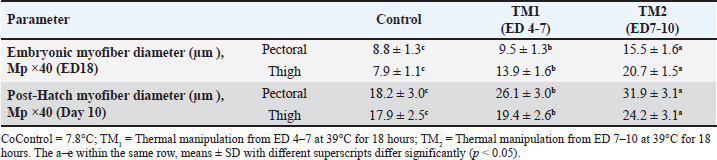
Statistical analysis The chi-square test was used to investigate hatchability. However, body weight (BW), morphometric examination of the fiber diameter of the pectoral and thigh muscles, and the mRNA gene expression of Hsp60, Hsp70, Hsp90, Hsp108, Myf-5, MyoD, MyoG, IGF-1, GH, and MyoS were expressed as means ± SE. Using IBM SPSS statistics 20 (IBM software, Chicago, USA), various parameters were examined for each treatment group using one-way ANOVA and all-pairs Bonferroni tests. When p < 0.05, a difference is deemed significant. Ethical approval All protocols about samples, experimental incubation, hatching management, and circumstances were approved by the KFU-ACUC. ResultsEffects of thermal manipulation on BW and hatchabilityTable 2 summarizes the impact of TM during embryogenesis on myofiber diameter, BW, and hatchability. Compared with the control group, thermal modification of TM1 and TM2 at ED4-7 and ED7-10 improved the hatchability rate. In addition, the TM groups showed improved embryonic BW in ED7 compared with the control group. Conversely, at ED10 and ED18, neither TM1 nor TM2 significantly affected embryonic BW. Compared with the control and TM1 groups, the TM2 group had considerably greater BW on posthatching days 1, 10, and 15. Impact of heat treatment on myofiber diameterThe myofiber cells of the pectoral and thigh muscles were analyzed in each group. The diameters of the myofiber in the embryonic pectoral and thigh muscles varied from 8.8–15.5 to 7.9–20.7 μm, respectively (Table 3). On the other hand, the myofiber diameters of the posthatched pectoral and thigh muscles varied from 18.2–31.9 to 17.9–24.2 μm, respectively (Table 3, Fig. 1). Furthermore, at both the embryonic and post-hatch ages, there were significant differences in pectoral myofiber diameters across all treatment groups, with TM2 exhibiting a more significant difference than TM1 and the control. Additionally, TM1 markedly increased pectoral myofiber diameters compared to the control at both the embryonic and post-hatch ages. The thigh myofiber measurement revealed significant variations in myofiber diameters between the TM1, TM2, and the control group, with TM2’s myofiber diameters showing greater significant differences than TM1’s and the control group (Table 3 and Fig. 2). At ED18, the comparative histometrical and histological results between the pectoral and thigh myofiber diameters showed that the thigh myofiber diameter was remarkably higher only in the TM1 and TM2 groups compared with the myofiber diameter of the pectoral muscle, with the highest value of the thigh myofiber diameter in the TM2 group (Figs. 1 and 2, Table 3). The comparative histometrical and histological results of post-hatch day 10 showed that the pectoral myofiber diameters were significantly higher in the TM1 and TM2 groups compared with the myofiber diameters of the thigh muscle in all treated groups, with the most significant increase in the pectoral myofiber diameter in the TM2 group (Figs. 1 and 2, Table 3). Table 2. The effects of TM on hatchability and BW.
Table 3. The effect of TM on myofiber diameter.
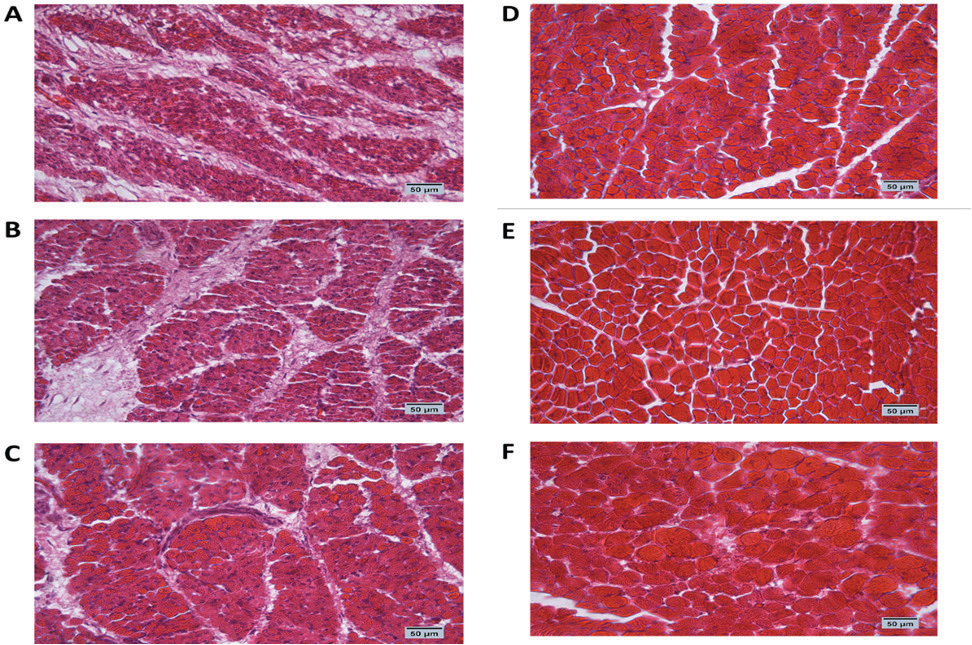
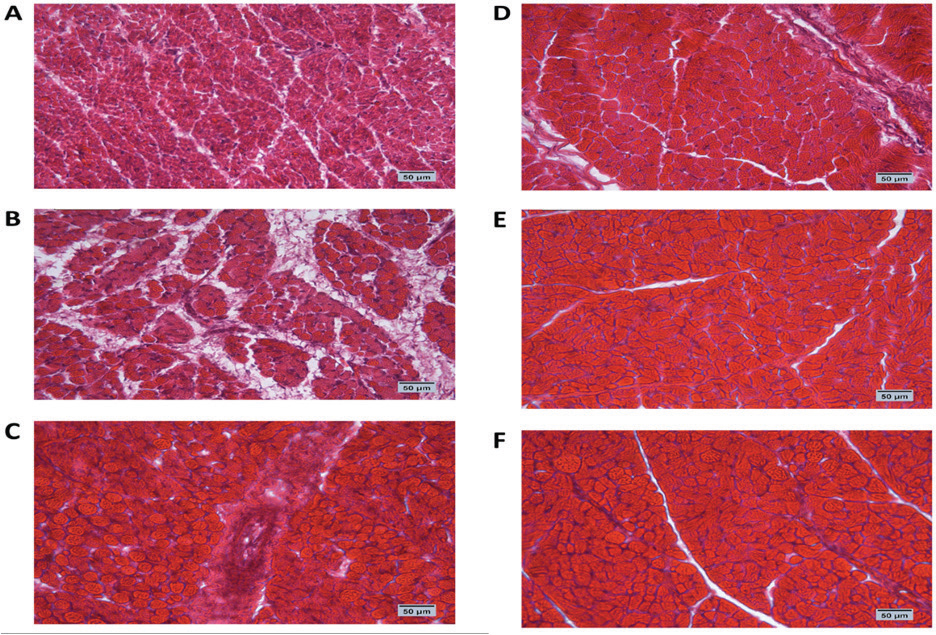
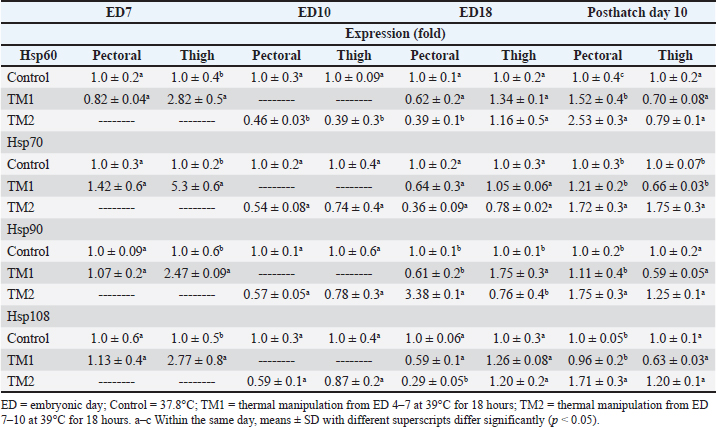
Fig. 1. Cross-section of pectoral muscle at ED18 and post-hatching at day 10 stained by H&E stain under magnification power ×40 showing differences in myofiber diameter by light microscopy. (A) Control at ED18=37.8°C; (B) TM1 at ED18 from ED 4–7 at 39°C for 18 hours; (C) TM2 at ED18 from ED 7–10 at 39°C for 18 hours. The three figures showed clear connective tissue formation that appeared as white fibers within the muscular tissues. (D) the control at post-hatch at day 10 at 37.8°C; (E) TM1 at post-hatch day 10 from ED 4–7 at 39°C for 18 hours; (F) TM2 at post-hatch day 10 from ED 7–10 at 39°C for 18 hours. The D, E, and F figures showed that the connective tissues were replaced with clear myocyte, indicating an increased mass of the muscular tissues. Impact of heat treatment on Hsp60, Hsp70, Hsp90, and Hsp108 mRNA expressionThe mRNA expression of HSPs (Hsp60, Hsp70, Hsp90, and Hsp70) in TM1 varied between the pectoral and thigh muscles due to heat manipulation (Table 4). Compared to the group’s pectoral muscle (across the EDs), the thigh mRNA gene expression of the genes in TM1 was noticeably higher. In addition, no statistically significant differences were observed in the mRNA expression of genes between the pectoral TM1 and control groups. In the thigh muscle, TM1 expression was significantly higher expression than the control group, with Hsp70 showing a higher expression level than Hsp60, Hsp90, and Hsp108. In contrast to the control, the results for ED10 showed that temperature modification had no appreciable effect on Hsp70, Hsp90, or Hsp108 in the TM2 pectoral and thigh muscles. The expression of Hsp60 was significantly reduced in the pectoral and thigh muscles of TM2 compared with the control. There was no detectable effect of heat treatment of TM1 in ED18 on the mRNA expression of Hsp60, Hsp70, and Hsp108 in thigh muscle. Furthermore, TM1 significantly upregulated Hsp90 expression in the thigh muscle compared with the control. The results also demonstrated that TM had no appreciable effect on the mRNA expression of Hsp60, Hsp70, Hsp90, and Hsp108 in the pectoral muscle of TM1 compared with the control. Additionally, there were no discernible changes in the mRNA gene expressions of Hsp60, Hsp70, Hsp90, and Hsp108 in the thigh muscle when TM of TM2 at ED18 was applied compared with the control. Furthermore, compared with the control, TM of TM2 at ED18 dramatically increased the expression of Hsp90 in the pectoral muscle alone. Table 4 illustrates the considerable decrease in the mRNA expression of Hsp60, Hsp70, and Hsp108 in the pectoral muscle of TM2 compared with the control. The results of posthatching analysis on day 10 showed that the pectoral muscle of TM2 had considerable mRNA expression of Hsp60, Hsp70, Hsp90, and Hsp108 compared with the control and TM1. Furthermore, compared with the control and TM1, TM2 has dramatically increased the expression of Hsp70 in the thigh muscle alone. Table 4 showed that there was no discernible variation in the mRNA gene expressions of Hsp60, Hsp90, and Hsp108 in either the pectoral or thigh muscle when compared with the control to TM1 or TM2.
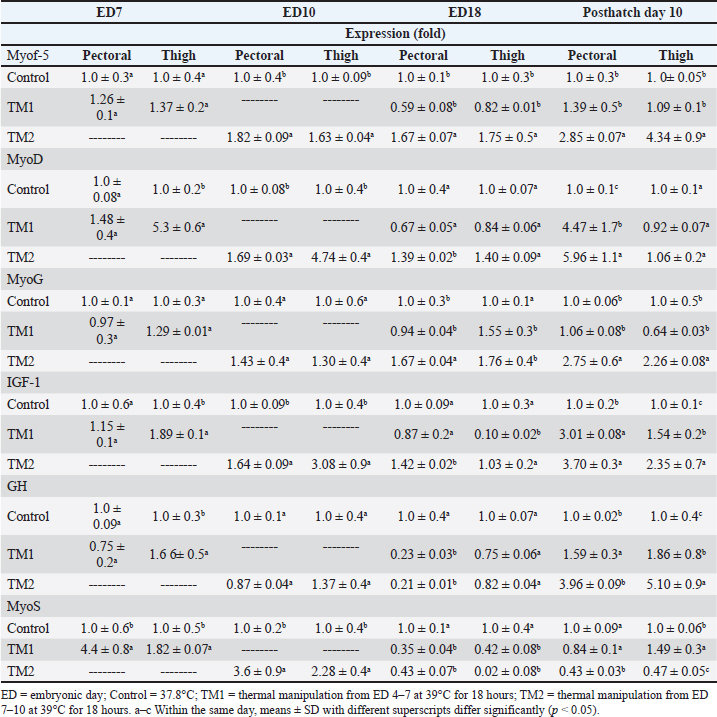
Fig. 2. Cross-section of thigh muscle at ED18 and post-hatching at day 10 stained by H&E stain under magnification power ×40 showing differences in myofiber diameter by light microscopy. (A) Control at ED18=37.8°C; (B) TM1 at ED18 from ED 4–7 at 39°C for 18 hours; (C) TM2 at ED18 from ED 7–10 at 39°C for 18 hours. The three figures showed clear connective tissue formation that appeared as white fibers within the muscular tissues. (D) the control at post-hatch at day 10 at 37.8°C; (E) TM1 at post-hatch day 10 from ED 4–7 at 39°C for 18 hours; (F) TM2 at post-hatch day 10 from ED 7–10 at 39°C for 18 hours. The D, E, and F figures showed that the connective tissues were replaced with clear myocyte, indicating an increased mass of the muscular tissues. Impact of heat treatment on Myf-5, MyoD, MyoG, IGF-1, GH, and MyoS mRNA gene expressionFor the pectoral muscle, the heat manipulation results in ED7 revealed that MyoS mRNA expression in TM1 was significantly higher than that in the control group. However, the mRNA expression of Myf-5, MyoD, MyoG, IGF-1, and GH in TM1 did not differ significantly from that in the control group (Table 5). In the thigh muscles, the MyoD, IGF-1, GH, and MyoS mRNA gene expressions in TM1 were significantly higher than those of the control group, whereas Myf-5 and MyoG mRNA gene expressions were not statistically different from those of the latter group. The results of ED10 showed that the mRNA expression of Myf-5, MyoD, IGF-1, and MyoS in TM2 was considerably higher than that in the control group in the pectoral and thigh muscles. GH and MyoG mRNA gene expression in the TM2 pectoral and thigh muscles did not differ significantly from that in the control. Heat treatment of TM2 increased Myf-5 and MyoG mRNA expression in the ED18 pectoral and thigh muscles compared with the control and TM1 muscles. Additionally, compared with the control group, the pectoral and thigh muscles of TM1 and TM2 exhibited significantly lower MyoS gene expression. Additionally, the results demonstrated that there was no discernible impact of TM on MyoD and IGF-1 mRNA expression in the pectoral and thigh muscles of TM1 and TM2, in contrast to the control group. When comparing the expression of GH in TM1 or TM2 with the control in the thigh muscle, Table 5 shows that there was no discernible change. Furthermore, compared with the control, the mRNA expression levels of GH in the pectoral muscle were significantly lower after TM of TM1 and TM2 at ED18. Myf-5, MyoD, MyoG, IGF-1, and GH mRNA gene expression was significantly higher in the TM2 pectoral and thigh muscles than in the control group, according to the results of post hatch day 10. Furthermore, compared with the control group, Table 5 showed that TM2 considerably reduces MyoS expression in the pectoral and thigh muscles. In addition, the TM1 data revealed a significant increase in the expression of MyoD and IGF-1 mRNA genes in pectoral muscle compared with the control. However, there was minimal difference between the control and TM1 thigh muscles in terms of Myf-5, MyoD, MyoG, IGF-1, GH, and MyoS mRNA expression. Table 4. Muscle Hsp60, Hsp70, Hsp90, and Hsp108 mRNA relative quantification duringembryogenesiss (ED7 and ED10) and posthatch day 10.
DiscussionOne of Saudi Arabia’s most promising sectors is the poultry business, and the kingdom consumes more chicken meat than other animal products. This might be due to the fact that chicken meat is more affordable than the meat of sheep and cattle and has higher nutrients, such as unsaturated fats (Hussein, 2018). Native Saudi chickens are important socioeconomic elements to smallholder communities because of their flavorful meat and eggs, resistance to a variety of infectious illnesses, and tolerance to extreme heat, (Fathi et al., 2017). However, with global climate change, high temperatures have a negative effect on broiler and native chicken productivity and create stressful conditions that lower body performance, increase the risk of heat-related illnesses, and increase mortality. These regions include Saudi Arabia and tropical and subtropical regions (Hsiao-Mei et al., 2016; Apalowo et al., 2024). For laying hens, the ideal temperature range is probably 19 to 22°C, while for developing broilers it is likely 18 to 22°C (Charles, 2002). Depending on the strain, feathering, diet, and production technique, heat stress may arise when a hen’s thermo requirement is not met (Apalowo et al., 2024). Thermal manipulation is one of the main strateimplementedat have been put out to combat the negative consequences of heat stress, particularly in with hot temperatures. This low-cost method genetically improves heat tolerance trait (Boonkum et al., 2024). In poultry raised for meat production, the main edible portions are concentrated in skeletal muscles; therefore, optimal muscle development is essential for good commercial production. Myogenesis is a complex process that can be divided into two phases: embryonic and postnatal muscle development (Liu et al., 2023). Therefore, the goal of the current investigation was to examine how storage temperature and storage time affected the amplification of biological specimens with either high or low levels of mRNA expression during embryogenesis. A previous study investigated how high heat manipulation during embryonic development affected a local chicken breed’s embryo weight, specific organ weights, hatchability, hatched body temperature, and some blood biochemical and hormonal changes (Badran et al., 2012). However, the processes of heat manipulation that manage heat stress during embryonic development after hatching are not well understood. Consequently, it has been suggested that high-temperature incubation toward the end of embryonic development may cause physiological alterations that could serve as epigenetic thermal adaptation (Badran et al., 2012). Previously, it was shown that applying the thermal manipulation approach to native and broiler chickens can boost their BW and muscular growth (Al-Rukibat et al., 2017; Dasari et al., 2021). Therefore, improving the genetic makeup of locally produced Saudi native chicken meat through heat tolerance and growth may lead to an earlier market age and increased input efficiency in commercial native chicken production, as well as an improvement in the growth rate and meat output. Table 5. Relative quantification of muscle Myf-5, MyoD, MyoG, IGF-1, GH, and MyoS mRNAlevels duringg embryogenesis and posthatch day 10.
Meat texture and tenderness resulting from the type of muscle structures, such as myofibrillar proteins, fiber diameter, and intramuscular connective tissue, may be influenced by heat stress (Warner et al., 2022). This study aimed to close information gaps by enhancing body growth performance during Saudi native chicken embryogenesis and examining the impact of heat manipulation on hatchability and body performance. This study focused on the effects of heat manipulation on the fiber diameters of the pectoral and thigh muscles. Prior research by Tazawa et al. (2004) demonstrated that variations from the ideal incubation temperature range of 37°C to 37.5°C may have an impact on embryo size, organ and skeleton growth, metabolic rate, physiological development, and success in hatching. It has been shown that embryonic growth and development are enhanced under high-temperature incubation (Yalcin and Siegel, 2003). On the other hand, Yalčın et al. (2005) discovered that when eggs were exposed to 39.6°C for 6 hours per day from ED10 to ED18 of incubation, the weights of the embryos were lower on ED18 and that the weights at hatch were either the same as or slightly lower than the controls. Our findings revealed that thermal manipulation of TM1 and TM2 at ED7-ED11 and ED11-ED15 resulted in an improvement in hatchability rate compared with the control, with no significant effects on embryonic BW at ED10 and ED18. On the other hand,compared witho the control and TM1, the TM2 group had considerably greater BW on posthatch days 1, 10, and 15. As a result, temperature modification during embryogenesis in native Saudi chickens was a safe procedure that had no negative effects on the BW or hatchability of the embryo. According to previous research, broilers’ absolute pectoral muscle weight at 42 days of age was higher in TM on ED16 to ED18, the time of satellite cell population development for 3 hours at 38.5°C or 39.5°C, than in controls. However, TM between ED8 and ED10 had no impact (Halevy et al., 2006; Collin et al., 2017; Ana Patrícia Alves Leão et al., 2024). Whereas fetal myoblasts were most prevalent in chicks between ED8 and ED12, embryonic myoblasts were most abundant in ED5 (Stockdale, 1992). During late embryogenesis (ED15 onwards), individual myofibers are enclosed by a basement membrane, and at this point, satellite cells may be distinguished based on their appearance and location (Ana Patrícia Alves Leão et al., 2024). Satellite cells, which are widely distributed in the skeletal muscle at hatch, are the primary source of myogenic precursors in the postnatal muscle (Marciano et al., 2021). Post-hatching, muscle development requires only an increase in myofiber size, not an increase in myofiber quantity (Remignon et al., 1995), which is linked to higher levels of DNA and protein (Wang et al., 2022). Consequently, a crucial aspect of post-hatch skeletal muscle development is DNA accumulation (Mohammadabadi et al., 2021). The number of satellite cells that undergo mitosis is responsible for the increased DNA content of myofiber (Goldring et al., 2002; Morgan and Partridge, 2003). The satellite cell population’s role in healthy skeletal muscle growth is to proliferate and then supply nuclei to the growing myofiber (Bazgir et al., 2017). The genetic machinery responsible for age-related increases in myofiber size is provided by satellite cell fusion. According to a previously published report, at least two stages of postnatal myofiber development occur in avian species (Gu et al., 2024). Early in life, during the initial phase of myofiber formation, high levels of satellite cell mitotic activity are observed. However, with age, the amount of satellite cell mitotic activity decreases, and the expansion of the cytoplasm around each nucleus is nearly the only mechanism by which muscle fibers expand (Mozdziak et al., 2000). Because the capacity of the muscle to reach its maximum genetic potential size may be governed by the application of heat manipulation during the early stages of embryonic development, our work focused on the potential triggers of satellite cell mitotic activity. In this study, the comparative histometrical and histological findings between the two thermally treated groups and the control group demonstrated the safety of thermal manipulation and its ability to increase myofiber diameter in both the thigh and pectoral muscles. Additionally, only in TM1 and TM2 are the diameters of the thigh myofiber substantially greater than those of the pectoral muscle. Our findings also demonstrated that native Saudi chickens respond effectively to heat stress during embryogenesis, maintaining their physical performance both before and after hatching. This observation is consistent with earlier research on birds (Wang et al., 2023). The present study examined the gene expression profiles of many HSPs as well as genes linked to muscle development. The levels of mRNA expression were measured and analyzed using semiquantitative real-time RT-PCR during development and the subsequent TC at 35 days post-hatching. HSPs were thus overexpressed in the TM1 thigh muscle compared with the control group, with Hsp70 expressing more than Hsp60, Hsp90, and Hsp108. In contrast, there was no appreciable change in the mRNA expression of Hsp60, Hsp70, and Hsp108 in the thigh muscle when TM1 was heated in the ED18 group. MyoS gene expression in TM1 was substantially higher than that in the control group, as demonstrated by heat manipulation in ED7. Myf-5, MyoD, MyoG, IGF-1, and GH mRNA gene expression in TM1 was not substantially different from that of the control pectoral muscle. Compared with the control group, the TM2 pectoral and thigh muscles displayed highly significant mRNA expressionn of Myf-5, MyoD, MyoG, IGF-1, and GH at posthatch day 10. Moreover, TM2 significantly decreased MyoS expression in the pectoral and thigh muscles compared with the control. Both the morphometric analysis and qRT-PCR analyses yielded consistent results. These findings are consistent with earlier studies on broiler chicks (Dalab et al., 2022). ConclusionFor the first time, we identified several key genes that may be responsible for the differences in thigh and pectoral muscle growth during hatchability and embryonic BW of indigenous chicken. The best protocol that commercial breeders in the Kingdom of Saudi Arabia may use to improve the muscle growth of native chickens without negatively impacting their hatchability rate and embryonic BW is to manipulate the temperature during ED 7–10 for 18 hours at 39°C. The results of this study might offer strategies for enhancing growth efficiency, which could increase the total amount of meat produced by native Saudi chickens. AcknowledgmentsThis work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, under the Annual Research Track (KFU242481). The authors also thank the staff of the University’s research station for their Assistance. Conflict of interestThe authors declare no conflict of interest. FundingNone. Authors’ contributionsThis work was conducted mainly by the author and an assistant to the staff of the university research station. Data availabilityAll data were provided in the manuscript. ReferencesAlagar Boopathy, L.R., Jacob-Tomas, S., Alecki, C. and Vera, M. 2022. Mechanisms tailoring the expression of heat shock proteins to proteostasis challenges. J. Biol. Chem. 298(5), 101796. Ali, A.M., Dalab, A.S., Althnaian, T.A., Alkhodair, K.M. and Al-Ramadan, S.Y. 2022. Molecular investigations of the effects of thermal manipulation during embryogenesis on muscle heat shock protein 70 and thermotolerance in broiler chickens. R. Bras. Zootec. 51, e20210011. Al-Rukibat, R.K., Al-Zghoul, M.B., Hananeh, W.M., Al-Natour, M.Q. and Abu-Basha, E.A. 2017. Thermal manipulation during late embryogenesis: effect on body weight and temperature, thyroid hormones, and differential white blood cell counts in broiler chickens. Poult. Sci. 96, 234–240. Ana Patrícia Alves Leão, A.P., Vinhas de Souza, A., Barbosa, D.R., Gomes da Silva, C.F., Alvarenga, R.R., Sousa de Araújo, I.C., Geraldo, A., Resende, C.O. and Zangeronimo, M.G. 2024. Thermal manipulation during the embryonic stage and the post-hatch characteristics of broiler chickens. Animals (Basel) 14(23), 3436. Apalowo, O.O., Ekunseitan, D.A. and Fasina, Y.O. 2024. Impact of heat stress on broiler chicken production. Poultry 3(2), 107–128; doi: 10.3390/poultry3020010 Badran, A.M., Desoky, A., Abou-Eita, E.M. and Stino, F. 2012. The epigenetic thermal adaptation of chickens during late embryonic development. Egypt. Poult. Sci. 32, 675–689. Bancroft, J.D. and Stevens, G.A. 1990. Theory and practice of histological techniques, 2nd ed. London, UK: Churchill Livingstone,. Bazgir, B., Fathi, R., Rezazadeh Valojerdi, M., Mozdziak, P. and Asgari, A. 2017. Satellite cell contribution to exercise-mediated muscle hypertrophy and repair. Cell 18, 473–484. Boonkum, W., Chankitisakul, V., Kananit, S. and Kenchaiwong, W. 2024. Heat stress effects on the genetics of growth traits in Thai native chicken (Pradu Hang dum). Anim. Biosci. 37(1), 16–27. Charles, D.R. 2002. Responses to the thermal environment. In Poultry environment problems, a guide to solutions. Eds., Charles, D.A. and Walker, A.W. Nottingham, UK: Nottingham University Press, 1-16. Collin, A., Berri, C., Tesseraud, S., Rodon, F.E., Skiba-Cassy, S., Crochet, S., Duclos, M.J., Rideau, N., Tona. K., Buyse, J., Bruggeman, V., Decuypere, E., Picard, M. and Yahav, S. 2017. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 86, 795–800. Dalab, A.S., Ali, A.M., Althnaian, T.A., Alkhodair, K.M. and Al-Ramadan, S.Y. 2022. Molecular and ultrastructural investigations of the effect of thermal manipulation during embryogenesis on pectoral and thigh muscle growth factors in broilers. J. Appl. Poult. Res. 31, 100188. Dasari, H. P., Desamsetti, S., Langodan, S., Viswanadhapalli, Y. and Hoteit, I. 2021. Analysis of outdoor thermal discomfort over the Kingdom of Saudi Arabia. Geohealth 5(6), e2020GH000370. Fathi, M.M., Al-Homidan, I., Motawei, M.I., Abou-Emera, O.K. and El-Zarei, M.F. 2017. Evaluation of genetic diversity of Saudi native chicken populations using microsatellite markers. Poult. Sci. 96, 530–536. Goldring, K., Partridge, T. and Watt, D. 2002. Muscle stem cells. J. Path. 197, 457–467. Gu, S., Huang, Q., Jie, Y., Sun, C., Wen, C. and Yang, N. 2024. Transcriptomic and epigenomic landscapes of muscle growth during the postnatal period of broilers. J. Anim. Sci. Biotechnol. 15, 91. Halevy, O., Yahav, S. and Rozenboim, I. 2006. Enhancement of meat production by environmental manipulations in embryos and young broilers. World’ Poult. Sci. J. 62, 485–497. Hsiao-Mei, L.D.L., Yan-Der, H., Tsung-Ping, H., Hsiu-Luan, C., Cheng-Yung, L. and Hsi-Hsun, K.H. 2016. Association of heat shock protein 70 gene polymorphisms with acute thermal tolerance, growth, and egg production traits of native chickens in Taiwan. Arch. Anim. Breed. 59, 173–181. Hussein, M. 2018. Poultry and products annual: Saudi Arabia. Washington, DC: USDA Foreign Agricultural Service, GAIN Report No. SA1810. Liu, L., Yin, L., Yuan, Y., Tang, Y., Lin, Z. and Liu, Y. 2023. Developmental characteristics of skeletal muscle during the embryonic stage in Chinese yellow quail (Coturnix japonica). Animals 13(14), 2317. Loyau, T., Berri, C., Bedrani, L., Metayer-Coustard, S., Praud, C., Duclos, M.J., Tesseraud, S., Rideau, N., Everaert, N., Yahav, S., Mignon-Grasteau, S. and Collin, A. 2013. Thermal manipulation of the embryo modifies the physiology and body composition of broiler chickens reared in floor pens without affecting breast meat processing quality. J. Anim. Sci. 91, 3674–3685. Marciano, C.M.M., Ibelli, A.M.G., Marchesi, J.A.P., de Oliveira Peixoto, J., Fernandes, L.T., Savoldi, I.R., Kamilla, B.C. and Mônica, C.L. 2021. Differential expression of myogenic and calciumsignaling–related genes in broilers affected with white striping. Front. Physiol. 12, 712464. Mohammadabadi, M., Bordbar, F., Jensen, J., Du, M. and Guo, W. 2021. Key genes regulating skeletal muscle development and growth in farm animals. Animals (Basel) 11(3), 835. Morgan, J.E. and Partridge, T.A. 2003. Muscle satellite cells. Int. J. Biochem. Cell Biol. 35, 1151–1156. Mozdziak, P., Pulvermacher, P. and Schultz, E. 2000. Unloading of juvenile muscle results in a reduced muscle size at week 9 after reloading. J. Appl. Physiol. 88, 158–164. Mozdziak, P.E., Schultz, E. and Cassens, R.G., 1994. Satellite cell mitotic activity in posthatch turkey skeletal muscle growth. Poult. Sci. 73, 547–555. Nawaz, A.H., Amoah, K., Leng, Q.Y., Zheng, J.H., Zhang, W.L. and Zhang, L. 2021. Poultry response to heat stress: physiological, metabolic, and genetic implications of meat production and quality, including strategies to improve broiler production in a warming world. Front. Vet. Sci. 8, 699081 Remignon, H., Gardahaut, M., Marche, G. and Ricard, F. 1995. Selection for rapid growth increases the number and size of muscle fibers without changing their typing in chickens. Muscle Res. Cell Motil. 16, 95–102. Sharma, A., Kumar, B.S., Dash, S., Singh, S. and Verma, R. 2020. Heat shock protein B1 expression is associated with age at sexual maturity in Rhode Island Red and Punjab Red layers under heat stress. Int. J. Biometeorol. 64, 1133–1143. Stockdale, F.E. 1992. Myogenic cell lineages. Dev. Biol. 154, 284–298. Tazawa, H., Chiba, Y., Khandoker, A., Dzialowski, E.M. and Burggren, W. 2004. Early development of thermoregulatory competence in chickens: responses of heart rate and oxygen uptake to altered ambient temperatures. Avian Poult. Biol. Rev. 15, 166–176. Wang, Y.H., Lin, J., Wang, J., Wu, S.G., Qiu, K., Zhang, H.J. and Qi, G.H. 2022. The role of incubation conditions in the regulation of muscle development and meat quality in poultry. Front. Physiol. 13, 495–498. Wang, Z., Tian, W., Wang, D., Guo, Y., Cheng, Z., Zhang, Y., Xinyan, L., Yihao, Z. Li, D., Li, Z., Jiang, R., Li, G., Tian, Y., Kang, X., Li, H., Dunn, I.C. and Liu, X. 2023. Comparative analyses of dynamic transcriptome profiles reveal key response genes and dominant isoforms for muscle development and growth in chicken. Genet. Sel. Evol. 55(1), 73. Warner, R.D., Wheeler, T.L., Ha, M., Li, X., Bekhit, A.E., Morton, J., Vaskoska, R., Dunshea, F.R., Liu, R., Purslow, P. and Zhang, W. 2022. Meat tenderness: advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 185, 108657. Yacoub, H.A. and Fathi, M.M. 2013. Phylogenetic analysis using the d-loop marker mtDNA of Saudi native chicken strains. Mitochondrial DNA 24, 538–551. Yalčın, S., Özkan, S., Cabuk, M., Buyse, J.J., Decuypere, E. and Siegel, P. 2005. Effects of pre- and postnatal conditioning on the induction of thermotolerance on body weight, physiological responses, and relative asymmetry of broilers originating from young and old breeder flocks. Poult. Sci. 84, 967–976. Yalcin, S. and Siegel, P. 2003. Exposure to cold or heat during incubation affects the developmental stability of broiler embryos. Poult. Sci. 82, 1388–1392. | ||
| How to Cite this Article |
| Pubmed Style Ibrahim Albokhadaim. Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Vet J. 2025; 15(4): 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 Web Style Ibrahim Albokhadaim. Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. https://www.openveterinaryjournal.com/?mno=229307 [Access: July 31, 2025]. doi:10.5455/OVJ.2025.v15.i4.7 AMA (American Medical Association) Style Ibrahim Albokhadaim. Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Vet J. 2025; 15(4): 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 Vancouver/ICMJE Style Ibrahim Albokhadaim. Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Vet J. (2025), [cited July 31, 2025]; 15(4): 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 Harvard Style Ibrahim Albokhadaim (2025) Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Vet J, 15 (4), 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 Turabian Style Ibrahim Albokhadaim. 2025. Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Veterinary Journal, 15 (4), 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 Chicago Style Ibrahim Albokhadaim. "Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles." Open Veterinary Journal 15 (2025), 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 MLA (The Modern Language Association) Style Ibrahim Albokhadaim. "Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles." Open Veterinary Journal 15.4 (2025), 1565-1575. Print. doi:10.5455/OVJ.2025.v15.i4.7 APA (American Psychological Association) Style Ibrahim Albokhadaim (2025) Saudi native chicken response to embryonic thermal manipulation: Comparative morphometric analysis of myofiber diameter of the pectoral and thigh muscles. Open Veterinary Journal, 15 (4), 1565-1575. doi:10.5455/OVJ.2025.v15.i4.7 |