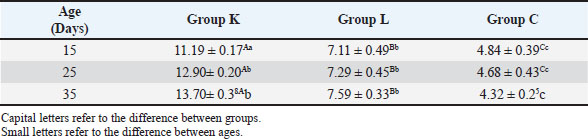| Research Article | ||
Open Vet. J.. 2025; 15(1): 388-394 Open Veterinary Journal, (2024), Vol. 15(1): 388-394 Research Article Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chickenRand Jaleel Edan* and Rawaa Saladdin JumaaDepartment of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Rand Jaleel Edan. Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: rand.eidan2203m [at] covm.uobaghdad.edu.iq Submitted: 03/11/2024 Accepted: 29/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
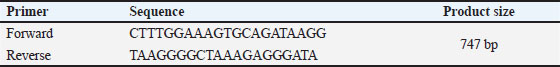
AbstractBackground: Newcastle disease virus is a virus that causes a significant economic impact on the poultry industry and is primarily controlled through vaccination. Most of the vaccinations with the LaSota strain are either live attenuated or inactivated vaccines, both of which are from the Lentogenic pathotype. Aim: This study aimed to investigate the influence of vaccine type and genetic variations on Chicken Interleukin-4 (ChIL4) activation. Methods: Three groups were examined: Group K received the killed vaccine, Group L received the live vaccine, and Group C served as the control. Blood samples were collected on days 15, 25, and 35 post-vaccinations to assess ChIL4 levels using Enzyme-Linked Immunosorbent Assay and for molecular analysis using polymerase chain reaction. Results: Group K showed a gradual increase in ChIL4 levels from 11.19 ± 0.17 to 13.70 ± 0.38, while Group L exhibited an initial increase on day 15 followed by stabilization on days 25 and 35. In contrast, ChIL4 levels in Group C declined over time from 4.84 ± 0.39 to 4.32 ± 0.25. Molecular analysis revealed four genetic variations of the single nucleotide polymorphism (SNPs) type at locations (3044, 3132, 3261, 3499) bp, with the third SNP at location (3261 bp) resulting in an amino acid change from valine to isoleucine. Analysis indicated that variants for most of these SNPs occur more likely in Group K compared to the other two groups. Conclusion: These findings suggest that genetic variations, particularly SNPs, may play a significant role in ChIL4 activation, potentially impacting vaccine efficacy and immune response. Keywords: Single nucleotide polymorphism, Cytokine, PCR technique. IntroductionNewcastle disease (ND) is an acute and highly contagious viral disease with elevated pathogenicity that affects the respiratory, digestive, and nervous systems of poultry at all ages, resulting in significant consequences for the economy (Al-Azawy et al., 2019). The Newcastle disease virus (NDV) is avian orthoavulavirus-1 (also known as avian paramyxovirus serotype-1), which is a member of the paramyxoviridae virus family and the Avulavirinae subfamily belonging to the Mononegavirales order. The viruses of this subfamily have been isolated from a variety of bird species (Gogoi et al., 2015; Ahmed and Odisho, 2019; Hassan et al., 2020). NDV is classified into five pathotypes based on the strain’s pathogenicity: Asymptomatic enteric strains include lentogenic, mesogenic, viscerotropic, and neurotropic velogenic strains. An infection with the velogenic NDV pathotype can result in neurological symptoms and significant mortality in chickens (Ulaiwi, 2023). The virus strain, as well as flock management, concomitant illnesses, and acquired immunity from previous paramyxovirus infections, all influence clinical symptoms (Ahmed et al., 2018). The viral form of NDV is pleomorphic. It is an enveloped, single-stranded, non-segmented negative-sense RNA virus with a length of about 15 kb. The genome encoded six structural proteins, including large RNA-dependent polymerase protein (L), phosphoprotein (P), fusion protein (F), matrix protein (M), nucleocapsid protein, and hemagglutinin-neuraminidase, as well as two additional non-structural proteins (W and V) that were produced in the P gene during mRNA transcription (Kapczynski et al., 2013; Al-Zuhariy et al., 2017). Several direct and indirect diagnostic procedures are used to identify NDV, including the hemagglutination inhibition test, serum neutralization test, Enzyme-Linked Immunosorbent Assay (ELISA) test, and polymerase chain reaction (PCR) (Al-Amri et al., 2015; Shauqi and Omran, 2019). In general, PCR is a powerful tool for both direct and indirect diagnosis of viruses (Jumaa et al., 2020; Jbr and Jumaa, 2024). Poultry flocks inoculated against NDV have a strong defense against pathogenic strains. It is well known that live attenuated vaccines produce an average to moderate immune response, are inexpensive, and are simple to deliver. ND vaccination has been approved for use in a number of countries throughout the world, and it is available in live, inactivated, and attenuated forms. Vaccination with LaSota strain is usually of Lentogenic pathotype (live attenuated or inactivated) (Mayers et al., 2017). Chicken Interleukin-4 (ChIL-4) is a cytokine that is a strong immune regulator released largely by mast cells, activated Th2 cells, eosinophils, and basophils. It is a critical regulator of humoral and adaptive immunity when faced with extracellular pathogens and helminth parasite infections (Gadani et al., 2012; Dai et al., 2018). It has been shown to be the first Cytokine produced by mast cells during an immune response (McLeod et al., 2015). ChIL-4 plays a role in type 2 immune response, along with the other signature cytokines IL-5, IL-9, and IL-13, and in the development of Allergic reactions (Sahoo et al., 2016). Materials and MethodsExperimental animals and designOne-day-old chicks (Gallus gallus domesticus) were kept in the animal houseCollage of Veterinary MedicineUniversity of Baghdad for the duration of the experiment in a disinfected room. The room was divided into sections to accommodate the three groups. It was then fumigated with the floor bedding using an environmental disinfectant. The temperature was maintained at 33°C for the first 2–3 days and then was stabilized at 36°C using heaters and air fans. Sixty chicks were divided into three groups with 20 chicks in each one: Group 1 (K) was vaccinated Intramuscularly with inactivated Newcastle vaccine La Sota strain on the second day of the experiment. Group 2 (L) was vaccinated via ocular installation with live attenuated Newcastle vaccineLa Sota strain on days 10, 20, and 30. Group 3 (C) served as a control group. Whole blood and serum samples were collected from all groups on days 15, 25, and 35 of the experiment to measure ChIL-4 levels and for molecular analysis. Enzyme-linked immunosorbent assayThe YL biont sandwich ELISA kit was used to measure the level of ChIL4 in serum. PrimersThe primer used in this study for ChIL-4 was designed according to The National Center for Biotechnology Information. as shown in Table 1. DNA extractionThe Monarch® Genomic DNA Purification kit was used to extract DNA from frozen whole blood. Molecular detectionThe reaction was done under the optimal touchdown PCR conditions for amplification of the ChIL-4 gene as demonstrated in Table 2. Each PCR cycle involved a progressive decrease in the annealing temperature. This approach was created in response to the need for greater primer binding specificity in clinical research. This decreases the amount of off-target primer binding since their rigid binding requirements only allow them to attach to exactly complementary areas on the target DNA (McDonald et al., 2024): SequencingThe PCR product from each sample has been sent to Macrogen, Korea, for sequencing using the Sanger method. Analysis of FASTA sequence files was performed using Geneious Prime software and aligned to RefSeq of ChIL-4 gene with accession number (NC_052544). Table 1. Primer design for ChIL-4 gene.
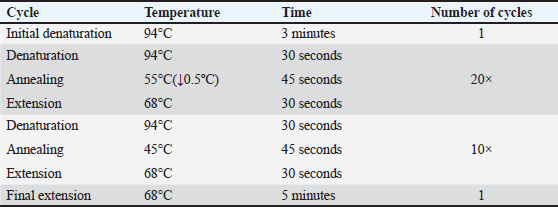
Table 2. PCR conditions for amplification of the ChIL-4 gene.
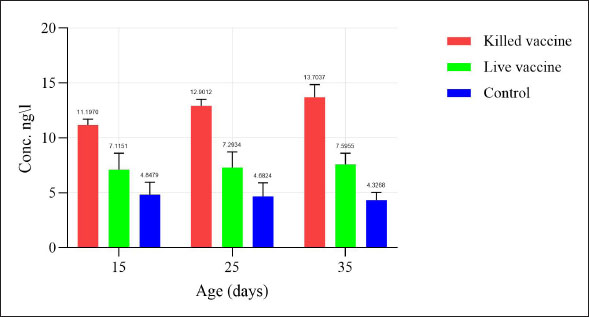
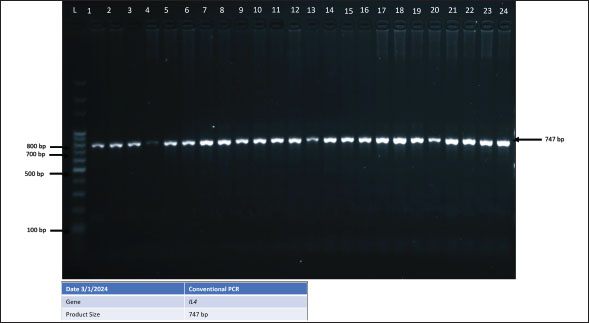
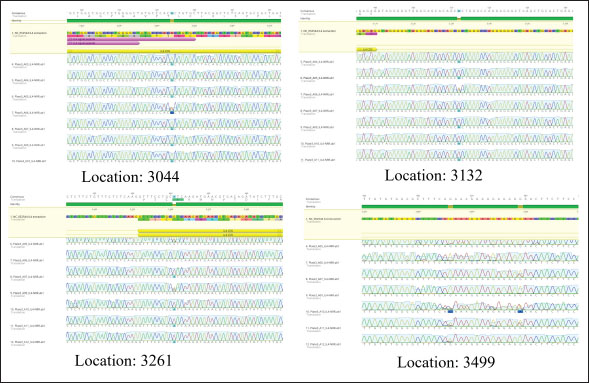
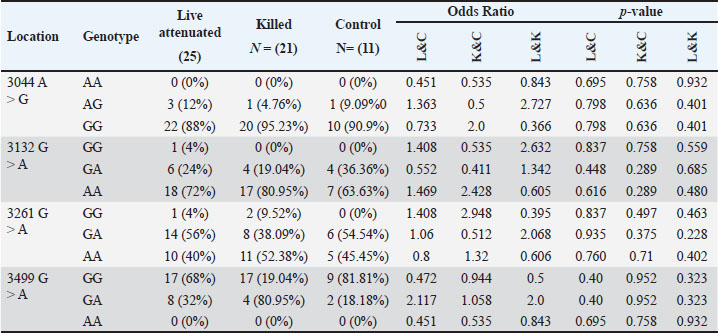
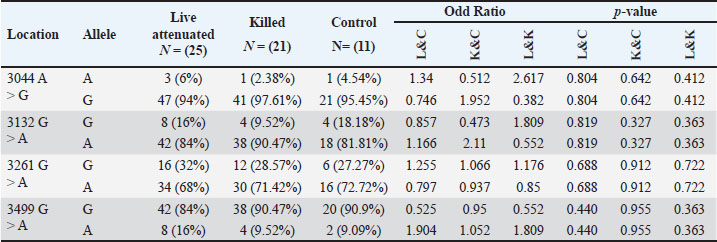
Statistical analysisThe GraphPad Prism software version 10.2.2 (San Diego, CA, United States) was used to statistically analyze the obtained data by Two-way ANOVA. Whenever ANOVA results were significant, post hoc analysis was performed using Tukey’s test. Number of animals represented by (n). Data are presented as mean ± standard error. Statistically significant was accepted when (p < 0.05) (Kim, 2014). Odds ratios were calculated using a free online calculator (MedCalc). Ethical approvalThe Experimental Design and Protocols used in the Current Study were reviewed and approved in accordance with the Animal Welfare Ethical. Measures by the Scientific Committee of the Department of Veterinary Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq (p.o.3052024212). ResultsChIL4 levelsThe results demonstrated in Table 3 and Figure 1 indicated Group (K) had a gradual increase in ChIL-4 levels from 11.19 ± 0.17 on day 15 to 13.70 ± 0.38 on day 35. while group L exhibited a significant increase on day 15, this increase plateaued during subsequent observations, yet it still had higher levels than group C, which exhibited a significant decrease (p ˂ 0.05), with the lowest among the three groups being on day 35 at 4.32 ± 0.25. Molecular detectionGel electrophoresis showed bands of amplified DNA for the ChIL-4 gene as exhibited in Figure 2: SequencingSequence analysis indicated the presence of four genetic variants of the single-nucleotide polymorphism (SNPs) type (Fig. 3) within the area that was amplified. These variants were detected at locations 3044, 3132, 3261, and 3499 bp. Three of these are in the non-coding region, while site 3261 bp was in a coding region where in the instances where a homozygous mutant was demonstrated, the amino acid was changed from valine (Val) to isoleucine (Ile). Genotypically, Group K indicated the highest percentage of homozygous mutations at locations 3044, 3132, and 3261 bp. While Group L had the highest percentage of heterozygous mutations at locations 3044 and 3261 bp. The control group had the highest heterozygous mutant at 3132 bp and the highest homozygous wild at 3499 bp as shown in Table 4. The allele frequency in Group K had the highest percentage of mutant alleles at sites 3044 and 3132 bp. Group L had the highest percentage at site 3499 bp and the highest wild allele at site 3261 bp. The control group had the highest mutant allele at site 3261 bp and the highest wild allele at site 3132 bp which is demonstrated in Table 5. Sequencing results indicated that the alteration in the genotype in the first three locations (3044 A>G, 3132 G>A, 3261 G>A) occurred more likely in Group K compared with the other two groups, as shown in the odds ratio values in Table 4 this alteration in genotype resulted in an increase in the levels of Interleukin-4 that is produced. The last location (3499 G>A) indicated that the increase in Interleukin-4 happens more likely in group K than in group L (OR=0.843). The allele alteration at the first two locations (3044 A>G, 3132 G>A) highlighted similar results to the alteration in the genotype. Table 3. Mean ± S.E of ChIL4 titer of serum in ELISA test.
Fig. 1. ChIL4 levels of serum in ELISA test.
Fig. 2. PCR gel electrophoresis bands of ChIL4 gene. Amplification (747 bp).
Fig. 3. Genetic variants at multiple locations within the amplified area of the ChIL4 gene. Table 4. Genotype analysis at different locations in ChIL4 gene for the three groups.
Table 5. Allele frequencies at different locations in ChIL4 gene for the three groups.
DiscussionVaccination and biosecurity are employed to manage the disease. Although immunization does not prevent the virus from replicating and shedding, it can protect against some clinical symptoms (Al-Zuhariy, 2023). The most widely used ND vaccines are live vaccination viruses developed with strains identified in the 1940s and 1960s. The LaSota, B1, and VG/GA vaccines were developed using viruses that existed in poultry. All of these types of viruses are genotype II and have a high genetic and antigenic similarity (>98% nucleotide identity). LaSota vaccines possessed the strongest tropism and replication capacity in naïve hens, leading to higher levels of neutralizing antibodies than other strains (Dimitrov et al., 2017). One limitation of inactivated ND vaccines lies in the fact that they require a withdrawal period before vaccinated birds may be processed for human food, and each vaccine must be administered individually via subcutaneous or intramuscular injection. Birds injected with inactivated vaccines have larger humoral antibody levels, but they do not generate a significant cell-mediated immune response (Schijns et al., 2013). This is exhibited in The ChIL-4 levels measured by sandwich ELISA, where group K scored the highest among the three groups. Dai et al. (2018) considered ChIL-4 to be a key biomarker of humoral immunity. Therefore, through measurement of its level using ELISA, it works as an indicator of the functionality of the Inactivated vaccine as demonstrated in the ELISA results of the three groups. None of the genetic variants that were detected were registered in the European variant archive suggesting that these mutations are novel, all though these changes have shown to be statistically non-significant. When comparing the ELISA results with the genetic variants that were detected, a possible link can be established between ChIL-4 levels and the mutation in the gene itself. Both the percentage of homozygous mutation in three of the four locations and ChIL-4 levels were the highest in Group K, this can be explained as the mutation resulting in the activation of ChIL-4. Thus, increasing the effectiveness of the vaccine. In a study done by Dang et al. (2020), it was found that the change from Val to Ile in anti-Müllerian hormone might not be an important influence on egg production. The Val and Ile diverge by only one methyl group. Without structural comparison, it would be very hard to recognize that this minor modification between two hydrophobic residues drastically altered a protein’s ligand preference (Yuan et al., 2010). Non-coding area mutations that disrupt non-coding components have been linked to severe disease, as confirmed by numerous methods. This includes influencing splicing, transcription, translation, RNA processing, stability, and chromatin interactions (Ellingford et al., 2022). In comparison, studies on genetic variation in combination with vaccines discuss their impact on the immune response and vaccine effectiveness. These alterations are shown to be of the SNPs type, which are genetic variations that can affect the function or structure of a protein (Abdullah and Abed, 2024). In a study done by Xie et al. (2016) on HBV vaccination, it was concluded that minor alleles found in non-coding regions had an impact on the immune response following the vaccination in humans. These findings align with (Wen et al., 2021) in their study on Influenza vaccination, which that demonstrated people with a mutant genotype had a significantly lower risk of poor vaccine responsiveness. A comparable conclusion was reached by Luo et al. (2022) in their investigation of novel Coronavirus mutation, stating that mutations in the S protein receptor binding domain of mutant strains can lead to immunological escape of the virus. Thus, impacting the effectiveness of the commercially available vaccines. In (Gao et al., 2024), an engineered vaccine with a mutation in the VP5 protein of Infectious bursal disease virus (IBDV) was found to reduce the mortality of host cells and limit IBDV proliferation early after infection, postponing cell death. These studies, in combination with the findings of this study, may suggest a profound effect on gene regulation and immune responses. This underscores the necessity for additional research into the impact of genetic variations on vaccine efficacy in chickens. Integrating genomic data with vaccine strategies could lead to the development of more effective, targeted vaccines that account for both humoral and cell-mediated immune responses. These strategies might assist in overcoming present obstacles, such as the requirement for customized inactivated vaccine administration, while simultaneously tackling the problem presented by new NDV variations. ConclusionIn conclusion, there is a noticeable effect caused by genotyping and allele variations on ChIL4 activation, which is more obvious with inactivated vaccination than with live attenuated vaccine, and even less so with non-vaccinated chickens. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingNone. Authors’ contributionsContributions from each author were equal. ReferencesAbdullah, W.L. and Abed, R.M. 2024. Gene expression and single nucleotide polymorphism (rs1140713) of microRNA-126. Iraqi J. Agric. Sci. 55(Special), 1–11; doi:10.36103/ijas.v55iSpecial.1880 Ahmed, A.I. and Odisho, S.M. 2018. Isolation identification and pathotyping of newcastle disease viruses form naturally infected chickens in Iraqi Kurdistan region. Iraqi J. Agric. Sci. 49(1), 132–141; doi:10.36103/ijas.v49i1.216 Ahmed, A.I., Odisho, S.M. and Al-Gafari, R.N. 2018. Comparison of the immune response between locally manufactured and commercial inactivated newcastle disease virus vaccines in a challenge trial with field isolated newcastle disease virus. Iraqi J. Vet. Med. 42(1), 46–51; doi:10.30539/iraqijvm.v42i1.30 Al-Amri, S.A.H., Odisho, S.M. and Ibrahem, O.M.S. 2015. In vitro antiviral potential of ocimum basilicum and olea europaea leaves extract against newcastle disease virus of poultry. Iraqi J. Vet. Med. 39(1), 94–99; doi:10.30539/iraqijvm.v39i1.204 Al-Azawy, A.K., Al-Ajeeli, K.S. and Ismail, A. 2019. Isolation and identification of wild isolate of newcastle disease virus from broiler farm in diyala province: virological and histopathological study. Iraqi J. Vet. Med. 42(2), 41–49; doi:10.30539/iraqijvm.v42i2.286 Al-Zuhariy, M.T. 2023. Evalution of the best vaccinal routes against newcastle in the production stage of laying hens. Iraqi J. Agric. Sci. 54(3), 748–754; doi:10.36103/ijas.v54i3.1757. AL-Zuhariy, M.T.A., Abdulmaged, S.H., Rabee, R.H.S. and Al-Baldawi, A.A.A. 2017. Isolation and identification of the newcastle disease virus from field outbreaks in broiler and layer flocks in iraq: mushtaq tb al-zuhariy1; sahar hamdi abdulmaged1; raed hs rabee2 and amer aa al-baldawi3. Iraqi J. Vet. Med. 41(1), 23–27; doi:10.30539/iraqijvm.v41i1.73 Dai, H., Jh, C., Shen, Y., Xh, Z., Zz, X., Chen, X. and Xa, J. 2018. Production of monoclonal antibodies against chicken interleukin-4 and development of a capture enzyme-linked immunosorbent assay. Pak. Vet. J. 38, 286–290; doi:10.29261/pakvetj/2018.066 Dang, L., Liu, R., Zhao, W., Zhou, W., Min, Y. and Wang, Z. 2020. Investigating structural impact of a valine to isoleucine substitution on anti-müllerian hormone in silico and genetic association of the variant and amh expression with egg production in chickens. J. Integr. Agric. 19(6), 1635–1643; doi:10.1016/S2095-3119(20)63176-8 Dimitrov, K.M., Afonso, C.L., Yu, Q. and Miller, P. J. 2017. Newcastle disease vaccines—a solved problem or a continuous challenge? Vet. Microbiol. 206, 126–136; doi:10.1016/j.vetmic.2016.12.019 Ellingford, J.M., Ahn, J.W., Bagnall, R.D., Baralle, D., Barton, S., Campbell, C., Downes, K., Ellard, S., Duff-Farrier, C., FitzPatrick, D.R. and Greally, J.M. 2022. Recommendations for clinical interpretation of variants found in non-coding regions of the genome. Genome Med. 14(1), 73; doi:10.1186/s13073-022-01073-3 Gadani, S.P., Cronk, J.C., Norris, G.T. and Kipnis, J. 2012. IL-4 in the brain: a cytokine to remember. J. Immunol. 189(9), 4213–4219; doi:10.4049/jimmunol.1202246 Gao, H., Zhang, S., Chang, H., Guo, Y., Li, Z., Wang, Y., Gao, L., Li, X., Cao, H. and Zheng, S.J. 2024. Generation of a novel attenuated IBDV vaccine strain by mutation of critical amino acids in IBDV VP5. Vaccine 42(24), 126081; doi:10.1016/j.vaccine.2024.06.048 Gogoi, P., Ganar, K. and Kumar, S. 2015. Avian paramyxovirus: a brief review. Transbound. Emerg. Dis. 64(2), 279–297; doi:10.1111/tbed.12355 Hassan, S.A.H., Allawe, A.B. and Al-Shammari, A.M. 2020. In vitro oncolytic activity of non-virulent newcastle disease virus lasota strain against mouse mammary adenocarcinoma. Iraqi J. Sci. 61(2), 285–294; doi:10.24996/ijs.2020.61.2.5 Jbr, A. and Jumaa, R. 2024. Sequencing analysis of the n gene of canine distemper virus from infected dogs in baghdad city. Iraqi J. Vet. Med. 48(1), 41–47; doi:10.30539/n4wtde42 Jumaa, R.S., Allawi, A.B. and Jabbar, R.N. 2020. Genetic analysis of field isolates of infectious bursal disease virus in iraqi farms. Iraqi J. Vet. Med. 44(1), 18–28; doi:10.30539/ijvm.v44i1.931 Kapczynski, D.R., Afonso, C.L. and Miller, P.J. 2013. Immune responses of poultry to newcastle disease virus. Dev. Comp. Immunol. 41(3), 447–453; doi:10.1016/j.dci.2013.04.012 Kim, H.Y. 2014. Analysis of variance (ANOVA) comparing means of more than two groups. Restor. Dent. Endod. 39(1), 74–77; doi:10.5395/rde.2014.39.1.74 Luo, W., Wu, X., Wang, W., Yu, J., Chen, Q., Zhou, X., Huang, X., Pan, H., Liu, Z., Gao, Y. and He, J. 2022. Novel coronavirus mutations: vaccine development and challenges. Microb. Pathog. 173, 105828; doi:10.1016/j.micpath.2022.105828 Mayers, J., Mansfield, K.L. and Brown, I.H. 2017. The role of vaccination in risk mitigation and control of newcastle disease in poultry. Vaccine 35(44), 5974–5980; doi:10.1016/j.vaccine.2017.09.008 McDonald, C., Taylor, D. and Linacre, A. 2024. PCR in forensic science: a critical review. Genes 15(4), 438; doi:10.3390/genes15040438 McLeod, J.J., Baker, B. and Ryan, J.J. 2015. Mast cell production and response to IL-4 and IL-13. Cytokine 75(1), 57–61; doi:10.1016/j.cyto.2015.05.019 Sahoo, A., Wali, S. and Nurieva, R. 2016. T helper 2 and t follicular helper cells: regulation and function of interleukin-4. Cytokine Growth Factor Rev. 30, 29–37; doi:10.1016/j.cytogfr.2016.03.011 Schijns, V.E.J.C., van de Zande, S., Lupiani, B. and Reddy, S.M. 2013. Practical aspects of poultry vaccination. In Avian immunology. Eds., Schat, K.A., Kaspers, B. and Kaiser, P. Amsterdam, The Netherlands: Elsevier Science, pp: 345–362. Shauqi, H.H. and Omran, H.A. 2019. The immune response of broiler chickens fed diets supplemented with propolis and digestarom under heat stress condition. Iraqi J. Vet. Med. 42(2), 33–40; doi:10.30539/iraqijvm.v42i2.283 Ulaiwi, A.H. 2023. Diva molecular detection of suspected case of newcastle and encephalomyelitis disease in layers. Iraqi J. Agric. Sci. 54(3), 890–895; doi:10.36103/ijas.v54i3.1778 Wen, S., Wei, H., Liao, Q., Li, M., Zhong, S., Cheng, Y., Huang, W., Wang, D. and Shu, Y. 2021. Identification of two novel candidate genetic variants associated with the responsiveness to influenza vaccination. Front. Immunol. 12, 664024; doi:10.3389/fimmu.2021.664024 Xie, B., Zhang, P., Liu, M., Zeng, W., Yang, J. and Liu, H. 2016. Deltex1 polymorphisms are associated with hepatitis B vaccination non-response in Southwest China. PLoS One 11(2), e0149199; doi:10.1371/journal.pone.0149199 Yuan, X., Yin, P., Hao, Q., Yan, C., Wang, J. and Yan, N. 2010. Single amino acid alteration between valine and isoleucine determines the distinct pyrabactin selectivity by PYL1 and PYL2. J. Biol. Chem. 285(37), 28953–28958; doi:10.1074/jbc. M110.160192 | ||
| How to Cite this Article |
| Pubmed Style Edan RJ, Jumaa RS. Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Vet. J.. 2025; 15(1): 388-394. doi:10.5455/OVJ.2025.v15.i1.34 Web Style Edan RJ, Jumaa RS. Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. https://www.openveterinaryjournal.com/?mno=227215 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.34 AMA (American Medical Association) Style Edan RJ, Jumaa RS. Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Vet. J.. 2025; 15(1): 388-394. doi:10.5455/OVJ.2025.v15.i1.34 Vancouver/ICMJE Style Edan RJ, Jumaa RS. Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 388-394. doi:10.5455/OVJ.2025.v15.i1.34 Harvard Style Edan, R. J. & Jumaa, . R. S. (2025) Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Vet. J., 15 (1), 388-394. doi:10.5455/OVJ.2025.v15.i1.34 Turabian Style Edan, Rand Jaleel, and Rawaa Saladdin Jumaa. 2025. Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Veterinary Journal, 15 (1), 388-394. doi:10.5455/OVJ.2025.v15.i1.34 Chicago Style Edan, Rand Jaleel, and Rawaa Saladdin Jumaa. "Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken." Open Veterinary Journal 15 (2025), 388-394. doi:10.5455/OVJ.2025.v15.i1.34 MLA (The Modern Language Association) Style Edan, Rand Jaleel, and Rawaa Saladdin Jumaa. "Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken." Open Veterinary Journal 15.1 (2025), 388-394. Print. doi:10.5455/OVJ.2025.v15.i1.34 APA (American Psychological Association) Style Edan, R. J. & Jumaa, . R. S. (2025) Impact of Newcastle disease virus vaccines and genetic variations on interleukin-4 activation in broiler chicken. Open Veterinary Journal, 15 (1), 388-394. doi:10.5455/OVJ.2025.v15.i1.34 |