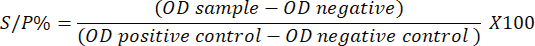| Research Article | ||
Open Vet. J.. 2025; 15(4): 1645-1653 Open Veterinary Journal, (2025), Vol. 15(4): 1645-1653 Research Article Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, IraqNuha Qasim Mohammed1* and Noaman Naji A’aiz21Research Unite for Zoonotic Disease, Faculity of Veterinary Medicine, AL-Qadisiyah University, Al Diwaniyah, Iraq 2Department of Microbiology, Faculity of Veterinary Medicine, AL-Qadisiyah University, Al Diwaniyah, Iraq *Corresponding Author: Nuha Qasim Mohammed. Research Unite for Zoonotic Disease, Faculity of Veterinary Medicine, AL-Qadisiyah University, Al Diwaniyah, Iraq. Email: Nuha.Allban [at] qu.edu.iq Submitted: 04/10/2024 Accepted: 16/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
AbstractBackground: Neospora caninum is one of the protozoa that have been identified in recent decades and plays an important role in causing disease in cattle, leading to abortions in pregnant animals. Therefore, to highlight the epidemiology of this parasite and the extent of genetic changes in local strains, this study was conducted. Aim: The goal of this study is to find out the infection of N. caninum, the commonly causing abortion in cattle in the Iraqi region of Al-Diwaniyah. Methods: Two hundred samples were collected from slaughterhouses and butcher shops. Indirect enzyme-linked immunosorbent assay antibodies had become used to perform the serological test involving the identification of N. caninum antibodies within 200 serum samples. Throughout the course of our study, 400 tissue samples divided into 200 samples each from the brain and muscles were gathered for genetic analyses using a primer set specific to the N. caninum gene via polymerase chain reaction. Results: The overall seroprevalence rate of N. caninum in analyzed serum samples was 7.5%. The prevalence rates were 17.5% in females and 5% in males. Also, the rates were 8.57% in the 2–6 years of age category and 15.62% in spring. The molecular investigations revealed that the prevalence rate of N. caninum in tissue samples was 6%. The serological and molecular approaches showed 75% and 96.8% sensitivity and specificity. Sequencing and phylogenetic analysis showed that the local isolates of N. caninum is closely related to the global isolates from China, Poland, Australia, Palestine, Brazil, the USA, and Tunisia. Conclusion: According to serological results, the infection rate was not significantly related to age and season (p > 0.05) but was significantly related to sex (p < 0.01). The results of the two methods confirmed that the serological and molecular approaches exhibited almost quite similar prevalence of N. caninum within tested samples. The present findings support an approach that is more economical, quick, repeatable, and efficient for determining the incidence and prevalence of N. caninum within herd populations during endemic or epidemic conditions. Keywords: ELISA, Neospora caninum, PCR, Serum, Tissue. IntroductionIn cattle, neosporosis has been caused by the parasite Neospora caninum, which is a member of apicomplexan protozoa (Dubey and Schares, 2011). In young cattle, this protozoan is spread either postnatally by the ingestion of milk polluted with N. caninum or during gestation when it passes from infected mothers with the neonates (Gharekhani et al., 2023). At most, cattle vertical transmission is considered the key infection method that was reported to have 93% efficiency (Dubey and Schares, 2011). In cattle, neosporosis is the main reason underlying miscarriage and unsuccessful reproduction (Ansari-Lari et al., 2017). The yearly financial shortfall estimated by Santos et al. (2012) because of an infection of cows with N. caninum was greater than US$1.3 billion globally. Consequently, searching for novel robust methodologies for diagnostics purposes has motivated many researchers in this field to reduce the annual loss of cattle due to neosporosis. Generally, there exist serological, histopathological, and molecular laboratory methods that have been well-established to help detect the pathogen in infected animals. These employed methods have been the main concern for several studies that are interested in selecting the appropriate method to detect the pathogen simply, quickly, accurately, and cost effectively. The literature review addresses the comparative issues among these diagnostic laboratory techniques. However, there were discrepancy encountered among these reports (Guido et al., 2016; Khan et al., 2020). From the standpoint of epidemiology and economic savings issues due to cattle losses exaggerated by neosporosis, the goal of the current study is focused on assessing the potential of two commonly used approaches: molecular and serological techniques in the identification of the pathogen N. caninum in cattle with related certain genetic aspects, regionally in Al-Diwaniyah city, Iraq. This may help in using the right tools and genetic profile to develop strategies for better control of the protozoan. Materials and MethodsSamplingBlood sera (200) and tissue (400) samples (brain and muscle) collected from cattle from butcher shops and slaughterhouses in Al-Diwaniyah, City (180 km south to Baghdad) (Fig. 1), Iraq were enrolled in this study from August 2022 to December 2023. Blood samples were collected for serological investigations. However, tissue samples were gathered for molecular investigation. The sex, age (˂,2 –>6 years), and season at which the sample was taken were three vital factors that were considered in statistical analyses to help infer any significance between the N. caninum infection rate and each factor separately. The blood was left for 1 hour to promote blood clotting which was then centrifuged at 3,000 rpm for a duration of 10 minutes. Then, the resultant serum was kept within 1.5 ml of Eppendorf tubes and preserved at –20°C until being processed (Ibrahim, 2013).
Fig. 1. Field of study (Ghawi, 2018). Serological investigation of N. caninumEnzyme-linked immunosorbent assay (ELISA) Indirect ELISA; Screen® N. caninum Competition (ID Co., Grabels, France), had been executed on 200 gathered sera samples according to the instructions of the kit’s manufacturer (ID.VET/France). At 450 nm, the microtiter plate was read, and the software saved the optical densities for further analysis. The calculations were performed according to the following equation:
The results were also evaluated as follows: S/P ≥ 50% and S/P ≤ 40 are considered as positive and negative, respectively. Molecular investigation of N. caninumGenomic DNA isolation Genomic DNA from the tissue samples: the brain and muscles were isolated using a DNA isolation Kit (Geneaid/ New Taipei City, Taiwan) according to the manufacturer’s instructions. The DNA obtained from the extraction process was evaluated for quality and quantity using a NanoDrop. The target DNA was positioned in a 1.5 ml Eppendorf tube and kept in –20°C storage. 5.8S rRNA primer setThe polymerase chain reaction (PCR) primer pair utilized for the identification of N. caninum were designed Primer3 and the NCBI websites. The primer sequences were NS-F-5ˊATTTATTCCTATATTA-3ˊ and NS-R-5ˊGGGCTTTACATACTACTACG-3ˊ.. PCRThe PCR had been performed in a total volume of 50 μl. The reaction mixture consisted of 6 μl DNA template (100 ng), 25 μl 2 × EasyTaq® PCR Super Mix, 3 μl forward primer, 3 μl reverse primer, and 15 μl nuclease-free water. The PCR conditions in the PCR thermocycler (Techne, Ramsey, MN) were set as follows: Initial denaturation: 95°C, 5 minutes, 30 cycles: Each cycle: Denaturation: 94°C, 30 seconds, Annealing: 50°C, 30 seconds, Extension: 72°C, 30 seconds, and a final extension of one cycle at 72°C for 10 minuted. After the termination of PCR, the PCR product was evaluated by 1% agarose gel electrophoresis. The expected PCR product of 236 bp was visualized using a UV-transilluminator. Statistical analysisThe obtained datum was exposed to a chi-square test to assess the various parameter values impacting the infection rate, with statistical significance specified at (p≤0.05) and (p≤0.01) levels (SAS, 2012). Ethical approvalIn this study, no live animals were harmed, as all samples were collected from animals that had already been slaughtered for commercial purposes. The handling of these samples strictly followed relevant national or institutional guidelines for biosafety and ethics. To protect privacy, all samples were anonymized to ensure that the identity of both the source locations and the animals was protected. Confidentiality was maintained throughout the process, and results were reported in a way that guaranteed anonymity for the businesses and animals involved. Participation in the study was voluntary, and shop or slaughterhouse owners/managers could refuse permission for sample collection without any consequences. Additionally, stringent biosafety protocols were followed to ensure the safety of all personnel and prevent contamination during sample collection. Table 1. Infectivity percent of N. caninum in cattle according to sex and age by ELISA.
Table 2. Percentage infectivity of N. caninum in cattle according to season.
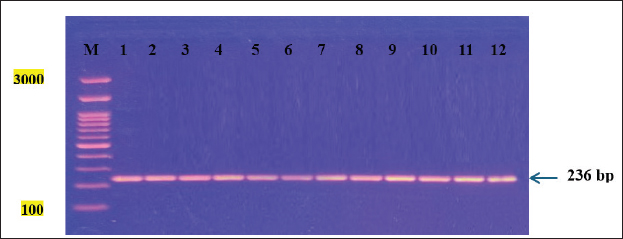
Fig. 2. Agarose gel electrophoresis showing PCR amplification of a partial fragment of the 5.8S rRNA gene in tissue samples from infected cattle with the parasite N. caninum. M: DNA Ladder (3,000 bp). Lanes 1–12: Positive samples. Table 3. Sensitivity and specificity of ELISA and PCR for detecting the prevalence of N. caninum in cattle.
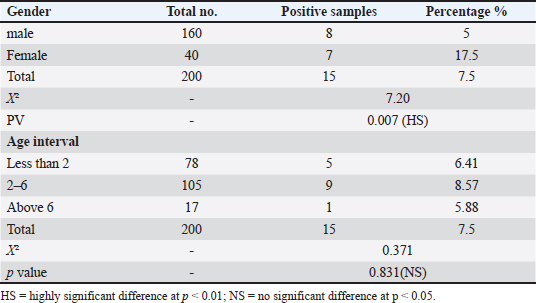
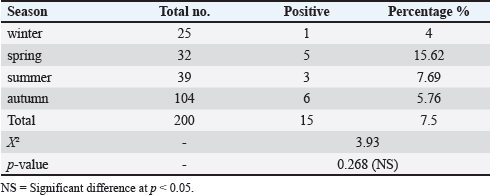
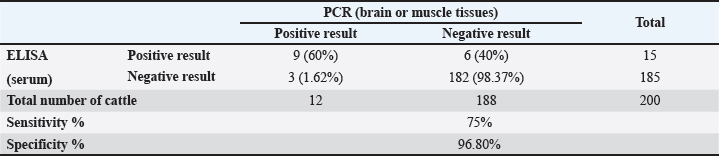
ResultsSeroprevalence of N. caninum by ELISATotal seroprevalence of N. caninum in cattle Data revealed that only 15 out of 200 cattle (7.5%) showed positive antibodies with N. caninum, and 185 cattle (92.5%) returned negative. Seroprevalence of N. caninum in cattle according to sex and age An aggregate of 200 cattle (160 males and 40 females) were used in the test. Eight calves out of 160 males tested positive (5% positivity rate) for N. caninum antibodies, and 7 out of 40 females tested positive (17.5%) in the sample, indicating a highly significant difference (p < 0.01) (Table 1). Among the three age groups studied, cows aged 2–6 years recorded the highest infection rate (8.57%), compared with the other two groups, but without statistically significant differences (p > 0.05) (Table 1). Seroprevalence of N. caninum in cattle according to season Obviously, the infection rate with N. caninum was season-dependent in the current study. The highest prevalence rate of infections was detected in spring (15.6%), in comparison with the declining rates of infection in other seasons. However, these were non-significant differences (p > 0.05) (Table 2). Prevalence of N. caninum based on molecular aspectA partial fragment of the 5.8S rRNA gene (236 bp) was amplified from genomic DNA isolated from brain and muscle tissue samples. Figure 2 shows the amplification of a partial fragment of the 5.8S rRNA gene in infected tissues. Then, the results were subjected to statistical analysis according to total prevalence as elucidated below. Total prevalence of N. caninum in cattleThe brain and muscle tissue samples exhibited 12 out of 200 cattle (6%) infectivity with N. caninum, while 188 out of 200 cattle (94%) were negative for both tissues. The statistical analysis revealed no significant difference (p =0.781). Sensitivity and specificity of ELISA and PCR for detecting the prevalence of N. caninumData inferred that there was an alignment between the results derived from serological and molecular analysis to decipher the presence of the parasite N. caninum in sera and tissue samples. This was elucidated in Table 3. Sequencing and phylogenetic analysisAfter conducting a genetic sequence examination of all positive samples by PCR, they were registered in the World Gene Bank under the following accession numbers:
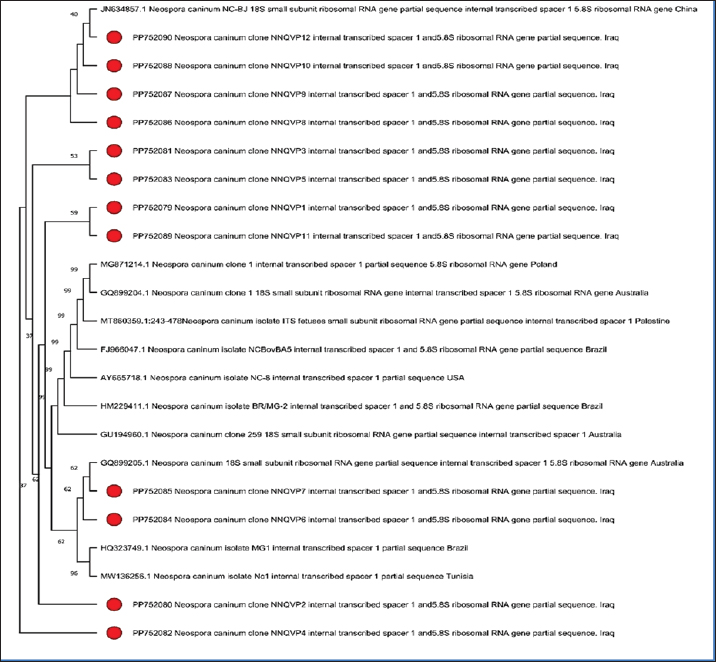
Fig. 3. Phylogenetic tree analysis of Neospora caninum from infected cattle based on PCR-amplified 5.8S ribosomal RNA gene. (PP752079, PP752080, PP752081, PP752082, PP752083, PP752084, PP752085, PP752086, PP752087, PP752088, PP752089, and PP752090). The local strains were subjected to bioinformatics analysis, and their phylogenetic tree was drawn (Fig. 3) to compare with the global strains. It was found that there is a similarity (97%–99%) with global isolates from China, Poland, Australia, Palestine, Brazil, the USA, and Tunisia. DiscussionThe intracellular protozoan parasite N. caninum is found all over the world. Neosporosis exhibited the following prevalence rates 10.7% to 19.6% in Africa (Ghalmi et al., 2012; Ibrahim et al., 2012), 5.7%–43% in Asia (Koiwai et al., 2006; Nazir et al., 2013), 0.5%–27.9% in Europe (Bartels et al., 2006; Imre et al., 2012), and 7.6%–76.9% in America (Sousa et al., 2012; Cedeão and Benavides, 2013). In China, Yi et al., (2024) recorded that IgG was found in 2.1% (21/985) of cattle. In Egypt, Ragab et al. (2024) recorded that 13.2% of the tested animals were positive to N. caninum. In central India, Hebbar et al. (2022) showed the presence of 24.8% (143/576) in the tested animals. Neosporosis can be a main reason for financial loss for livestock producers especially in the cattle sector (Dubey et al., 2007, Reichel et al., 2013). Neosporsis’ influence on animals is still unveiled in certain regions worldwide. In this context, some regions in Iraq like Al-Diwaniyah, were selected to determine the prevalence of N. caninum among cattle. Moreover, the aim of the research was to detect the infection of N. caninum, the common cause of abortion in cattle within the Iraqi region of Al-Diwaniyah. Cattle was selected as a major source for meat production, so the reproductive loss in this livestock would be disastrous. Furthermore, the choice of a reliable, reproducible, and cost-effective approach is an issue of paramount importance in diagnostics filed for the animal reproduction sector. The review of literature addressed a plethora of reports concerning the seroprevalence and molecular prevalence of N. caninum in animals applying either a single approach or dual approaches. Regarding the seroprevalence of N. caninum in cattle, our finding was 7.5%. Al-Gharban et al. (2017) from Middle Iraq determined the prevalence of antibodies to N. caninum in bovines with prevalence 27.22%. In 2019, a previous study conducted in north Tanzania on 3,015 cattle serum samples showed a seroprevalence of N. caninum of 21.5% using indirect ELISA-antibodies against the parasite (Semango et al., 2019). A study conducted in Sudan reported that antibodies against N. caninum were detected in 9.2% in females, while in males the positive infection rate was 1.5% (Ibrahim et al., 2012). In Mexico, Garcia-Vazquez et al. (2009) also documented a higher seroprevalence of N. caninum in Heifers-cows (11.8%) were more infected than bulls (5.6%). A recent study conducted in Iran has shown a seroprevalence percentage of 3.9%, with infection rate in >2-year-old bulls higher than ≤ 2-year-old (Gharekhani et al., 2023). In another study conducted in Pakistan in 2013, a high seroprevalence of neosporosis (43%) was recorded in cattle, and seroprevalence percentage of 47 and 55% among 2-year-old cattle and crossbreds, respectively, was noted (Nazir et al., 2013). In Romania in 2012, a seroprevalence of 27.7% neosporosis was observed without a statistical correlation of neosporosis in relation to age (Imre et al., 2012). In 2019, other previous study conducted in Kenya revealed a seroprevalence of neosporosis of 25.6% (Okumu et al., 2019). A recent study conducted in Egypt showed a high seroprevalence of neosporsis among cattle at 28.89% (Selim et al., 2023). Another study in Egypt has reported a total seroprevalence of 30.04% in cattle (Gaber et al., 2021). The result of the current research was in agreement with Mallah (2012) who reported a high seropositive percentage from March to June (20.576 %) and a low seropositive percentage from July to October (14.285%). Rinaldi et al., (2005) in southern Italy revealed that significantly high seroprevalence to N. caninum in cattle was recorded in spring. Talafha and Al-Majali (2013) in Jordan, documented infection rates among dairy herds and identified significant monthly differences related to serologic responses at 35% and 66.5 % during January and June, respectively. Similarly, a discrepancy among various studies regarding the molecular detection of N. caninum in cattle. For instance, 13.6% molecular prevalence was detected in Iran (Gharekhani et al., 2022). A recent study, conducted in Kurdistan region-Iraq, has showed the molecular prevalence of N. caninum of 20.6% and 17.9% among sheep and goats (Mohammed et al., 2023). A previous study had detected the presence of N. caninum in sheep in Brazil using PCR (Pena et al., 2007). The PCR’s greater specificity in detecting the parasite’s DNA might be more indicative of a live infection (Sager et al., 2006). Frössling et al. (2006) and Borsuk et al. (2011) found high sensitivity and specificity of ELISA in detecting the parasite. The inherent 75% sensitivity of the PCR test is in line with other studies that have recorded sensitivities of 70%–90% (Otranto et al., 2003; Collantes-Fernãndez et al., 2008). It is evident through these outcomes that more than one test is needed to diagnose N. caninum in a cow with good sensitivity and specificity. Therefore, ELISA as an infection screening test is recommended, but because of its low sensitivity and the risk of false positives, it should later be confirmed by a more specific test such as PCR (Dubey et al., 2007). High genetic variance in N. canninum could be essential to assay the rate of evolution and dispersal of the parasite and develop region-specific intervention programs to minimize the danger of the parasite. Comparison with a large number of isolates from China, Poland, Australia, Palestine, Brazil, the USA, and Tunisia further confirms the global distribution of N. caninum (Gondim et al., 2004; Sager et al., 2006; Dubey et al., 2007; Herman et al., 2007; Osman et al., 2020). The genetic similarities or differences among these isolates may help us understand the transmission routes and sources of infections, which is useful for controlling the disease by targeting the sources of infection. Reasons for the diversity in genes of Neospora include a broad spectrum of introduced host animals, the ability to have a sexual life stage, and the potential for genetic recombination (Dubey and Schares, 2011). Calarco et al. (2018) suggested that genes associated with the ability of strains to cause infection are expressed during the tachyzoite phase of the N. caninum life cycle. Discrepancies in seroprevalence and/or molecular prevalence of N. caninum across different studies might be attributed to some aspects, of most paramount are the sample size used in each study, rearing place (wildlife area or others), the environmental factors, such as shrub and forest habitats, climatic changes, in the study area that may facilitate the parasite’s spread, different serological methods used with various cutoff, different genetic markers used in the molecular identification method, and the lack of results validation. Some of the limitations that might be highlighted are that there is some degree of cross-reactivity of the protozoan with other microorganisms, such as Toxoplasma spp. (Gondim et al., 2017; Hebbar et al., 2022). AcknowledgmentsThe authors express appreciation to the College of Veterinary Medicine, University of AL-Qadisiyah, for facilitating this study to be conducted. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study did not receive external funding. Authors’ contributionNuha Qasim Mohammed was responsible for data collection and curation of the study; Noaman Naji A’aiz supervised the completion of the research work. Data availabilityAll data obtained during this investigation are available in the manuscript. ReferencesAl-Gharban, H.A.J., Al-Eodawee, E.M.M. and AlShabbani, A.H.A. 2017. Seroepidemiological and molecular identification of Neospora caninum in cattle in Wasit Province. Basra J. Vet. Res. 16(2), 172–183. Ansari-Lari, M., Ghasrodashti, A.R., Jesmani, H., Masoudian, M. and Badkoobeh, M. 2017. Association of Neospora caninum with reproductive performance in dairy cows: a prospective study from Iran. Vet. Res. Forum. 8(2), 104–119. Bartels, C.J.M., Arnaiz-Seco, J.I., Ruiz-Santa-Quitera, A., Björkman, C., Frössling, J., Blumröder, D., Conraths, F.J., Schares, G., Maanen, C., Wouda, W. and Ortega-Mora, L.M. 2006. Supranational comparison of Neospora caninum seroprevalences in cattle in Germany, The Netherlands, Spain and Sweden. Vet. Parasitol. 137, 17–27. Borsuk, G., Smielewska-Los, E. and Gryzinska, M. 2011. Comparative assessment of serological and molecular diagnostic techniques for detecting Neospora caninum infection in cattle. Parasitol. Res. 108(5), 1031–1035. Calarco, L., Barratt, J. and Ellis, J. 2018. Genome wide identification of mutational hotspots in the apicomplexan parasite Neospora caninum and the implications for virulence. Genome Biol. Evol. 10(9), 2417–2431. Cedeão, Q.D. and Benavides, B.B. 2013. Seroprevalence and risk factors associated to Neospora caninum in dairy cattle herds in the municipality of Pasto, Colombia. Rev. MVZ Cordoba, 18, 3311–3316. Collantes-Fernãndez, E., Arnaiz-Seco, I., Burgos, B.M., Rodríguez-Bertos, A., Aduriz, G., Fernãndez-García, A., and Ortega-Mora, L.M. 2008. Comparison of Neospora caninum distribution, parasite loads and lesions between epidemic and endemic bovine abortion cases. Vet. Parasitol. 158(1–2), 62–70. Dubey, J.P., Schares, G. and Ortega-Mora, L.M. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20, 323–367. Dubey, J.P. and Schares, G. 2011. Neosporosis in animals-the last five years. Vet. Parasitol. 180(1–2), 90–108. Fereig, R.M., Salama, D.B., Salem, F.K., Rouby, S.R., Shaapan, R.M., Draz, S., Elsawy, B.S.M., Elgioushy, M.M., Altwaim, S.A., Aboelhadid, S.M. and Frey, C.F. 2024. Frequency of Besnoitia besnoiti and Neospora caninum antibodies in cattle and small ruminants from greater Cairo and Beni Suef governorates, Egypt. Vet. Parasitol. Reg. Stud. Rep. 53, 101078. <AQ1> Frössling, J., Sternberg Lewerin, S., Lindberg, A. and Björkman, C. 2006. Evaluation of a commercial ELISA for detecting antibodies to Neospora caninum in bulk milk. Vet. Parasitol. 137(1–2), 58–63. Gaber, A., Hegazy, Y., Oreiby, A. and AL-Gaabary, M. 2021. Neosporosis: a neglected abortifacient disease in Egypt, seroprevalence and farmers’ knowledge, attitudes and practices. J. Hellenic Vet. Med. Soc. 72, 3109–3116. Garcia-Vazquez, Z., Rosario-Cruz, R., Mejia-Estrada, F., Rodriguez-Vivas, I., Romero-Salas, D., Fernandez-Ruvalcaba, M. and Cruz-Vazquez, C. 2009. Seroprevalence of Neospora caninum antibodies in beef cattle in three southern states of Mexico. Trop. Anim. Health Prod. 41(5),749–753. Ghalmi, F., China, B., Ghalmi, A., Hammitouche, D. and Losson, B. 2012. Study of the risk factors associated with Neospora caninum seroprevalence in Algerian cattle populations. Res. Vet. Sci. 93, 655–661. Gharekhani, J., Yakhchali, M. and Heidari, R. 2022. Molecular detection and phylogenetic analysis of Neospora caninum in various hosts from Iran. Comp. Immunol. Microbiol. Infect. Dis. 80, 101737. Gharekhani, J., Mohammed, R.R., Heidari, R., Hajipour, N., Trotta, M. and Villanueva-Saz, S. 2023. Assessment of Neospora caninum infection in bulls using serological and molecular techniques. Vet. Parasitol. Reg. Stud. Rep. 46, 100940. Ghawi, A.H. 2018. Study on the development of household wastewater treatment unit. J. Ecol. Eng. 19(2), 63–71. Gondim, L.F., McAllister, M.M., Pitt, W.C. and Zemlicka, D.E., 2004. Coyotes (Cani slatrans) are definitive hosts of Neospora caninum. Int. Parasitol. 34, 159–161. Gondim, L.F.P., Mineo, J.R., and Schares, G. 2017. Importance of serological cross-reactivity among Toxoplasma gondii, Hammondia spp., Neospora spp., Sarcocystis spp. and Besnoitia besnoiti. Parasitology 144(7), 851–868. Guido, S., Katzer, F., Nanjiani, I., Milne, E. and Innes, E.A. 2016. Serology based diagnostics for the control of bovine neosporosis. Trends Parasitol. 32(2), 131–143 Hebbar, B.K., Mitra, P., Khan, W., Chaudhari, S., Shinde, S., and Deshmukh, A.S. 2022. Seroprevalence and associated risk factors of Toxoplasma gondii and Neospora caninum infections in cattle in Central India. Parasitol. Int. 87, 102514. Herman R.K., Molestina, R.E., Sinai, A.P., Daniel, K. and Howe, D.K. 2007. The apicomplexan pathogen Neospora caninum inhibits host cell apoptosis in the absence of discernible NF-kappa B activation. Infect. Immun. 75(9), 4255–4262. Ibrahim, H.M. 2013. Seroprevalence of Neospora caninum antibodies in chicken samples from Delta Egypt using a recombinant NcSAG1 protein-based ELISA. Egypt J. Immunol. 20, 29–37. Ibrahim, A.M.E., Elfahal, A.M. and El-Hussein, A.R.M. 2012. First report of Neospora caninum infection in cattle in Sudan. Trop. Anim. Health Prod. 44, 769–772. Imre, K., Morariu, S., Ilie, M.S., Imre, M., Ferrari, N., Genchi, C. and Dărăbuş, G. 2012. Serological survey of Neospora caninum infection in cattle herds from Western Romania. J. Parasitol. 98(3), 683–685. Khan, A., Shaik, J.S., Sikorski, P., Dubey, J.P. and Grigg, M.E. 2020. Neosporosis: an overview of its molecular epidemiology and pathogenesis. Engineering 6(1), 10–19. Koiwai, M., Hamaoka, T., Haritani, M., Shimizu, S., Zeniya, Y., Eto, M., Yokoyama, R., Tsutsui, T., Kimura, K. and Yamane, I. 2006. Nationwide seroprevalence of Neospora caninum among dairy cattle in Japan. Vet. Parasitol. 135(2), 175–179. Mallah, M.O. 2012. Immunological and molecular study of Neospora caninum among dogs and cattle in Al-Muthana Province. Ph.D. Sc. Veterinary Medicine-University of Al-Qadisiyah, Al Diwaniyah, Iraq. Mohammed R.R., Tavassoli1, M., Sidiq, K.R. and Esmaeilnejad, B. 2023. Prevalence of Neospora caninum as an etiologic agent of animal abortion in Kurdistan Region of Iraq. Polish J. Vet. Sci. 26(3), 349–357. Nazir, M.M., Maqbool, A., Khan, M.S., Sajjid, A. and Lindsay, D.S. 2013. Effects of age and breed on the prevalence of Neospora caninum in commercial dairy cattle from Pakistan. J. Parasitol. 99, 368–370. Okumu, T.A., John, N.M., Wabacha, J.K., Tsuma, V. and Van-Leeuwen, J. 2019. Seroprevalence of antibodies for bovine viral diarrhoea virus, Brucella abortus and Neospora caninum, and their roles in the incidence of abortion/foetal loss in dairy cattle herds in Nakuru District, Kenya. BMC Vet. Res. 15(1), 95. Osman, S.A., Elshahidy, M.H., Abdelrahman, K.A. and Abdelrahman, S.I. 2020. Molecular detection and genetic characterization of Neospora caninum in cattle in Palestine. Vet. World 13(5), 1009. Otranto, D., Llazari, A., Testini, G., Traversa, D., Di Regalbono, A.F., Badan, M. and Capelli, G. 2003. Seroprevalence and associated risk factors of Neosporosis in beef and dairy cattle in Italy. Vet. Parasitol. 118, 7–18. Pena, H.F.J., Soares, R.M., Ragozo, A.M.A., Monteiro, R.M., Yai, L.E.O., Nishi, S.M. and Gennari, S.M. 2007. Isolation and molecular detection of Neospora caninum from naturally infected sheep from Brazil. Vet. Parasitol. 147(1–2), 61–66. Ragab, M.F., Amira, M.M., Azzah, S.A., Mona, Z.A., Mosaab, A.O., Abdulaziz, M.A., Mohamed, E., Hend, I.E., Kamel, S., Caroline, F.F. and Gamal, W. 2024. Seroprevalence of antibodies to Brucella spp. and Neospora caninum in cattle from Delta Region of Egypt: correlation of seropositivity with abortion history. Immuno 4(4), 374–384; doi:10.3390/immuno4040024 Rinaldi, L., Fusco, G., Musella, V., Veneziano, V., Guarino, A., Taddei, R. and Cringoli, G. 2005. Neospora caninum in pastured cattle: determination of climatic, environmental, farm management and individual animal risk factors using remote sensing and geographical information systems. Vet. Parasitol. 128(3–4), 219–230. Reichel, M.P., Ayanegui-Alcérreca, M.A., Gondim, L.F.P. and Ellis, J.T. 2013. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int. J. Parasitol. 43, 133–142. Sager, H., Fischer, I., Furrer, K., Strasser, M., Waldvogel, A., Boerlin, P. and Gottstein, B. 2006. A Swiss case-control study to assess Neospora caninum-associated bovine abortions by PCR, histopathology and serology. Vet. Parasitol. 139(1–3), 15–27. Santos, R.R.D., da Rocha, C.M.B.M., Gonãalves, T.M. and Gonçalves, A.M. 2012. Quantification of vertical transmission of Neospora caninum in dairy cows in Minas Gerais, Brazil. Rev. Bras. Parasitol. Vet. Jaboticabal 21(3), 294–297. Selim, A., Alshammari, A., Gattan, H.S., Marzok, M., Salem, M. and Al-Jabr, O.A. 2023. Neospora caninum infection in dairy cattle in Egypt: a serosurvey and associated risk factors. Sci. Rep. 13(1), 15489. Semango, G., Hamilton C.M., Kreppel, K., Katzer, F., Kibona, T., Lankester, F., Allan, K.J., Thomas, K.M., Claxton, J.R., Innes, E.A., Swai, E.S., Buza, J., Cleaveland, S. and de Glanville, W.A. 2019. The Sero-epidemiology of Neospora caninum in cattle in Northern Tanzania. Front. Vet. Sci. 6, 327. Sousa, M.E., Porto, W.J.N., Albuquerque, P.P.F., Neto, O.L.S., Faria, E.B., Júnior, J.W.P. and Mota, R.A. 2012. Seroprevalence and risk factors associated with infection by Neospora caninum of dairy cattle in the state of Alagoas, Brazil. Pesq. Vet. Bras 32, 1009–1013. Talafha, A.Q. and Al-Majali, A.M. 2013. Prevalence and risk factors associated with Neospora caninum infection in dairy herds in Jordan. Prev. Vet. Med. 112(1–2), 92–98. Yi, X.L., Yang, W.H., Zheng, H.L., Cao, M.L., Xiong, J., Chen, W.C., Zhou, Y.J., Li, F., Zhu, X.Q. and Liu, G.H. 2024. Seroprevalence and molecular detection of Toxoplasma gondii and Neospora caninum in beef cattle and goats in Hunan province, China. Parasit. Vectors 17(1), 195. Author Queries <AQ1> Please provide in-text citation for the reference “Fereig et al., 2024”y | ||
| How to Cite this Article |
| Pubmed Style Mohammed NQ, A'aiz NN. Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Vet. J.. 2025; 15(4): 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 Web Style Mohammed NQ, A'aiz NN. Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. https://www.openveterinaryjournal.com/?mno=223178 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.16 AMA (American Medical Association) Style Mohammed NQ, A'aiz NN. Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Vet. J.. 2025; 15(4): 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 Vancouver/ICMJE Style Mohammed NQ, A'aiz NN. Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 Harvard Style Mohammed, N. Q. & A'aiz, . N. N. (2025) Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Vet. J., 15 (4), 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 Turabian Style Mohammed, Nuha Qasim, and Noaman Naji A'aiz. 2025. Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Veterinary Journal, 15 (4), 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 Chicago Style Mohammed, Nuha Qasim, and Noaman Naji A'aiz. "Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq." Open Veterinary Journal 15 (2025), 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 MLA (The Modern Language Association) Style Mohammed, Nuha Qasim, and Noaman Naji A'aiz. "Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq." Open Veterinary Journal 15.4 (2025), 1645-1653. Print. doi:10.5455/OVJ.2025.v15.i4.16 APA (American Psychological Association) Style Mohammed, N. Q. & A'aiz, . N. N. (2025) Serological and molecular investigation of Neospora caninum in cattle in Al-Diwaniyah province, Iraq. Open Veterinary Journal, 15 (4), 1645-1653. doi:10.5455/OVJ.2025.v15.i4.16 |