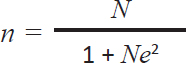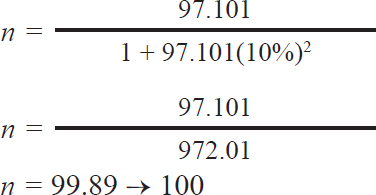| Research Article | ||
Open Vet J. 2025; 15(4): 1549-1556 Open Veterinary Journal, (2025), Vol. 15(4): 1549-1556 Research Article Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati–Pasuruan, East Java, IndonesiaPoedji Hastutiek1,2*, Lucia Tri Suwanti1,3, Endang Suprihati1, Nunuk Dyah Retno Lastuti1,4, April Hari Wardhana5, Munawer Pradana6, and Muhammad Ahdi Kurniawan61Department of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Entomology Study Group, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia 3Toxoplasma Study Group, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia. 4Postgraduate School, Universitas Airlangga, Surabaya, Indonesia. 5The Research Organization for Health, National Research and Innovation Agency, Bogor, Indonesia 6Student Master's Program in Veterinary Medicine Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Poedji Hastutiek. Laboratory of Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Laboratory of Entomology, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia. Email: poedji-h [at] fkh.unair.ac.id Submitted: 30/09/2024 Accepted: 05/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Coccidiosis, caused by Eimeria spp., is a protozoan infection that rapidly spreads in the gastrointestinal tract of cattle. Coccidiosis requires attention from all parties, including the government, because it remains a neglected disease that impacts milk and meat production, potentially compromising food security and nutrition. The disease is frequently observed on farms with poor management systems, inadequate housing sanitation, and suboptimal environmental conditions. Eimeria spp. primarily cause mortality in calves less than 1 year old. Infection occurs when cattle consume sporulated oocysts that contaminate their water and feed, and this is the primary mode of transmission. Aim: This study aimed to identify various species of Eimeria spp. oocysts, followed by molecular characterization to identify pathogenic Eimeria spp. in dairy cattle. Methods: Fresh fecal samples were collected from 100 dairy cattle. Microscopic examination was performed to detect Eimeria using floatation. Molecular characterization of pathogenic Eimeria spp. by Polymerase Chain Reaction. Polymerase chain reaction (PCR) targeting of the ribosomal RNA gene’s Internal Transcribed Spacer 1 (ITS-1) region. Result: The analysis indicated that 47 samples tested positive for the presence of Eimeria spp. oocysts. The analysis revealed five different species of Eimeria. Four samples with oocyst concentrations ranging from 250 to 2,500/ml were selected for DNA extraction and amplification, and using conventional PCR methods, Eimeria bovis (238 bp) was identified. Positive results for the molecular characterization of pathogenic Eimeria spp. were obtained using the ITS-1 gene at 238 bp. Conclusion: This study investigated cases of bovine coccidiosis and the molecular characterization of Eimeria species. The prevalence of coccidiosis in dairy cattle is 47%. E. bovis has been characterized using ITS-1, which measures 238 bp, in dairy cattle located in Grati-Pasuruan, East Java, Indonesia. Keywords: Coccidiosis, Dairy cattle, East Java, Eimeria species, Grati. IntroductionIn 2018, Indonesia reported a cattle population exceeding 16.5 million for beef production and over 0.5 million for dairy production (MoARI, 2018). Notably, 42.6% of beef cattle and 98.9% of dairy cattle are concentrated on the island of Java (The Statistical Book on Livestock and Animal Health 2017). Indonesia has initiated a strategic plan to achieve self-sufficiency in food and milk production, as part of a revitalization effort to reduce beef and milk imports and boost domestic production. Unfortunately, cattle farming in rural areas is primarily traditional, with most farms having fewer than five cows. The success of self-sufficiency in food and milk production may be impeded by gastrointestinal parasites that affect beef and dairy cattle. Bovine coccidiosis caused by infection with Eimeria spp. is a prevalent parasitic disease affecting cattle. There are approximately 21 Eimeria species in cattle, of which Eimeria bovis and Eimeria zuernii are the most pathogenic (Tomczuk et al., 2015). Coccidiosis is often asymptomatic in adult animals, but it can act as a reservoir for calves (Bangoura et al., 2012). Infection in calves causes diarrhea, dehydration, dysentery, debilitation, and death in severe cases (Lopez-Osario et al., 2020). Although most infections are nonpathogenic, Eimeria spp. can cause intestinal tissue damage and decrease the productivity of meat and milk (Ekawasti et al., 2019). In addition, Eimeria spp. infection in cattle can increase their vulnerability to other infectious diseases, including pneumonia and bacterial and viral infections (Fox et al., 1985; Gräfner et al., 1985). The global economic impact of coccidiosis in cattle has been estimated at USD 400 million worldwide. In Mexico, coccidiosis affects the economics of large and small ruminants, with annual losses of up to USD 23.7 million (Gräfner et al., 1985). Furthermore, the annual economic losses associated with both clinical and subclinical coccidiosis have been steadily rising, with current estimates exceeding USD 723 million worldwide, as reported by Koutny et al. (2012). The risk of environmental pollution can be decreased, and subclinical production losses can be avoided by preventing Eimeriosis (Keeton and Navarre 2018). Coccidiosis cases are easily found in managed farms in dirty environments contaminated by Eimeria oocysts. Cattle are infected with Eimeria spp. through the ingestion of sporulated oocysts that contaminate water and feed as the main source of transmission (Lassen et al., 2014). Factors related to the prevalence of Eimeria spp. infection in cattle include farm management, age, and environmental temperature (Lee et al., 2018). In Indonesia, agricultural management relies primarily on traditional systems that are overseen by family units. To the best of our knowledge, there is no data regarding the economic losses due to bovine coccidiosis in Indonesia because most studies only focused on poultry coccidiosis. Only a few studies have reported Eimeria infection in cattle, particularly in Indonesia. Ananta et al. (2014) reported a 22.4% prevalence of Eimeria in cattle in West Java Province, and Hamid et al. (2016) reported a 15.5% prevalence of eimeria in Central Java Province. Coccidiosis in Madura cattle was also reported microscopically by Hastutiek et al. (2019), with a prevalence of 75.07%. Eimeria species on a dairy farm in Bandung, Indonesia (Sufi et al., 2017). However, in almost all studies, Eimeria spp. were only observed morphologically via microscopic examination. The first report of Eimeria species based on molecular identification in Indonesia was done by Ekawasti et al. (2019), who reported that the prevalence of each species was 10.4%, 2.8%, 2.1%, 1.4%, 1.1%, and 0.4% for E. bovis, Eimeria ellipsoidalis, Eimeria alabamensis, E. zuernii, Eimeria auburnensis, and Eimeria cylindrical. Therefore, this study aimed to identify various species of Eimeria spp. oocysts, followed by molecular characterization to identify pathogenic Eimeria spp. in dairy cattle. Materials and MethodsSampling techniqueThe research was conducted between June and September 2023. Fresh fecal samples were obtained from dairy cattle in Grati-Pasuruan, East Java, Indonesia. These samples were analyzed microscopically at the Laboratory of Veterinary Parasitology within the Faculty of Veterinary Medicine at Universitas Airlangga. Polymerase chain reaction (PCR) testing was performed by the Institute of Tropical Disease at Universitas Airlangga. Determining the number of samples using the Slovin formula (Sugiyono, 2006), the Slovin formula was utilized to calculate the sample in this investigation:
n: Number of samples N: amount of populations e: 10% accuracy The following computations were performed:
The calculation based on Slovin’s formula yielded the sample size required for this study. The minimum number of necessary samples was 99.89, and the total number of dairy cattle involved in this study was 100. Fecal sample collectionA total of 100 fecal samples were gathered for this study, with samples obtained from bulls and cows and grouped according to age. One to five fecal samples were collected randomly at each farm. Most stools were normal, except for 2 diarrhea and 3 soft ones. Samples were taken directly from areas near the animal enclosures, placed in small zip-lock polythene bags, and preserved with 2.5% potassium bichromate for further analysis. Each bag was labeled with a unique sample number and stored in an ice-filled container. Subsequently, the fecal samples were analyzed using the sugar centrifugal flotation technique, as described by Matsubayashi et al. (2005). Fecal examinationFecal samples were analyzed using the sugar centrifugal flotation technique, as described by Matsubayashi et al. (2005). In this method, approximately 1 g of fecal matter was centrifuged at 800 × g for 5 minutes. After discarding the supernatant, a sugar solution with a specific gravity of 1.2 was added, followed by another round of centrifugation. The upper layer of the solution was then transferred onto a glass slide for examination under a light microscope. Eimeria parasites were identified based on morphological features, such as size, shape, number of sporozoites, and other notable characteristics (Soulsby, 1986). A qualitative microscopic analysis was performed to detect the presence or absence of oocysts. For purification, a positive sample was selected. Eimeria oocysts were isolated using sugar flotation as described by Kawahara et al. (2010). The oocysts were collected from the sugar solution by taking about 1–2 ml from the surface of the centrifuge tube. One milliliter contains between 750 and 1,000 oocysts. The supernatant was subsequently washed three times with distilled water, and the resulting pellet was resuspended in 1–2 ml of PBS and stored at 4°C. Molecular identificationThe PCR analysis in this study was performed using positive Eimeria samples with the highest oocysts per gram (OPG) values. Four morphologically positive samples were subjected to molecular analysis. The selection of molecular samples was based on the number of oocysts containing 250–25,000 oocysts per milliliter of fecal solution. DNA extraction was performed using DNAzol (Molecular Research Center, Cincinnati, Ohio, USA), following the manufacturer’s protocol with the addition of five freeze-thaw cycles before extraction. To identify Eimeria species, including pathogenic types (the ITS-1 target for E. bovis is 238 bp and E. zuernii is 344 bp), PCR amplification targeting the internal transcribed spacer 1 (ITS-1) region of the ribosomal RNA gene was performed as described by Kawahara et al. (2010). The resulting PCR products were separated using agarose gel electrophoresis, stained with ethidium bromide, and visualized using a UV transilluminator. Data analysisMicroscopic and molecular test data were analyzed qualitatively and explained descriptively, as well as the prevalence of Eimeria spp. infection based on sex, age, species variation, and severity indicators. The prevalence was expressed as a percentage using the following formula: p=(Number of cases: Total of samples) × 100%. The infection distribution was categorized by age in dairy cattle: 6 months, 6–24 months, and >24 months. The number of OPG of feces was calculated using a modified McMaster technique (Koutny et al., 2012). The degree of protozoan infection is divided into three categories: mild infection (1–499 oocysts/gram), moderate infection (500–5,000 oocysts/gram), and severe infection (>5,000 oocysts/gram).
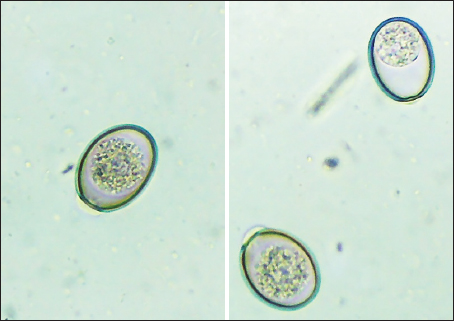
Fig. 1. Oocysts of Eimeria spp. were not sporulated using a light microscope 400x). Table 1. Summary of study sex, ages of dairy cattle, and Eimeria spp., identification, and prevalence.
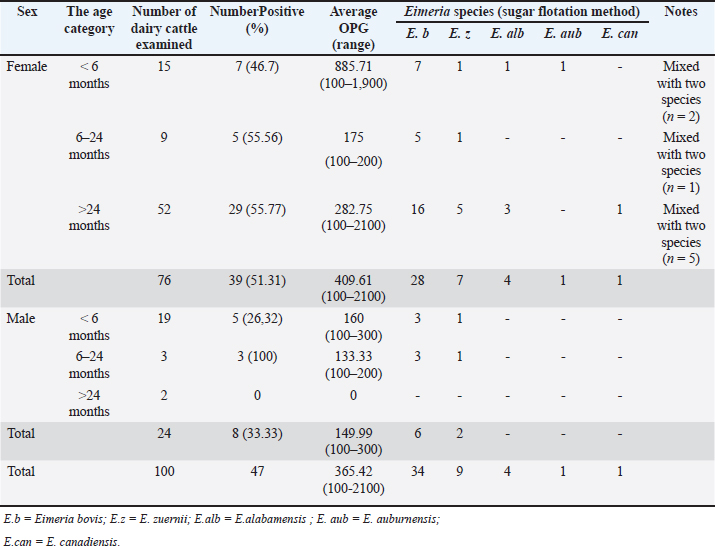
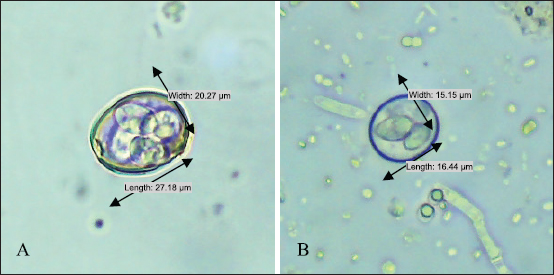
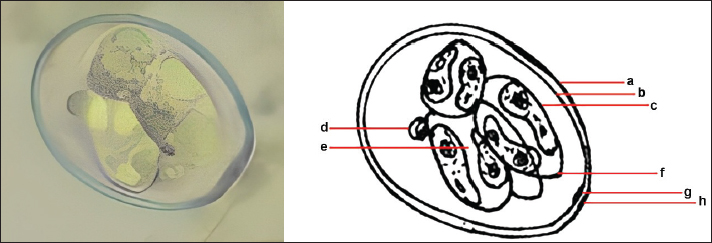
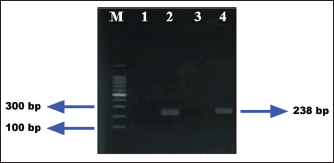
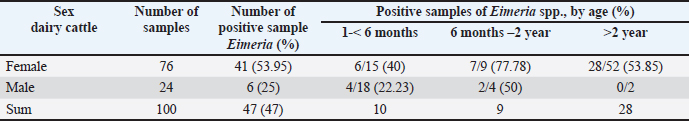
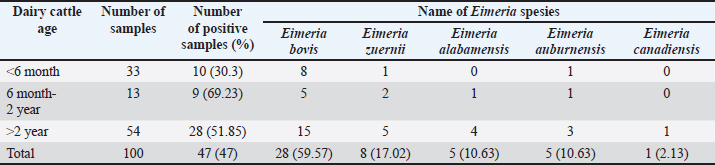
Ethical approval No ethical approval was required because the samples were collected for diagnostic purposes alone. This study did not use dairy cattle as the sample, but only fresh fecal samples were collected from around the enclosures. However, sample collection was conducted in the field with permission from the Animal Husbandry Department in Pasuruan. ResultsQuantitative examination was performed using the sugar floating method for morphological identification, and OPG was determined using the McMaster method. Observations under a light microscope revealed the morphology of Eimeria spp., with representative oocysts shown in Figure 1. Out of 100 fecal samples examined, 47 tested positive for Eimeria spp., resulting in a prevalence of 47%. Morphological analysis identified six species: E. bovis, E. zuernii, E. alabamensis, E. auburnensis, and E. canadiensis. The prevalence of Eimeria spp. infection was higher in cows 51.31% (39/76) than in bulls 33.33% (8/24), as shown in Table 1. Among the identified species, E. bovis had the highest prevalence at 72.34% (34 samples), followed by E. zuernii at 19.15% (9 samples), E. alabamensis at 8.51% (4 samples), and E. auburnensis and E. canadiensis at 2.28% each (1 sample). Due to their high prevalence, further morphological analysis focused on E. bovis and E. zuernii (Fig. 2). The prevalence of infection also varied by age group (Table 3). Calves under 6 months of age showed a prevalence of 30.3%, and three species were identified (E. bovis, E. zuernii, and E. auburnensis). Calves aged 6–24 months showed a prevalence of 69.23%, showed the presence of E. bovis, E. zuernii, E. alabamensis, and E. auburnensis. Dairy cattle over 24 months of age showed a prevalence of 51.85%, and five species were detected (E. bovis, E. zuernii, E. alabamensis, E. auburnensis and E. canadiensis), with E. bovis being the most frequently identified species across all age groups. Infection severity was categorized according to OPG levels: light (1–499), moderate (500–5,000), and high (>5,000). The average OPG across the 47 positive samples was 365.42, indicating a predominance of light-to-moderate infections, with only five samples showing OPG values between 500 and 5,000. Tables 2 and 3 show the results of the prevalence of Eimeria spp based on sex and age. Infections in males were 25% (6/24) lower than in females, 53.95% (41/76) (Table 2). In male and female dairy cattle, the highest prevalence of Eimeria was observed at the age of 6 months to 2 years. Images of E. bovis were captured using a Lucida microscope, with schematic representations provided in Figure 3. The morphology of the observed E. bovis oocysts is ovoid, featuring micropiles and double-layered walls. Molecular identification of Eimeria spp. by polymerase chain reactionPCR analysis was conducted to confirm the microscopic results for Eimeria spp. Most samples exhibited mild infections with OPG values of 1–499, whereas only a limited number demonstrated moderate infection with OPG values between 500 and 5,000. PCR analysis was performed on fecal samples from dairy cattle containing 250–2,500 oocysts per milliliter, which were subsequently purified. PCR analysis confirmed the identification of E. bovis (238 bp) (Fig. 4).
Fig. 2. Photographs of the oocysts of (A) Eimeria bovis and (B).
Fig. 3. Sporulation of E. bovis. a. Outer layer of oocyst wall. b. Inner layer of the oocyst wall. c. Sporocyst wall. d. Polar granule. e. Sporozoite. f. Stieda body. g. Micropyle. h. Polar cap.
Fig. 4. Polymerase chain reaction DNA product of E. bovis from fecal samples of dairy cattle in Grati, Pasuruan. M=DNA ladder; Number 1–4: sample number, Samples 1 and 3 are negative. 2 and 4 are positive. DiscussionNumerous studies have documented the prevalence of Eimeria spp. in cattle in different countries using a standard microscopy examination to detect oocysts (Lopez-Osario et al., 2020). The prevalence of Eimeria infection differed in each country; the prevalence was 75.5% in Colombia (Lopez-Osario et al., 2020), 22.1% in South Korea (Lee et al, 2018), 47.09% in Pakistan (Rehman et al., 2011), and 11.97% in India Das et al. (2015). Ekawasti et al. (2019) reported a 52.3% prevalence of Eimeria in Java Island. Furthermore, bovine coccidiosis has been reported in Maluku Island as the highest (94.1%) prevalence, followed by Kalimantan (83%), Sumatra (70.3%), Sulawesi (68.9%), Papua (62.3%), and Nusa Tenggara (58.5%) (Ekawasti et al., 2021). The variation in prevalence and type of infection can differ depending on individuals’ various infection rates and shedding intensities. These differences may be due to geographical conditions, feed sources, and feeding behaviors (de Andrade et al., 2012). Based on the findings from molecular diagnostics, two samples tested positive. E. bovis was this study’s most frequently detected organism, followed by E. zuernii, E. auburnensis, and E. cylindrica. In South Korea, Lee et al. (2018) reidentified E. bovis in 9% and E. zuernii in 66% of samples. Ekawasti et al. (2019) also reported that E. bovis (10.4%) is the most prevalent species in Java Island, Indonesia. Using PCR as a molecular approach, Lee et al. (2018) successfully identified three species of Eimeria, specifically E. bovis, E. zuernii, and E. auburnensis. In their study, Ekawasti et al. (2019) recognized E. bovis, E. ellipsoidalis, E. alabamensis, E. zuernii, E. auburnensis, and E. cylindrica. In addition, it is noteworthy that not all samples that were positive during microscopic examination yielded positive PCR results in this study, which may be attributable to the limited number of oocysts present in the fecal samples. These findings were supported by the findings of Carvalho et al. (2011) Mirhashemi et al. (2016), and Ekawasti et al. (2019), who explained that a small number of oocysts was not sufficient for species identification using the PCR method. Contaminants may have inhibited the PCR process, as noted by Kawahara et al. (2010). Table 2. Prevalence of Eimeria spp., based on sex and age of dairy cattle in Grati sub-district, Pasuruan.
Table 3. Identification of Eimeria species from dairy cattle feces samples in the Grati sub-district based on age and prevalence.
Bovine coccidiosis can cause not only growth delays but also decreases in body performance and cattle production. These clinical signs also affect the quality of adult cattle, resulting in high morbidity and mortality in calves and inhibiting the sustainability of livestock production (Heidari and Gharekhani, 2014). Theoretically, coccidiosis is a pathogenic disease of young animals, but poor nutritional and environmental management can be potential risk factors for older animals. Adult cattle with chronic infection are frequently diagnosed with anorexia, weight loss, emaciation, bloody diarrhea, and blood-stained dung in the perineum and tail part (Sudhakara et al., 2015). In this study, dairy cattle were infected with either single or mixed Eimeria species. Coccidiosis is typically caused by more than one species of Eimeria. The Madura cattle in this study were infected with either single or mixed Eimeria. Bangoura et al. (2012) reported that a single infection in calves caused 48.6% of diarrhea cases, and 51.4% of cases involved mixed infections. Morgoglione et al. (2020) also reported that 71.2% of cattle were infected with more than 1 Eimeria species. Previous results are similar to those of our study, which showed that mixed infections were recorded more frequently than single infections. However, our study diverged from previous results, as it demonstrated that single infections were more prevalent than mixed infections. In addition, the management of cages and sanitation in the sampling area is inadequate because feces are disposed of in close proximity to the cages, potentially increasing the risk of infection and reinfection (Marskole et al., 2016). The majority of cages are also traditional and are not equipped with feces and urine disposal lines. Management patterns also affect the occurrence of Eimeria spp. infection, such as sanitation methods, drainage systems, population density, cage structures, feeding systems, and drinking sources (Makau et al., 2017). The incidence of infection and intensity of Eimeria spp. in cattle were also recorded at a lower percentage in cages than in pasture (Jäger et al., 2005). Therefore, cattle shed a lot of oocysts through feces in their closed cages every day during the patent period, which can increase the risk of transmission and increase the development cycle of Eimeria spp. The clinical signs of bovine coccidiosis frequently appear 2–3 weeks after infection in contaminated environments (Hussin, 2016). Studies on E. bovis molecularly in cattle have indeed been conducted in Indonesia, but in beef cattle (Hamid et al., 2016; Ekawasti et al. 2021; Hastutiek et al., 2022), in dairy cattle, it is still limited to morphological observations (Sufi et al., 2017). The results of this study support previous studies and confirm that dairy cattle are infected with E. bovis morphologically and molecularly. Although the number of samples in our study was limited, we revealed that the Eimeria spp. samples could be identified at the species level using a molecular method. Therefore, comprehensive studies are required to further investigate Eimeria spp’s pathogenicity. infection in dairy cattle improves productivity through improved and integrated livestock management practices. ConclusionAlthough the number of samples in the present study was limited, the samples were identified at the species level by morphological analysis. The number of bovine coccidiosis cases in dairy cattle in Grati was 47%. E. bovis was predominant among the detected species by PCR using specific species primers. Based on these findings, dissemination programmes regarding coccidiosis should be delivered to farmers and veterinarians for effective and efficient action to prevent or cure the disease. Biosecurity measures among traditional farmers must be strengthened to control the transmission of Eimeria spp. in dairy cattle. AcknowledgmentThe authors sincerely thank the Faculty of Veterinary Medicine, Universitas Airlangga, for their support in this study. Our appreciation also goes to Rias Nawang Kartika, DVM, from Koperasi Usaha Tani dan Ternak (KUTT) in Grati-Pasuruan. This article was partially supported by the Penelitian Dasar Unggulan (PDU) funding from Airlangga University, Indonesia, in the 2023 fiscal year, under grant number 1289/UN.3.6/PT/2023. Conflict of interestThe authors declare that they have no conflicts of interest. FundingProvided by the Penelitian Dasar Unggulan (PDU) funding from Universitas Airlangga, Indonesia. Authors’ ContributionsMP and MAK were responsible for collecting fecal samples. PH, LTS, ES, and NDRL conducted microscopic analysis. PH, LTS, MP, MAK, and AHW performed molecular identification. PH and LTS wrote the initial draft and revised the manuscript. All authors have reviewed and approved the final version of the manuscript. Data availabilityAll the information supporting the discoveries in this regard are accessible in the manuscript. ReferencesAnanta, S.M., Hidayat, A. and Matsubayashi, M., 2014. Survey on gastrointestinal parasites and detection of Cryptosporidium spp. in cattle in West Java, Indonesia. Asian Pac. J. Trop. Med. 7(3), 197–201. Bangoura, B., Mundt, H.C., Schmãschke, R., Westphal, B. and Daugschies, A., 2012. Prevalence of Eimeria bovis and Eimeria zuernii in German cattle herds and factors influencing oocyst excretion. Parasitol. Res 110, 875–881. Carvalho, F.S., Wenceslau, A.A., Teixeira, M., Carneiro, J.A.M., Melo, A.D. and Albuquerque, G.R. 2011. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet. Parasitol. 176(2 3), 95–100. Das, M., Deka, D.K., Islam, P.C., Islam, S. and Sarma, S. 2015. Diversity of Eimeria spp. in dairy cattle of Guwahati, Assam, India. Vet. World, 8(8), 941. De Andrade, A.L.F., Da Silva, P.C., De Aguiar, E.M. and Santos, F.G.A. 2012. Use of coccidiostat in mineral salt and study on ovine eimeriosis. Rev Bras. Parasitol. Vet. 21(1), 16–21. Ekawasti, F., Nurcahyo, W., Wardhana, A.H., Shibahara, T., Tokoro, M., Sasai, K. and Matsubayashi, M. 2019. Molecular characterization of highly pathogenic Eimeria species among beef cattle on Java Island, Indonesia. Parasitol. Int. 72, 101927. Ekawasti, F., Nurcahyo, R.W., Firdausy, L.W., Wardhana, A.H., Sawitri, D.H., Prastowo, J. and Priyowidodo, D., 2021. Prevalence and risk factors of Eimeria species infection in cattle of different geographical regions of Indonesia. Vet. World. 14(9), 2339. Fox, J.E. 1985. Coccidiosis in cattle. Mod. Vet. Pract. 66(1), 113–116. Gräfner, G., Graubmann, H.D., Schwartz, K., Hiepe, T. and Kron, A. 1985. Investigation of the occurrence and epizootiol ogy of Eimeria as a coccidiosis agent in cattle under conditions of intensive husbandry [Weitere untersuchungenzu vorkommen, epizootiologie und bekãmpfung der Eimeria kokzidiose des rindesunter den bedingungenintensivenstall haltung]. Monatsh Vet. Med. 40(1), 41–44. Hamid, P.H., Kristianingrum, Y.P., Prastowo, J. and Da Silva, L.M.R. 2016. Gastrointestinal parasites of cattle in Central Java. Am. J. Anim. Vet. Sci. 11(3), 119–124. Hastutiek, P., Yuniarti, W.M., Djaeri, M., Lastuti, N.D.R., Suprihati, E. and Suwanti, L.T. 2019. Prevalence and diversity of gastrointestinal protozoa in Madura cattle in Bangkalan Regency, East Java, Indonesia. Vet. World, 12(2), 198–204. Hastutiek, P., Lastuti, N.D.R., Suwanti, L.T., Sunarso, A., Suprihati, E., Kurniawati, D.A. and Matsubayashi, M. 2022. Coproparasitological examinations and molecular determination of Eimeria species in Madura cattle reared on Madura Island, Indonesia. Parasitol. Int. 86, 102478. Heidari, H and Gharekhani, J. 2014. Detection of Eimeria species in Iranian native cattle. Int. J. Adv. Res. 2(7), 731–734. Hussin, A.G. 2016. Prevalence and risk factors of Eimeria spp. In cattle of Baghdad, Iraq. J. Appl. Anim. Sci. 9(1), 37–44. Jãger, M., Gualy, M., Bauer, C., Failing, K., Erhardt, G. and Zahner, H. 2005. Endoparasites in calves of beef cattle herds: management systems dependent and genetic influences. Vet. Parasitol. 131(3-4), 173–191. Kawahara, F., Zhang, G., Mingala, C.N., Tamura, Y., Koiwa, M., Onuma, M. and Nunoya, T. 2010. Genetic analysis and development of specific PCR assays based on the ITS-1 region of rRNA in bovine Eimeria parasites. Vet. Parasitol. 174(1–2), 49–57. Keeton, S.T.N. and Navarre, C.B. 2018. Coccidiosis in large and small ruminants. Vet. Clin. North Am. Food Anim. Pract. 34, 201–208. Koutny, H., Joachim, A., Tichy, A. and Baumgartner, W., 2012. Bovine Eimeria species in Austria. Parasitol. Res. 110, 1893–1901; doi:10.1007/s00436-011-2715-7 Lassen, B., Lepik, T. and Jãrvis, T. 2014. Seasonal recovery of Eimeria oocysts from soil on naturally contaminated pastures. Parasitol. Res. 113, 993–999. Lee, S.H., Kim, H.Y., Lee, H., Kim, J.W., Lee, Y.R., Chae, M.J., Oh, S.I., Kim, J.H., Rhee, M.H., Kwon, O.D., Goo, Y.K., Kim, T.H., Geraldino, P.J.L. and Kwak, D. 2018. Eimeria species in cattle with diarrhea in the Republic of Korea regarding age, season, and nature of diarrhea. Vet. Rec. 183(16), 504–504. Lopez-Osorio, S., Villar, D., Failing, K., Taubert, A., Hermosilla, C. and Chaparro-Gutierrez, J.J. 2020. Epidemiological survey and risk factor analysis on Eimeria infections in calves and young cattle up to 1 year old in Colombia. Parasitol. Res. 119, 255–266. Makau, D.N., Gitau, G.K., Muchemi, G.K., Thomas, L.F., Cook, E.A., Wardrop, N.A., Fevre, E.M. and de Glanville, W. 2017. Environmental predictors of bovine Eimeria infection in western Kenya. Trop. Anim. Health Prod. 49(2), 409–416. Marskole, P., Verma, Y., Dixit, A. K. and Swamy, M. 2016. Prevalence and burden of gastrointestinal parasites in cattle and buffaloes in Jabalpur, India. Vet. World, 9(11), 1214. Matsubayashi, M., Takami, K., Kimata, I., Nakanishi, T., Tani, H., Sasai, K. and Baba, E. 2005. Survey of Cryptosporidium spp. and Giardia spp. infections in various animals at a zoo in Japan. J. Zoo. Wildl. Med. 36(2), 331–335. MoARI. 2018. Livestock and Animal Health Statistics. Directorate General of Animal Husbandry and Animal Health Ministry of Agriculture of the Republic of Indonesia, Indonesia. Mirhashemi, M.E., Zintl A, Grant, T., Lucy, F., Mulcahy, C. and Waal T.D. 2016. Molecular epidemiology of Cryptosporidium species in livestock in Ireland. Vet. Parasitol. 216, 18–22. Morgoglione, M.E., Bosco, A., Maurelli, M.P., Alves, L.C., Saralli, G., Bruni, G., Cringoli, G. and Rinaldi L. 2020. A 10-year surveillance of Eimeria spp. in cattle and buffaloes in a Mediterranean area. Front. Vet. Sci. 7, 410. Rehman, T.U., Khan, M.N., Sajid, MS, Abbas, R.Z., Arshad, M., Iqbal, Z. and Iqbal, A. 2011. Epidemiology of Eimeria and associated risk factors in cattle of district Toba Tek Singh, Pakistan. Parasitol. Res. 108(5), 1171–1177. Soulsby, East J.L. 1986. Helminths, Arthropods, and Protozoa of Domestic Animals. 7th ed. Bailliere Tindall, pp: 582–585. Sudhakara, R.B., Sivajothi, S. and Rayulu, V.C. 2015 Clinical coccidiosis in adult cattle. J. Parasit. 2015 Dis. 39(3), 557–559. Sufi, I.M., Cahyaningsih, U. and Sudarnika, E. 2017. Eimeria species composition and factors influencing oocyst shedding in a dairy farm, Bandung, Indonesia. Biotropia. 24(2), 104–113. Sugiyono. 2006. Statistika Untuk Penelitian. Bandung, Indonesia: Allfabeta, p: 57. Tomczuk, K., Grzybek, M., Szczepaniak, K., Studzińska, K., Demkowska-Kutrzepa, M., Roczeń-Karczmarz, M. and Klockiewicz, M. 2015. Analysis of intrinsic and extrinsic factors influencing the dynamics of bovine Eimeria spp. in central-eastern Poland. Vet. Parasitol. 214, 22–28. The Statistical Book on Livestock and Animal Health. 2017. Livestock and Animal Health Statistics. Directorate General of Livestock and Animal Health Service, Ministry of Agriculture, Indonesia. | ||
| How to Cite this Article |
| Pubmed Style Hastutiek P, Suwanti LT, Suprihati E, Lastuti NDR, Wardhana AH, Pradana M, Kurniawan MA. Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Vet J. 2025; 15(4): 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 Web Style Hastutiek P, Suwanti LT, Suprihati E, Lastuti NDR, Wardhana AH, Pradana M, Kurniawan MA. Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. https://www.openveterinaryjournal.com/?mno=222486 [Access: July 31, 2025]. doi:10.5455/OVJ.2025.v15.i4.5 AMA (American Medical Association) Style Hastutiek P, Suwanti LT, Suprihati E, Lastuti NDR, Wardhana AH, Pradana M, Kurniawan MA. Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Vet J. 2025; 15(4): 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 Vancouver/ICMJE Style Hastutiek P, Suwanti LT, Suprihati E, Lastuti NDR, Wardhana AH, Pradana M, Kurniawan MA. Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Vet J. (2025), [cited July 31, 2025]; 15(4): 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 Harvard Style Hastutiek, P., Suwanti, . L. T., Suprihati, . E., Lastuti, . N. D. R., Wardhana, . A. H., Pradana, . M. & Kurniawan, . M. A. (2025) Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Vet J, 15 (4), 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 Turabian Style Hastutiek, Poedji, Lucia Tri Suwanti, Endang Suprihati, Nunuk Dyah Retno Lastuti, April Hari Wardhana, Munawer Pradana, and Muhammad Ahdi Kurniawan. 2025. Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Veterinary Journal, 15 (4), 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 Chicago Style Hastutiek, Poedji, Lucia Tri Suwanti, Endang Suprihati, Nunuk Dyah Retno Lastuti, April Hari Wardhana, Munawer Pradana, and Muhammad Ahdi Kurniawan. "Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia." Open Veterinary Journal 15 (2025), 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 MLA (The Modern Language Association) Style Hastutiek, Poedji, Lucia Tri Suwanti, Endang Suprihati, Nunuk Dyah Retno Lastuti, April Hari Wardhana, Munawer Pradana, and Muhammad Ahdi Kurniawan. "Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia." Open Veterinary Journal 15.4 (2025), 1549-1556. Print. doi:10.5455/OVJ.2025.v15.i4.5 APA (American Psychological Association) Style Hastutiek, P., Suwanti, . L. T., Suprihati, . E., Lastuti, . N. D. R., Wardhana, . A. H., Pradana, . M. & Kurniawan, . M. A. (2025) Bovine coccidiosis and molecular characterization of pathogenic Eimeria species in dairy cattle on Grati—Pasuruan, East Java, Indonesia. Open Veterinary Journal, 15 (4), 1549-1556. doi:10.5455/OVJ.2025.v15.i4.5 |