| Research Article | ||
Open Vet. J.. 2025; 15(1): 151-161 Open Veterinary Journal, (2025), Vol. 15(1): 151-161 Research Article Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1βHevi Wihadmadyatami1*, Muhammad Ali Zulfikar2, Herawati Herawati3, Srikanth Karnati4, Golda Rani Saragih1, Dinda Aliffia1, Dyah A.O.A. Pratama5, Nurrahmi Handayani2, Ulayatul Kustiati6, Dewi Ratih Tirtosari7, and Yudy Tjahjono81Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 22Department of Chemistry, Faculty of Mathematics and Science, Institut Teknologi Bandung, Bandung, Indonesia 3Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 4Institute of Anatomy and Cell Biology, Julius Maximillian University, Wuerzburg, Germany 5Laboratory of Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 6Laboratory of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 7Department of Pharmacy, Faculty of Medical Science, Universitas Ibrahimy, Situbondo, Indonesia 8Faculty of Pharmacy, Widya Mandala Catholic University Surabaya, Surabaya, Indonesia *Corresponding Author: Hevi Wihadmadyatami. Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: heviwihadmadyatami [at] ugm.ac.id © 2025 Open Veterinary Journal
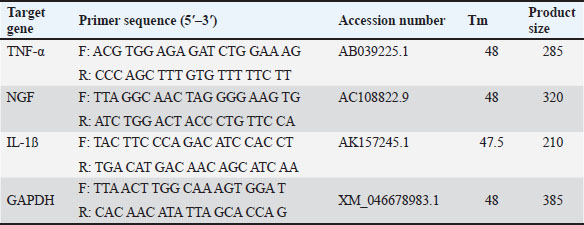
AbstractBackground: Neurodegenerative diseases (NDDs) are distinguished by impairment and depletion of nerve cells; one of the most common NDDs is Alzheimer’s disease (AD), which can appear in early onset or late onset. In recent years, the secretome or conditioned medium of mesenchymal stem cells has provided new hope for improving conditions and preventing AD. One of the secretomes is bovine umbilical mesenchymal stem cells-conditioned medium (BUMSC-CM), where BUMSC is predicted to promote neuronal proliferation potentially. Aim: This study analyzes the therapeutic efficiency of conditioned medium or secretome produced from BUMSC-CM in treating neurodegeneration in animal models of AD. Methods: Five groups consisting of 12 male rats were assigned: untreated (Group A, n=5), positive control group given normal saline 1 ml/100 g BW (Group B, n=5), AD rats model followed by Donepezil treatment (Group C, n=5), AD rats model with BUMSC-CM 0.2 ml/kg BW post-trimethyltin (TMT) induction (Group D, n=5), and AD rats model with BUMSC-CM 0.5 ml/kg BW post-TMT induction (Group E, n=5). The brain samples were analyzed for neuronal density using cresyl violet staining. The expression and activity of brain-derived neurotrophic factor (BDNF) were analyzed by ELISA; in addition, interleukin 1beta (IL-1β), tumor necrotic factor-alpha (TNF-α), and neural growth factor (NGF) were analyzed by quantitative polymerase chain reaction. Interactions between the main substances of BUMSC-CM and beta-amyloid protein were visualized using in silico molecular docking. Results: Our result demonstrated that BUMSC-CM with the dosage of 0.5 ml/kg BW significantly increased BDNF concentration. We also found that BUMSC-CM with dosage 0.2 ml/kg BW and 0.5 ml/kg BW down-regulated IL-1β and TNF-α and upregulated NGF expression. Additionally, the number of neurons in AD rats post-treated with BUMSC-CM was significantly increasing. Furthermore, the amino acids in BUMSC-CM, including isoleucine, leucine, and valine, bind to the amyloid beta protein via interactions that are hydrophobic and hydrogen-bonded. Conclusion: In this study, the neuroprotective potential of BUMSC-CM was demonstrated by its ability to upregulate BDNF and NGF while downregulating IL-1β and TNF-α. Additionally, BUMSC-CM showed potential to promote neuron proliferation in the hippocampus regions of a rat AD model. The main constituents in BUMSC-CM adhere to amyloid beta protein, hence diminishing the likelihood of ND disorders, specifically AD. Keywords: Alzheimer’s disease, BUMSC-CM, Rat model, IL-1β, TNF-α. IntroductionNeurodegenerative diseases (NDDs) are the dysfunction and progressive loss of nerve cells as the main targets, causing damage to neurons’ essential functions, such as signal transmission and network integration (Lu et al., 2019). It is characterized by the most popular NDDs in aging populations, such as amyotrophic lateral sclerosis, frontotemporal lobar dementia, Parkinson’s disease, and Alzheimer’s disease (AD), and among all the NDDs. AD is the most prevalent, and it occurs not only in humans but also in canines and is known as canine cognitive impairment or canine Alzheimer’s-like (Wihadmadyatami et al., 2023). One of the most prevalent risk factors for each of these illnesses is aging. As a result, because of the increasing global life expectancy, this disease will likewise increase, placing a substantial financial burden on people, families, and communities. Treatment of these diseases is still symptomatic, and there is no treatment based on the available mechanisms (Spillantini and Goedert, 2013; Ransohoff, 2016; Gan et al., 2018). Common cellular pathways of neurodegeneration mechanisms include oxidative stress resulting in DNA damage, increased lipid and protein oxidation, poor mechanisms for controlling and degrading proteins, altered balance of mitochondria, stressed granules, and maladaptive responses of the innate immune system (Giordano et al., 2014; Gan et al., 2018). Stem cell therapy has been applied in treating diverse ailments, such as neurological illnesses (Marsh and Blurton-Jones, 2017). However, stem cell therapy continues to face various obstacles, including the potential for tumor formation, rejection by the immune system, the production of blood clots, the transfer of infections, and the presence of diverse types of cell lines with varying characteristics (Yamanaka, 2020). Furthermore, the tumorigenic potential of stem cells is not limited to embryonic or induced pluripotent stem cells. Research by Kooreman and Wu (2010) has indicated that the tumorigenic properties of pluripotent cells pose significant challenges in clinical applications, as evidenced by a case where fetal neural stem cells led to the development of multifocal glioneuronal tumors in a patient. This highlights the necessity for rigorous evaluation of the tumorigenic potential of stem cells before their therapeutic use. In addition to the intrinsic properties of stem cells, external factors such as the tumor microenvironment and specific signaling pathways have a crucial role in modulating their tumorigenic potential. For instance, the Wnt/β-catenin signaling pathway had been implicated in enhancing stemness and tumorigenicity of various cancer types, involving colorectal and breast cancers (Wang et al., 2010; Dong et al., 2016). This pathway’s activation can lead to an enrichment of stem-like cell populations, further complicating the landscape of tumorigenesis. Cell-free therapy using the secretome provides some main advantages compared to using stem cells: there are safer considerations compared to the transplantation of living cells, it functions similarly to traditional pharmaceutical agents, it can be stored for long periods without losing potency, and it is more economical and practical for clinical application, possible for mass-production, immediate treatment, and modification, reduced time, and cost of expansion of cultured stem cells (Vizoso et al., 2017). Previous studies have examined the therapeutic potential of bovine umbilical vein endothelial cells-conditioned media (BUVEC-CM) in the treatment of neurodegeneration, and it is known that BUVEC-CM can inhibit an apoptotic on the neuronal mediated by depletion of both caspase-9 and caspase-3 (Larasati et al., 2022). BUVEC-CM contains several protein fractions, including cytokines and growth factors, and vesicular fractions, including microvesicles and exosomes, which have therapeutic effects. Growth factors included platelet-derived growth factor, vascular endothelial growth factor (VEGF), and epidermal growth factor. Fibroblast growth factor (FGF) is also included in this list. In addition, a study using the liquid chromatography—mass spectrometry revealed that bovine umbilical mesenchymal stem cells– conditioned medium (BUMSC-CM) contain many amino acids, such as isoleucine, leucine, valine, arginine, tryptophan (Kusindarta and Wihadmadyatami, 2021), beside an ELISA also provides insight that some growth factors such as VEGF and FGF are described in BUMSC-CM (Kusindarta and Wihadmadyatami, 2021). A review of neural stem cell secretomes has shown a bystander effect such as neuroprotection and immune regulation, preventing tissue damage, disrupting pathogenic processes, or rescuing endogenous nerve cells (Zhang et al., 2020). Based on those capabilities of the stem cells and its derivate (secretome), this research focuses on the ability of the secretome or condition media derived from mesenchymal stem cells in the umbilical cord of bovine, as a therapy for neurodegeneration mainly AD, on the in-vivo model (rat model) AD induced by trimethyltin (TMT). Materials and MethodsEthical declarationIn this work, we adhered to the accepted ethical standards for the use and care of animals. Moreover, the Research Ethics Committee of the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Indonesia (Ethical Clearance No. 119/EC-FKH/Int./2023) approved all of the experimental protocols. As mentioned earlier, the animals’ natural habits were considered, and we placed them in suitable conditions to minimize any harm, including pain and distress. Preparation of BUMSC-CMFour umbilical cords had been collected from healthy pregnant cows throughout normal delivery. The samples that had been accumulated in phosphate-buffered saline (PBS) (Gibco, New York,NY) in the presence of 100 U/ml penicillin (Gibco, New York, NY) and 100 µg/ml streptomycin (Gibco, New York, NY) thereafter redeployed to the laboratory. The samples were then transferred, rinsed with PBS, and cut into 5 cm2 segments. The segments had been sliced lengthwise, and the blood vessels had been repealed. The segments were transferred to 25 cm2 flasks and cultivated in DMEM added with 100 µg/ml streptomycin and 100 U/ml penicillin as supplementation. Three media were supplemented with 10% FBS (Gibco, New York, NY), and every sample had been cultured. In a 5% CO2 conditioned environment, flasks were incubated at 37°C and were left undisturbed for 7 days alone. The medium was then replaced for the first time, and every 3 days thereafter. Two weeks later, the stem cells were stable and DMEM with serum-free (Gibco, New York, NY) was used and the cultivation was continued; the conditioned medium was harvested every 3 days. The secretome was characterized using the ELISA method, a technique validated by previous publications (Kusindarta and Wihadmadyatami, 2021), ensuring the reliability of our results. Experimental designRattus norvegicus strain Sprague Dawley rats were used as test animals acquired from the Integrated Laboratory for Research and Testing, Universitas Gadjah Mada. The rats used were male and weighed about 180–220 g. The rats were kept in a regulated condition featuring a heat of 21°C, a relative dampness ranging from 45% to 65%, and a 12-hour light and 12-hour dark rotation. They were acclimated for 1 week before treatment. Using Ps power, the sample size with 90% power was determined and obtained a total of 25 rats. This study was implemented in the Laboratory of Animal Experiments, Integrated Research and Testing Laboratory, Universitas Gadjah Mada. The rats were categorized into 5 different groups. 1. Group A (untreated) was given normal saline 1 ml/100 g BW. 2. Group B (positive group) was given TMT 8 mg/kg intraperitoneally on the first day. 3. Group C was induced with TMT 8 mg/kg intraperitoneally on the first day and Donepezil 10 mg/kg orally from the second day for 14 days. 4. Group D was induced with TMT 8 mg/kg intraperitoneally on the first day and BUMSC-CM 0.2 ml/kg BW intraperitoneally since the second day for 14 days. 5. Group D was induced with TMT 8 mg/kg intraperitoneally on the first day and BUMSC-CM 0.5 ml/kg BW intraperitoneally from the second day for 14 days. TMT and Donepezil are dissolved in normal saline. Animal models with AD were induced using TMT 8 mg/kg BW intraperitoneally. Rat behavior was observed twice a day. After 15 days of treatment, the rats were euthanized using high doses of Ketamine (Kepro, Maagdenburgstreat, Holland) and Xylazine (Kepro, Maagdenburgstreat, Holland). The brain samples obtained were subjected to ELISA and qRT-PCR. Cresyl violet stainingThe Nissl staining procedure was conducted using cresyl violet (Sigma, Steinhem, Germany). The brain slices on slides were immersed in a solution of 0.4% cresyl violet for 30 minutes. The slides were cleaned with purified water for 1 minute and then subjected to dehydration using different ethanol concentrations. 70%, 80%, and 90%, followed by absolute ethanol I and II and xylene I, II, and III. The slides were mounted with Balsam Canada and covered with a coverslip. A light microscope (Nikon, Tokyo, Japan) was used to investigate the hippocampus area in brain sections at a 40x magnification. Cell counts in the hippocampal region (mm2) were determined using Optilab Image Raster Software (Optilab, Yogyakarta, Indonesia). In silico molecular dockingIn silico retrieval and preparation of 3D protein targetValine (CID 1182), isoleucine (CID 791), and leucine (CID 6106) ligand targets, alongside the control drug donepezil (CID 3152), were sourced from the PubChem NCBI database. The B-amyloid protein (PDB ID 1AAP) was retrieved from the Protein Data Bank and prepared using Molegro Virtual Docker version 5.0. The protein’s active side was predicted with a maximum van der Waals binding cavity of 5 as a parameter. The binding cavity was determined with Molegro Virtual Docker version 5.0. Docking simulation The necessary amino acids were docked with the target protein beta amyloid using Molegro Virtual Docker version 5.0. This process was performed on a specific grid of beta-amyloid proteins. The beta-amyloid protein grid used for docking includes X=11.98, Y=18.92, Z=36.63, and radius 12. Docking parameters consisted of setting default evaluator, MolDock SE instance, MolDock evaluator: MolegroGridEvaluator, and Setting optimizer default. The MolDock SE optimizer’s parameters are as follows: populace size 50; randomize ligand 1; cavity 1; recombine 1; creation energy threshold 100; simplex distance factor 1; simplex steps 300; step size [0.6, 1.66667, 0.05, 0.5]; mutate-factor 0,3; debug 0; pre-optimize 0; mutate 2 0; mutate 1 0; de-optimize 0; local optimize 0; maximum simplex 750; pose generator [10, 10, 30]; and evaluator default. Data analysis The data were analyzed and visualized using PyMol 2.3 and Discovery Studio version 21.1.1. The analysis encompassed 3D and 2D structure displays, elucidating interactions between ligands and proteins and assessing binding energies. Brain lysate preparationThe tissue pieces of the brain were homogenized in RIPA lysis buffer (Thermofisher, Waltham, MA) with tissue weight (g) divided by RIPA lysis buffer (ml) equals 1:9. The suspension was sonicated with an ultrasonic cell disrupter. The homogenates were centrifuged to get supernatant in 5,000 × g at 8°C. The brain lysate was stored in the fridge at −80°C. ELISA for brain-derived neurotrophic factor (BDNF)This research used sandwich ELISA methods to get rat BDNF concentration. The procedure was based on a manual manufactured kit (Elabscience Houston, Texas, USA). The plate was coated with BDNF antibody. Each well was supplemented with 100 µl of standard and brain lysate and subsequently maintained for an hour and a half at 37°C. The liquid was aspirated and rinsed with washing buffer twice. Administered 100 µl of Biotinylated Detection Antibody/Antigen and allowed it to react for 60 minutes at 37°C. After an hour, remove the liquid and rinse 3 times. After rinsing, 100 µl avidin-horseradish peroxidase was introduced and incubated for 30 minutes at 37°C. Subsequently, the liquid is removed, and the object is rinsed 5 times. The substrate reagent was introduced into each well, with a volume of 90 µl, and left to incubate for 15 minutes at a temperature of 37°C. An ELISA reader operating at 450 nm wavelength was used to quantify the optical density after the stop solutions were added. Isolation of RNABefore q-PCR, the brains were stored at −20°C in RNAlater (Sigma-Aldrich, Darmstadt, Germany). As directed by the product’s procedure, 50 mg of each rat’s brain tissue was extracted for RNA using the MiniPrep Plus Quick-RNA Kit (Zymo Research, Tustin, CA). The specimen was crushed before being homogenized by using a mortar and pestle and then treated with a DNA/RNA shield. After adding proteinase K and PK digestion buffer, the sample was incubated at ambient temperature for 30 minutes. Following incubation, the material is centrifuged, and RNA lysis buffer is added. After adding pure ethanol to the supernatant, the mixture was centrifuged. The supernatant was then disposed of. Before subjecting the column to DNase I treatment, it was purified using RNA wash buffer. After that, the column was filled with DNase I and digestion buffer, and it was centrifuged. After that, RNase-free water, RNA wash buffer, and RNA preparation buffer were introduced to the column, followed by centrifugation. The Spark® Multimode Microplate Reader (TECAN, Switzerland) was used to ascertain the amount of RNA that was derived. The eluted RNA was thereafter stored at −80°C until it was reverse-transcribed into cDNA. Reverse transcriptaseThe isolated RNA reverse transcribed into cDNA using SensiFAST cDNA Synthesis Kit (Bioline, TN, USA). TransAmp buffer, RNA, RNase-free water, and reverse transcriptase are combined. The temperature variations consisted of a 25°C cycle lasting 10 minutes, followed by a 42°C cycle lasting 15 minutes, then an 85°C cycle lasting 5 minutes, and finally a constant temperature of 4°C. Afterward, the cDNA concentration was quantified and stored at −20°C using a Spark® Multimode Microplate Reader. A concentration of 50 ng/μl of cDNA was maintained for quantitative RT-qPCR gene expression analysis. Primer designThe following sequences were designed to be the tumor necrotic factor, neural growth factor (NGF), interleukin 1beta (IL-1ß), and GAPDH primers (Integrated DNA Technologies, IA) (Table 1). Quantitative polymerase chain reaction for gene expressionThe subsequent data represent the volume composition of each tube: 1-μl forward primer, 1-μl reverse primer, 7-μl nuclease-free water, 1-μl cDNA, and 10-μl SsoFast EvaGreen Kit (Bio-Rad, CA, USA). The AriaMx Real-time PCR System (Agilent, CA, USA) was used for RT-PCR In terms of temperature, the enzyme was activated for 30 seconds at 95°C. After that, 40 thermal cycles were initiated, which included denaturation for 5 seconds at 95°C, annealing/extension for 5 seconds at 47.5°C, and a gradual melt curve at 65°C to 95°C with 0.5°C increments every 5 seconds. The Agilent Aria software v2.0 program and Microsoft Excel were used to evaluate the data. In order to assess the relative expression of each gene, the measurement cycle (Cq) of the target gene in the treatment group is subtracted from the Cq of GAPDH to obtain the ΔCt of the treatment group. Subsequently, the Cq value of the target gene in the non-treated group is subtracted from the Cq value of GAPDH to determine the ΔCt of the non-treated group. The ΔCt is subsequently obtained by subtracting the treatment group’s ΔCt from the non-treated group’s ΔCt. The standardized expression ratio can be calculated by applying the formula: 2 raised to the negative power of ΔΔCt. The fold change increased if the normalized expression ratio was greater than one because the value corresponded to the normalized expression ratio.
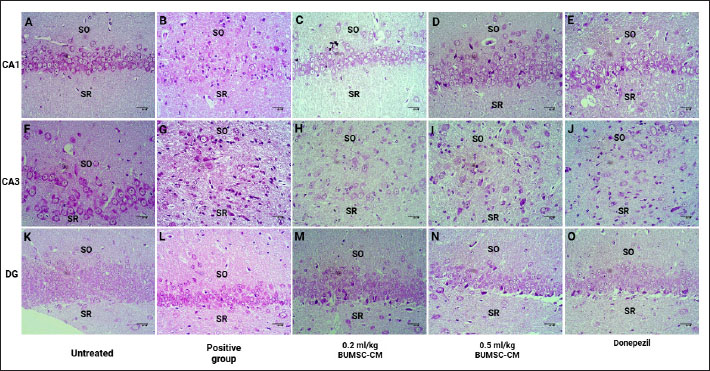
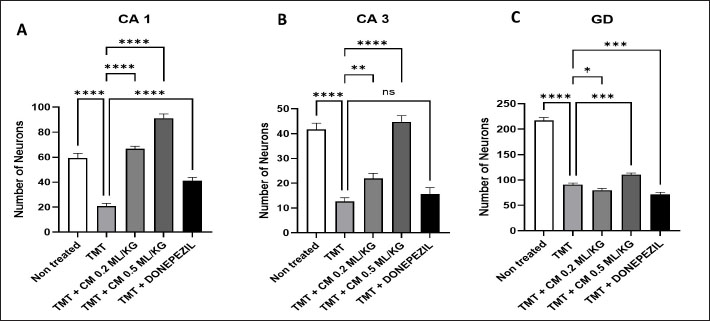
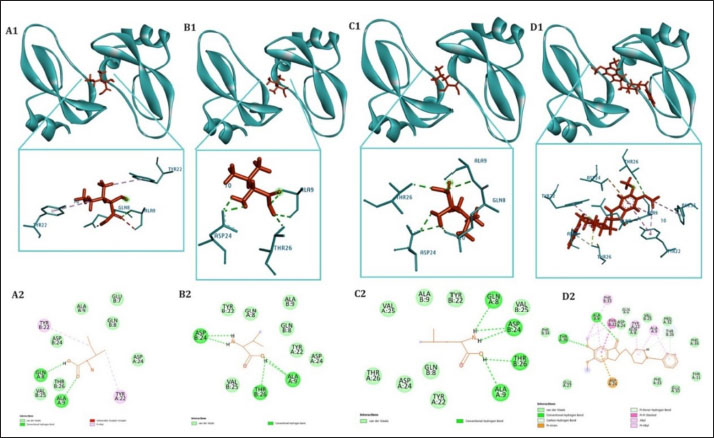
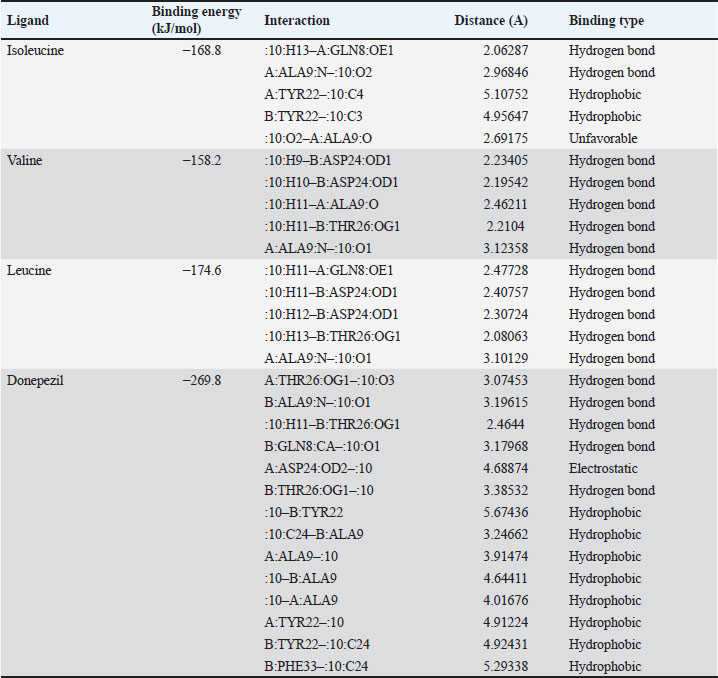
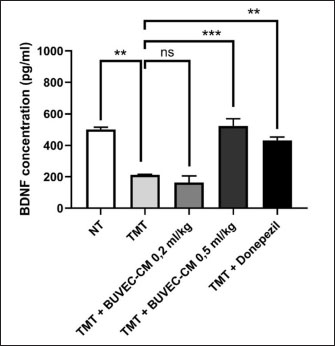
Table 1. The primer sequence ResultsThe application of bovine umbilical mesenchymal stem cell-conditioned medium increases the number of neuron cells in the cornu ammonis 1 (CA1), cornu ammonis 3 (CA3), and dentate gyrus (DG) areas of the hippocampus in an animal model of ADCresyl violet staining revealed that compared to the untreated group, rats given TMT had noticeably reduced nerve cell density in the hippocampus region, particularly in CA1, CA3, and DG. The administration of donepezil, as a commercial drug control in TMT-induced mice, showed increased neuron cell density in the CA1, CA3, and DG areas. Similarly, there is an increase in neuron cells in CA1. CA3 and DG were observed in the group treated with BUMSC-CM (Figs. 1 and 2). Nerve cell density increased with progressively higher doses of BUMSC-CM. In the TMT-induced neurodegeneration animal model, treatment with 0.2 ml/kg BW of BUMSC-CMs significantly increased neuron cell density in the CA1, CA3, and DG areas. The group treated with 0.5 ml/kg BW BUMSC-CM showed the highest increase in neuron cell density, with a significant value. Bovine umbilical mesenchymal stem cell-conditioned media interacts with B-amyloid in an in silico modelConditioned media from BUMSC consist of many amino acids, including isoleucine, leucine, and valine. The outcomes of computational testing indicate that amino acids such as isoleucine, leucine, and valine attach to amyloid beta protein in similar location as donepezil. Isoleucine binds to B-amyloid at GLN8 and ALA9 residues with hydrogen bonds, TYR22 with hydrophobic interactions, and ALA9 through weak bonds (Fig. 3) Valine interacts with multiple B-amyloid residues via hydrogen bonds, including ASP24, ALA9, and THR26. Likewise, leucine demonstrates five active sites with hydrogen bonds, involving GLN8, ASP24, THR29, and ALA9 as the active site residues. The active site bound by the third essential amino acid was also identified in the active site of Donepezil (Table 2). Bovine umbilical mesenchymal stem cell-conditioned medium increased brain-derived neurotrophic factor concentrationTo determine the result of BUMSC-CM in stimulating the development of new neurons, BDNF concentration was measured using the ELISA method. The ELISA results explained that TMT-induced animals will reduce BDNF concentrations, significantly decreasing BDNF levels compared to non-treated ones. The administration of donepezil as a commercial drug comparison and BUMSC-CM showed increased BDNF concentration compared to the TMT group with various concentrations. The administration of BUMSC-CM to enhance BDNF concentration will be most effective at a dosage of 0.5 ml/kg BW (Fig. 4).
Fig. 1. The neuronal density in the CA1, CA3, and DG regions of the hippocampus in the rat model of AD. Cresyl violet staining shows the (A, F, K) control group; (B, G, L) positive control (TMT); (C, H, M) treatment A (TMT + BUMSC-CM 0.2 mg/kg BW); (D, I, N) ) treatment B (TMT + BUMSC-CM 0.5 mg/kg BW); (E, J,O) commercial drug comparator (TMT + Donepezil); SO=stratum oriens; SR=stratum radiatum.
Fig. 2. The graphic represents the quantification of neuron cell density in the CA1, CA3, and DG areas. There is an increase of nerve cells in the non-treated group, the comparison drug group (Donepezil), and the BUMSC-CM treatment group with doses of 0.2 and 0.5 mg/kg BW compared to the positive control (TMT) (*: p < 0.0001), ns=no significant.
Fig. 3. Interaction between the essential amino acids isoleucine, valine, leucine, and donepezil on beta-amyloid protein. Tosca represents beta-amyloid protein, red indicates target ligand. A. isoleucine—B-amyloid, B. valine—B-amyloid. C. leucine—B-amyloid, D. donepezil—B-amyloid. Both the 3D and 2D structures of the ligand-protein complex are symbolized as 1 and 2, respectively.
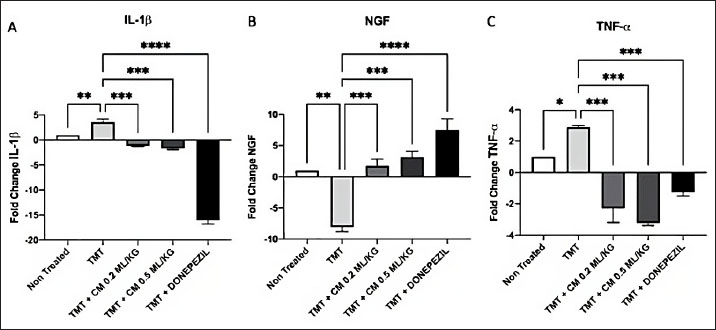
Table 2.Interaction between the essential amino acids isoleucine, valine, leucine, and donepezil on beta-amyloid protein. Bovine umbilical mesenchymal stem cell-conditioned medium down-regulated IL-1ß, tumor necrotic factor-alpha (TNF-a), and up-regulated NGF expressionIn order to evaluate the impact of BUMSC-CM on inflammation in AD, we analyze the manifestation of IL-1ß and TNF-α expression. To assess the ability of BUMSC-CM to stimulate the proliferation of neurons, we measured the expression of NGF using quantitative real-time PCR. According to the IL-1ß expression data (Fig. 5A), the positive control group (TMT-treated) had 3.6-fold higher IL-1ß expression than the non-treated group. The groups receiving a dosage of 0.2 and 0.5 ml/kg of BUMSC-CM experienced a drop in IL-1ß expression by a factor of 1.1 and 1.7, respectively. On the other hand, the group that was compared (Donepezil) exhibited a 16-fold reduction in IL-1ß expression (positive control group vs. non-treated, BUMSC-CM 0.2 ml/kg, 0.5 ml/kg and comparison groups: p=0.0060, p=0.0004, p=0.0002, and p < 0.0001, respectively).
Fig. 4. BUMSC-CM graph increases the BDNF concentration in the brain of rats modeled for AD induced with TMT. Mice were induced with TMT single dose intraperitoneally as an Alzheimer’s model animal, and donepezil was given orally 14 days post-TMT induction as a commercial drug comparison group, BUMSC-CM with 0.2 ml/kg, and 0.5 ml/kg dosage was given 14 days intraperitoneally post-TMT induction as a treatment group. Rat brain lysate was analyzed for BDNF by sandwich ELISA. The concentration data were computed using one-way ANOVA and then analyzed further using Tukey’s post hoc test (NT=non-treated; **, and ***, are the statistically significant values of the TMT group as positive control group with treatment and negative control groups, p values < 0.0038; 0.0008; n.s.=not significant, respectively). The findings of the NGF expression analysis (Fig. 5B) indicated that the positive control group (TMT-treated) showed a notable 8-fold reduction in NGF expression in contrast to the group that was not treated. NGF expression was increased by 1.7- and 3.1-fold in the BUMSC-CM dosage groups at 0.2 and 0.5 ml/kg, respectively. Meanwhile, the comparison group (Donepezil) had a 7.5-fold increased NGF expression (positive control group vs. non-treated, BUMSC-CM 0.2 ml/kg, 0.5 ml/kg and comparison groups: p=0.0010, p=0.0007, p=0.0004, and p < 0.0001, respectively). The results of the TNF-α expression analysis (Fig. 5C) indicated that the group of positive controls (TMT-treated) exhibited a 2.8-fold higher TNF-α expression than the non-treated group. The BUMSC-CM 0.2 and 0.5 ml/kg dosage groups had a 2.3- and 3.2-fold decreased TNF-α expression, respectively. Meanwhile, the comparison group (Donepezil) exhibited a 1.2-fold reduction in TNF-α expression (positive control group vs. non-treated, BUMSC-CM 0.2 ml/kg, 0.5 ml/kg and comparison groups: p=0.0191, p=0.0002, p=0.0001, and p=0.0006, respectively). DiscussionIn this work, we administer 2 different dosages of BUMSC-CM, specifically 0.2 and 0.5 ml/kg BW, to evaluate its effectiveness as a treatment in a live rat model induced with TMT. The study used the following parameters: BDNF, NGF, TNF-α, and IL-1ß. The ELISA results evaluating the concentration of BDNF in the rat’s brains indicated that the group treated with BUMSC-CM, at a dosage of 0.5 ml/kg BW exhibited a higher BDNF concentration compared to the non-treated and positive control groups. MSC-CM has been shown to enhance BDNF levels through various mechanisms. For instance, Cao et al. (2012) reported that nerve injury leads to increased BDNF levels in the dorsal root ganglia, suggesting that similar mechanisms could be activated by the application of MSC-CM, which contains a diversity of growth factors and cytokines that might stimulate BDNF production. This aligns with the understanding that MSC secrete factors that can modulate the local environment and promote neurotrophic support. BDNF is recognized for its involvement in synaptic plasticity, synaptic transmission, and synaptogenesis. This chemical is uniquely associated with synapse control in the human body. BDNF is important in facilitating synapse development by regulating axonal branching, dendritic growth, and activity-dependent synapse refinement. Research indicates that the control of synaptic plasticity and synaptic development by BDNF implies that it has a significant influence on cognitive functioning (Lu et al., 2013). Prior studies have demonstrated that the application of secretome generated from mesenchymal stem cells causes rats’ cortical and hippocampus neurons’ axons to grow longer; BDNF mediates this effect. (Martins et al., 2017). Furthermore, existing research indicates that BDNF is purportedly responsible for triggering the growth and specialization of neural stem cells into nerve cells and oligodendrocytes via stimulating the Wnt/β-catenin signaling pathway. Additionally, evidence indicates that the intravenous treatment of BDNF within the initial 5 days following an ischemic stroke can result in a notable enhancement of sensorimotor function for up to 6 weeks. Furthermore, it was shown that the administration of BDNF led to a substantial augmentation of neurogenesis within the dentate gyrus region. There was an increase in progenitor cell migration from the subventricular area to the ischemic hemisphere’s striatum in the region. The administration of mesenchymal stem cell secretome products containing BDNF is anticipated to facilitate neuroprotection by promoting the growth and specialization of stem cells into neurons. Moreover, consistent with the data acquired from BDNF expression, investigation using reverse transcriptase PCR revealed a distinct elevation in the expression of NGF. Similar to BDNF, NGF is essential for preserving and improving neuronal function in the brain. This protein facilitates the proliferation, viability, and specialization of neurons. NGF is a signaling molecule facilitating communication with neurons to promote their well-being and overall operation. NGF is synthesized by different cell types in the brain, such as astrocytes and microglia. It attaches to receptors on neurons, initiating a series of actions that enhance the growth and development of neurons. The signaling pathway controls vital processes such as synaptic plasticity, neurogenesis, and neuronal survival. NGF exerts neuroprotective effects by preventing neuronal degeneration and promoting synaptic plasticity, thereby facilitating the establishment of connections between neurons and other cells. It is especially significant in AD, as it involves a gradual reduction in synapses and a drop in cognitive function. NGF can decelerate or perhaps reverse these harmful consequences by stimulating the development of neurons and inhibiting cell death. Research suggests that decreased levels of NGF are associated with cognitive decline and increased susceptibility to ND conditions such as AD. Hence, discovering methods in this research through BUMSC-CM to augment the quantities of NGF in the brain could have substantial therapeutic ramifications for this debilitating ailment.
Fig. 5.BUMSC-CM reduced the expression of IL-1ß, TNF-α, and increased NGF expression in rats induced by neurotoxic agent TMT. Gene expression results were taken based on the fold change, and then calculated with one-way ANOVA followed by Tukey’s post hoc test (*, **, ***, and ****, are the statistically significant values of the TMT group as positive control vs. BUMSC-CM 0.2 ml/kg, BUMSC-CM 0.5 ml/kg, and Donepezil groups, with an optimal BUMSC-CM dosage of 0.5 ml/kg). Furthermore, the other significant contributors to the development and advancement of AD are IL-1β and TNF-α. Pro-inflammatory cytokines such as TNF-α and IL-1β are essential for the immunological response. This study shows that the pro-inflammatory cytokine’s expression can be reduced by BUMSC-CM. In normal conditions, the IL-1β and TNF-α help regulate various cellular processes involved in inflammation and immune function. However, in the context of AD, IL-1β can become overactive and contribute to neuroinflammation. Research has demonstrated that persons with AD had higher concentrations of IL-1β in their brain, particularly in areas affected by pathology such as amyloid plaques and neurofibrillary tangles. This chronic inflammatory response mediated by IL-1β can lead to neuronal damage and cognitive decline. It has been implicated in promoting neuroinflammation and disrupting synaptic plasticity and impairing memory formation. In line with IL-1β, TNF-α is found in elevated concentrations within the brain, resulting in persistent inflammation and neuronal impairment. Studies have shown that TNF-α may cause neurons to undergo apoptosis and promote the formation of amyloid plaques, which are distinctive characteristics of AD. It also disrupts synaptic function, impairing communication between brain cells. Thus, how can the BUMSC-CM suppress the expression of IL-1β and TNF-α? Some research proposes that mesenchymal stem cells provide an immunoregulatory effect; Therefore, it has the potential to reduce the output of IL-1β and TNF-α while concurrently increasing the expression of anti-inflammatory cytokines such as IL-10. The interruption with the nuclear factor-kappa B (NF-κB) signaling pathway is one of the main ways whereby mesenchymal stem cells (MSCM) stop the release of TNF-α and IL-1β. When NF-κB is active, it initiates the synthesis process of several pro-inflammatory cytokines, such as IL-1β and TNF-α. Treatment with MSCM demonstrated reducing oxidative stress, thereby repressing NF-κB activation and subsequently decreasing the expression of TNF-α (Fatmawati et al., 2019). This suggests that MSCM may exert similar effects by modulating oxidative stress levels. The interaction of MSCM with macrophages may have an important responsibility in suppressing IL-1β and TNF-α expression. Studies have revealed that conditioned media from stem cells can alter the polarization of macrophages to change their pro-inflammatory (M1) to anti-inflammatory (M2) phenotype. This shift is associated with decreased production of inflammatory cytokines. For instance, Son et al. (2013) found that certain compounds could suppress TNF-α and IL-1β production in macrophages, suggesting that MSCM may have similar effects by influencing macrophage behavior. A study was undertaken to analyze the metabolic content of BUMSC-CM using Liquid ChromatographyMass Spectrometry/ Mass Spectrometry, which accurately identified multiple crucial amino acids, including isoleucine, leucine, valine, arginine, and tryptophan (Kusindarta and Wihadmadyatami, 2021). Our in silico model study indicated that isoleucine, leucine, and valine were able to bind to the active side of amyloid beta protein with hydrogen bonds and hydrophobic interactions. Specifically, isoleucine interacts with Gln8, Ala9, and Tyr22, valine binds with Asp23, Ala9, and Thr26, and leucine interacts with Gln8, Asp24, Thr29, and Ala9. The amyloid β-peptide (Aβ) is the main component of amyloid fibrils that are present in the brain plaques of persons diagnosed with AD (Agarwal et al., 2013). The predominant forms of amyloid β-peptide are Aβ1-40 and Aβ1-42, which consist of 40 and 42 amino acids, respectively. Aβ1-42 has two more hydrophobic amino acids in comparison to Aβ1-40, which encourages the formation of larger fibrils in Aβ1-42, making it more harmful (Butterfield et al., 2013; Acosta et al., 2018). Aβ1-42 is generated from the amyloid precursor protein via enzymatic processing and cleavage by β- and γ-secretase enzymes. Environmental conditions such as pH, salts, and protein then promote Aβ1-42 to form monomers, dimers, soluble oligomers, and protofibrils, eventually leading to fibrils that accumulate in plaques (Acosta et al., 2018; Hampel et al., 2021) In consideration of the data, we did cresyl violet staining to examine the neuronal density particularly in the dentate gyrus, CA1, and CA3 regions of the hippocampus. Our finding shows that the administration of BUMSC-CM maintains and increases the number of nerve cells due to the administration of TMT as a neurotoxic organotin compound. Our study provides a novel insight of NDDs therapies, particularly AD and cortical cerebellar degeneration, by using cell-free therapy (BUMSC-CM). BUMSC-CM is able to give neuroprotectant on the rat model AD mediated by the upregulation of BDNF and NGF and, in line with this condition, also downregulation of TNF-α and IL-1β. ConclusionBUMSC-CM exhibit significant potential in the field of NDD, and their activities are under the influence of many cytokines and growth factors, which is proposed in this study to provide neuroprotection through upregulation of BDNF and NGF and also downregulation of TNF-α and IL-1β. This work offers a comprehensive review of the function of BDNF and NGF in the secretome on a rat model of AD. This work provides an identification of potential approaches to enhance the therapeutic benefits of BUMSC-CM. Meanwhile, further research still needs to be carried out considering that there is still very little research on CM originating from animals. AcknowledgmentsThe authors wish to thank Universitas Gadjah Mada, Bandung Institute of Technology, and Brawijaya University, for the Indonesian Collaboration Research 2024 (RKI 2024). Conflict of interestAll authors declared that there is no conflict of interest. Financial supportThis work was supported by the Research Collaboration Grant with the grant number 1874/UN1/DITLIT/PT.01.03/2024. Authors contributionConceptualization: HW, MAZ, and YT; Methodology: HW, MAZ, HH, DAOP SK, and DRT; Validation: HW, MAZ, and HH; Formal analysis: GRS, DA, NH, and UK; Investigation: GRS, DA, NH, and UK; Resources: HW, GRS, DA, NH, UK, and YT; Data curation: HW, GRS, DA, NH, UK, and DRT; Writing-original draft preparation: HW, GRS, DA, UK, and DRT; Writing-review editing: HW, GRS, DA, and UK; Visualization: GRS, UK, DA, and DRT; Supervision: HW, DAOP, and HH; Project administration: HW; and Funding acquisition: HW. Data availabilityThe manuscript contains all the data that support the findings of this study. References Acosta, D.M.A.V., Vega, B.C., Basurto, J.C., Morales, L.G.F. and Hernandez, M.C.R. 2018. Recent advances by in silico and in vitro studies of amyloid-β 1-42 fibril depicted a S-Shape conformation. Int. J. Mol. Sci. 19(8), 2415. Agarwal, V., Linser, R., Dasari, M., Fink, U., del Amo, J.M.L. and Reif, B. 2013. Hydrogen bonding involving side chain exchangeable groups stabilize amyloid quarternary structure. Phys. Chem. Chem. Phys. 15(30), 12551–12557. Butterfield, D.A., Swomley, A.M. and Sultana, R. 2013. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid. Redox. Signal. 19(8), 823–835. Cao, X. H., Chen, S. R., Li, L., and Pan, H. L. 2012. Nerve injury increases brain-derived neurotrophic factor levels to suppress bk channel activity in primary sensory neurons. J. Neurochem. 121(6), 944–953. Dong, H., Jang, G., Lee, H., Park, S., Kim, J., Nam, J. and Hong, I. 2016. The wnt/β-catenin signaling/id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 6(1), 1–13. Fatmawati, F., Erizka, E., and Hidayat, R. 2019. Royal jelly (bee product) decreases inflammatory response in wistar rats induced with ultraviolet radiation. OAMJMS. 7(17), 2723–2727. Gan, L., Cookson, M.R., Petrucelli, L., and La-Spada, A.R. 2018. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309. Giordano, S., Darley-Usmar, V. and Zhang, J. 2014. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox. Biol. 2, 82–90. Hampel, H., Hardy, J., Blennow, K., Chen, C., Perry, G., Kim, S., Villemagne, V., Aisen, P., Vendruscolo, M., Iwatsubo, T., Masters, C., Cho, M., Lannfelt, L., Cummings, J. and Vergallo, A. 2021. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry. 26, 5481–5503. Kooreman, N.G. and Wu, J.C. 2010. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J. R. Soc. Interface. 7(6), S753–S763. Kusindarta, D.L. and Wihadmadyatami, H. 2021. Conditioned medium derived from bovine umbilical mesenchymal stem cells as an alternative source of cell-free therapy. Vet. World. 14(10), 2588–2595. Larasati, V.A., Lembang, G.V., Tjahjono, Y., Winarsih, S., Ana, I.D. and Wihadmadyatami, H. 2022. In vitro neuroprotective effect of the bovine umbilical vein endothelial cell conditioned medium mediated by downregulation of IL-1β, caspase-3, and caspase-9 expression. Vet. Sci. 9(2), 1–13. Lu, B., Nagappan, G., Guan, X., Nathan, P.J. and Wren, P. 2013. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 401–416. Lu, J., Yang, Q., Zhang, H., Eslinger, P.J., Zhang, X., Wu, S., Zhang, B., Zhu, B. and Karunanayaka, P. 2019. Disruptions of the olfactory and default mode networks in alzheimer’s disease. Brain. Behav. 9(7) 1–13. Marsh, S.E. and Blurton-Jones, M. 2017. Neural stem cell therapy for neurodegenerative disorders: the role of neurotrophic support. Neurochem. Int. 106, 94–100. Martins, L.F., Costa, R.O., Pedro, J.R., Aguiar, P., Serra, S.C. and Teixeira, F.G. 2017. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 7(1), 1–13. Ransohoff, R.M. 2016. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 353, 772–777. Son, Y., Chung, H., and Pae, H. 2013. Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in raw264.7 macrophages. BioFactors. 40(1), 138–145. Spillantini, M.G. and Goedert, M. 2013. Tau pathology and neurodegeneration. Lancet. Neurol. 12(6), 609–622. Vizoso, F.J., Eiro, N., Cid, S., Schneider, J. and Perez-Fernandez, R. 2017. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 18(9), 1–24. Wang, K., Kao, A., Chang, C., Lee, J., Hou, M., Long, C., Chen, H., and Tsai, E. 2010. Increasing cd44+/cd24- tumor stem cells, and upregulation of cox-2 and hdac6, as major functions of her2 in breast tumorigenesis. Mol. Cancer. 9(1), 1–15. Wihadmadyatami, H., Zulfikar, M.A., Herawati, H., Pratama, D.A.O.A., Saragih, G.R., Kustiati, U., and Handayani, A.N. 2023. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine Alzheimer’s like mediated by inhibition of neuronal apoptotic. Open. Vet. J. 13(12), 1504–1516. Yamanaka, S. 2020. Pluripotent stem cell-based cell therapy—promise and challenges. Cell. Stem. Cell. 27(4), 523–531. Zhang, Q., Li, J., An, W., Fan, Y. and Cao, Q. 2020. Neural stem cell secretome and its role in the treatment of neurodegenerative disorders. JIN. 19(1), 179–185. | ||
| How to Cite this Article |
| Pubmed Style Wihadmadyatami H, Zulfikar MA, Herawati H, Karnati S, Saragih GR, Aliffia D, Pratama DA, Handayani N, Kustiati U, Tirtosari DR, Tjahjono Y. Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Vet. J.. 2025; 15(1): 151-161. doi:10.5455/OVJ.2025.v15.i1.14 Web Style Wihadmadyatami H, Zulfikar MA, Herawati H, Karnati S, Saragih GR, Aliffia D, Pratama DA, Handayani N, Kustiati U, Tirtosari DR, Tjahjono Y. Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. https://www.openveterinaryjournal.com/?mno=213898 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.14 AMA (American Medical Association) Style Wihadmadyatami H, Zulfikar MA, Herawati H, Karnati S, Saragih GR, Aliffia D, Pratama DA, Handayani N, Kustiati U, Tirtosari DR, Tjahjono Y. Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Vet. J.. 2025; 15(1): 151-161. doi:10.5455/OVJ.2025.v15.i1.14 Vancouver/ICMJE Style Wihadmadyatami H, Zulfikar MA, Herawati H, Karnati S, Saragih GR, Aliffia D, Pratama DA, Handayani N, Kustiati U, Tirtosari DR, Tjahjono Y. Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 151-161. doi:10.5455/OVJ.2025.v15.i1.14 Harvard Style Wihadmadyatami, H., Zulfikar, . M. A., Herawati, . H., Karnati, . S., Saragih, . G. R., Aliffia, . D., Pratama, . D. A., Handayani, . N., Kustiati, . U., Tirtosari, . D. R. & Tjahjono, . Y. (2025) Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Vet. J., 15 (1), 151-161. doi:10.5455/OVJ.2025.v15.i1.14 Turabian Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Srikanth Karnati, Golda Rani Saragih, Dinda Aliffia, Dyah A.o.a. Pratama, Nurrahmi Handayani, Ulayatul Kustiati, Dewi Ratih Tirtosari, and Yudy Tjahjono. 2025. Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Veterinary Journal, 15 (1), 151-161. doi:10.5455/OVJ.2025.v15.i1.14 Chicago Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Srikanth Karnati, Golda Rani Saragih, Dinda Aliffia, Dyah A.o.a. Pratama, Nurrahmi Handayani, Ulayatul Kustiati, Dewi Ratih Tirtosari, and Yudy Tjahjono. "Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β." Open Veterinary Journal 15 (2025), 151-161. doi:10.5455/OVJ.2025.v15.i1.14 MLA (The Modern Language Association) Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Srikanth Karnati, Golda Rani Saragih, Dinda Aliffia, Dyah A.o.a. Pratama, Nurrahmi Handayani, Ulayatul Kustiati, Dewi Ratih Tirtosari, and Yudy Tjahjono. "Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β." Open Veterinary Journal 15.1 (2025), 151-161. Print. doi:10.5455/OVJ.2025.v15.i1.14 APA (American Psychological Association) Style Wihadmadyatami, H., Zulfikar, . M. A., Herawati, . H., Karnati, . S., Saragih, . G. R., Aliffia, . D., Pratama, . D. A., Handayani, . N., Kustiati, . U., Tirtosari, . D. R. & Tjahjono, . Y. (2025) Neuroprotection effect of bovine umbilical mesenchymal stem cell-conditioned medium on the rat model of Alzheimer’s disease mediated by upregulation of BDNF and NGF and downregulation of TNF-α and IL-1β. Open Veterinary Journal, 15 (1), 151-161. doi:10.5455/OVJ.2025.v15.i1.14 |