| Research Article | ||
Open Vet. J.. 2025; 15(1): 162-170 Open Veterinary Journal, (2025), Vol. 15(1): 162-170 Research Article Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretomeTri Isyani Tungga Dewi1*, Deni Noviana2, Bambang Pontjo Priosoeryanto3, Gunanti Gunanti2 and Mawar Subangkit31Graduate Program in Animal Biomedical Sciences, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 2Division of Surgery and Radiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 3Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia *Corresponding Author: Tri Isyani Tungga Dewi. Graduate Program in Animal Biomedical Sciences, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia. Email: isyanitri [at] apps.ipb.ac.id Submitted: 01/08/2024 Accepted: 07/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
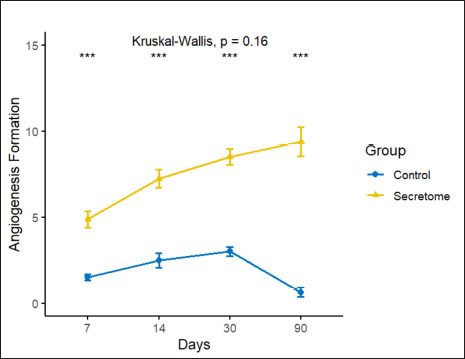
AbstractBackground: Pelvic organ prolapse increases in prevalence and incidence in older women and hypoestrogenic conditions. Treatment with native tissue surgery has a fairly high recurrence rate. Mesh-augmented surgery is one of the most promising treatments for pelvic organ prolapse, with high effectiveness and low recurrence. Mesh-augmented surgery has a side effect of tissue erosion. The addition of secretome is expected to improve tissue integrity and reduce tissue erosion. Aim: This study aimed to investigate the effect of adding the umbilical cord mesenchymal stem cell (UC-MSC) secretome on preventing tissue inflammatory responses, improving tissue integrity, and accelerating wound healing. Methods: A total of 32 female New Zealand white rabbit hypoestrogenic models were divided into two groups: the control group with normal mesh and the secretome group with artificial mesh. Hypoestrogenic models were created using the bilateral ovariectomy method. Mesh implantation was performed using a surgical method on hypoestrogenic rabbits. The animals were euthanized on days 7, 14, 28, and 90 after mesh implantation. Histopathology parameters included angiogenesis formation, fibroblast number, and collagen deposition area. Result: The results of this study showed that the number of angiogenesis, fibroblast, and collagen deposition data in the secretome group showed higher significantly (p < 0.05) than those in the control group on days 7, 14, 28, and 90 post mesh implantation. The formation of new blood vessels (angiogenesis) in the secretome group demonstrated a mean value of 9.81 ± 2.2 compared to 0.37 ± 0.03 in the control. The number of fibroblasts in the secretome group averaged 151.00 ± 8.14, in contrast to 34.00 ± 13.37 in the control group. Collagen formation in the secretome group was also higher, with a mean value of 80.02 ± 6.71 compared to 59.49 ± 4.61 in the control group over 90 days of observation. Conclusion: The administration of secretomes from UC-MSC improved tissue integrity and accelerated wound healing. Keywords: Hypoestrogenic, Angiogenesis, Mesh, Prolapse, Secretome. IntroductionUterine prolapse is a form of pelvic organ prolapse caused by weakness of the ligament and supporting fascia, leading to the exit of the uterus through the vagina. The prevalence of uterine prolapse varies depending on the population studied and the diagnostic methods employed; however, it is estimated to affect approximately 3%–6% of women worldwide in clinically diagnosed cases. When including milder and asymptomatic cases, the prevalence may increase to around 30%–50%, particularly among women over the age of 50 years. In the United States, approximately 11%–19% of women are expected to undergo surgery for prolapse or incontinence at some point in their lives. Pelvic organ prolapse is a frequent medical condition whose prevalence and incidence increase with age and hypoestrogenic conditions that result in weakness of the ligaments and fascia supporting the uterus. Estrogen plays a crucial role in the wound-healing process across all stages, including hemostasis/inflammation, proliferation, and remodeling. It helps by reducing the size of the wound, enhancing collagen deposition by regulating the levels of collagen I and III during the remodeling phase, and strengthening the tissue. Estrogen can also promote angiogenesis and regeneration by increasing the rate of epidermal cell mitosis. Low estrogen levels inhibit the wound-healing process. Pelvic organ prolapse treatment with native tissue surgery has a short-term anatomical repair rate of 35%–72% and recurrence is quite high, reaching 30%, due to the results of repair with native tissue showing that there are still weaknesses in the supporting connective tissue that have been repaired (Van Geelen and Dwyer, 2013). Mesh treatment is one of the most promising treatments for pelvic organ prolapse. The augmentation of prolapsed tissue with mesh-augmented repair is a reconstructive technique that provides satisfactory results, with an effectiveness rate of 76%–96% and a recurrence rate of 7.4% (Skala et al ., 2011). Mesh-augmented surgery has high efficacy and low recurrence. However, mesh-related complications leading to tissue erosion have been reported in 55% of cases, which is thought to be due to suboptimal wound healing and tissue integration with the mesh (Moen et al., 2014). The use of mesh has decreased dramatically with the U.S. Food and Drug Administration (FDA) warning, regarding postoperative complications, particularly mesh erosion/extrusion. In 2011, the FDA reported several complications arising after mesh insertion in pelvic organ prolapse repair, including mesh erosion, infection, bleeding, urinary tract problems, and organ perforation (Mancuso et al., 2019). To address the tissue damage associated with mesh implantation in pelvic organ prolapse, novel therapeutic approaches are being explored to improve outcomes. One promising strategy involves integrating reconstructive surgery with cell-based therapies, specifically leveraging stem cells because of their regenerative potential. Recent advancements in stem cell research have led to the use of the stem cell secretome, which contains bioactive molecules secreted by stem cells as progenitor agents for tissue repair and regeneration. In this study, the application of the stem cell secretome represents an innovative approach in preliminary trials aimed at combining stem cell-derived factors with reconstructive surgical techniques to enhance pelvic tissue integrity and function. The mesenchymal stem cell secretome contains a number of chemokine and cytokine growth factors, such as interleukin-6 and interleukin-8 (IL-6, IL-8), proteases and protease inhibitors, such as matrix metalloproteinase (MMP)-1 and 2, and extracellular matrix (ECM) molecules that assist in cell regeneration to accelerate the wound-healing process (Dilogo et al., 2020). Stem cells also provide growth factors that stimulate endothelial growth factors to accelerate angiogenesis (Amani et al ., 2019). In another field, the addition of stem cells accelerates muscle tissue regeneration by increasing the proliferation of myocyte cells (Bakhtiary et al., 2021). The addition of mesenchymal stem cell secretomes to the mesh can improve tissue integration and prevent excessive tissue inflammatory responses, thereby minimizing tissue erosion. This study aimed to analyze the inflammatory response and tissue healing after mesh insertion with the addition of umbilical cord mesenchymal stem cell (UC-MSC) secretome in hypoestrogenic rabbit models. Materials and MethodsEthical approvalAll procedures in this study were approved by the Animal Ethics Commission of the School of Veterinary Medicine and Biomedical Sciences, IPB University (certificate number: 002/KEH/SKE/VI/2022. MaterialsIn this study, we used stem cell secretome as the primary investigational material, with female New Zealand White rabbits serving as the animal model. The experimental procedures involved the use of an incubator (Labline, JPN), centrifuge (Labogene 1580R, JPN), Laminar Chamber (Thermo Scientific, JPN), Microtome (Leica, JPN), surgical instruments (Reinz, ITA), and sutures using Prolene® 3.0. Anesthesia was administered using a combination of ketamine (KET-A 100, PER) and xylazine (Xyla 20, USA) and EUTHASOL® (pentobarbital sodium and phenytoin sodium). Additionally, a mesh implant was used along with hematoxylin-eosin (HE) staining and Masson’s trichrome staining kits for histological analysis. Secretome preparationThe secretome was prepared at the Stem Cell and Tissue Engineering Research Cluster, Indonesian Medical Education and Research Institute, University of Indonesia, following standardized laboratory protocols for extraction from UC-MSCs. Five passage UC-MSCs were cultured in six 25 cm² flasks, each containing 5 ml of complete medium, with medium changes conducted every 2–3 days. The cell cultures were maintained in a controlled environment, with an incubator set to 37°C and 5% CO2 to ensure optimal cell growth. When cell confluency reached 80%–90%, the conditioned culture medium was harvested for secretome processing. A total volume of 30 ml of conditioned medium was collected from the six flasks. The conditioned medium was centrifuged at 3,500 rpm for 30 minutes to separate the cellular debris. The supernatant was then sequentially filtered through 0.45 and 0.22 µm filters to remove particulates, resulting in a purified secretome for downstream applications. Modeling a hypoestrogenic animalThis study used 32 female New Zealand white rabbits (Oryctolagus cuniculus) at 1 year of age, with body weights of 3.0–3.5 kg. Modeling of hypoestrogenic animal models was carried out using the bilateral ovariectomy method. The hypoestrogenic conditions were determined by measuring blood estradiol levels before ovariectomy surgery (day 0) as a baseline and 30-day post-ovariectomy. The results of the examination of estradiol levels showed that the rabbit models experienced hypoestrogenic conditions on the 30th day after ovariectomy, marked by a decrease in estradiol levels of more than 50% of those before ovariectomy. In addition, vaginal cytology was performed to ensure that the animal was no longer in the estrus phase. Vaginal cytology on day 30 revealed only small and large nucleated epithelial cells. Cornified epithelial cells were not observed. The animal model was created as described previously (Dewi et al., 2024). Mesh implantationHypoestrogenic rabbits were divided into two groups: the control and secretome groups. Each group contains 16 animals. Before mesh implantation, the animal models were anesthetized using a combination of ketamine (10 mg/kg BW and Xylazine 3 mg/kg BW) via intramuscular injection. After anesthesia, a 2-cm transversal incision was made on the anterior vaginal wall, followed by tissue dissection of 1.5–2.0 cm laterally and 3.0–3.5 cm longitudinally to the rectovaginal fascia. Mesh implantation was performed in the submucosal layer of the anterior vagina. All mesh corners were fixed to the tissue using prolene® 3.0 suture. In the treatment group, the mesh was supplemented with secretome. The peritoneum was closed with simple sutures, and the subcutaneous layer was sutured with continuous sutures using prolene® 3.0. Skin incisions were sutured using silk braided® 3.0. The subjects were followed for 90 days, and macroscopic and microscopic analysis was performed on days 7, 14, 3, and 90 post-mesh implantations. Animal models were euthanized with EUTHASOL® (pentobarbital sodium and phenytoin sodium) at a dose of 0.2 ml/kg body weight. We conducted euthanasia according to the rules of the AVMA Guidelines for the Euthanasia of Animals (AVMA, 2020). Histopathological and immunohistochemical analysisThe samples were fixed in 10% neutral buffered formalin and followed in tissue processed by paraffin-embedded methods. A 5-µm-thick tissue section was stained by HE. HE staining was employed to examine angiogenesis (formation of new blood vessels) and fibroblast proliferation during the observation. Angiogenesis and fibroblast numbers were counted manually in five fields of view in the mesh implantation area. Masson’s trichrome staining was used to observe collagen deposition in tissue with mesh implantation, as previously described. The aniline blue expression related to collagen was measured by calculating the proportion area and other objects using ImageJ (www.imagej.net). Data analysisThe data is statistically processed by comparing Control and Secretome group with paired t-test method and visualization graph was performed in R software ver. 4.4.0 (www.r-project.org). ResultsMeasurement of angiogenesis and fibroblast cellsThe measurement of angiogenesis volume is presented in Figure 1. Histopathological staining results are shown in Figure 2. The secretome group had a significantly higher all-day observation rate than the control group (p < 0.05). Angiogenesis increased consistently in the secretome group until the end of observation. Based on Figure 1, the formation of new blood vessels experienced an increasing trend during the observation period, in contrast to the control group, which experienced a decrease on day 90.
Fig. 1. Number of angiogenesis formation in the experimental rabbit after 90 days of observation in the control and secretome groups. Asterisks (*) imply significance value with p ≤ 0.05 (**p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001). Data represent mean ± SD.
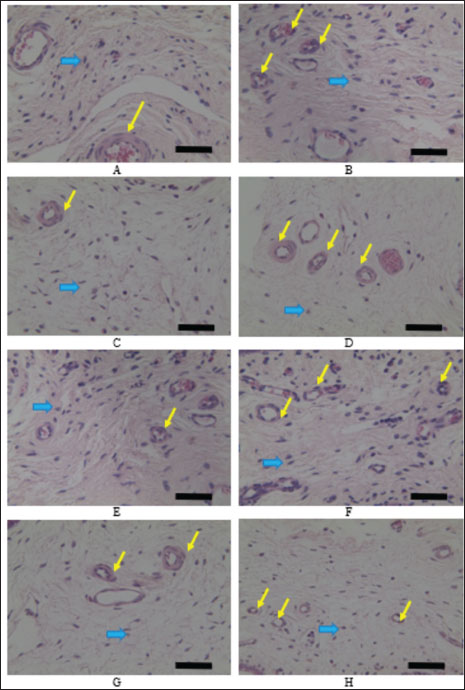
Fig. 2. Microscopic observation of angiogenesis and fibroblast cells using HE staining and observed using microscope with 100× magnify. A. Control groups on day 7, B. Secretome groups on day 7, C. Control group on day 14, D. Secretome groups on day 14, E. Control groups on day 30, F. Secretome groups on day 30, G. Control groups on day 90, H. Secretome groups on day 90. The yellow arrow indicates new vascular blood in the tissue, and the blue arrow indicates fibroblast cells in the tissue. The scale bar equals with the 100 μm. Fibroblast cell counts are presented in Table 1. In line with the angiogenesis data, the measurement of the number of fibroblasts showed that the secretome group had a higher number of fibroblasts than the control group. The results of measuring the number of fibroblasts showed a significant difference between the secretome and control groups on all days of observation (Fig. 2). The number of fibroblasts was higher in the secretome group and increased during the observation day, in contrast to the control group, which decreased on day 90. The observed increase in the formation of angiogenesis and the elevated number of fibroblasts indicate promising outcomes during tissue healing. The formation of new blood vessels is critical because it ensures an adequate supply of nutrients and oxygen to the damaged tissue, thereby facilitating repair and regeneration. Fibroblasts are essential for maintaining tissue integrity because they contribute to the ECM and play pivotal roles in the tissue structural framework. Their presence is vital for stabilizing tissue architecture and the overall healing process.
Table 1. Average number of fibroblasts in the control and secretome groups during the observation.
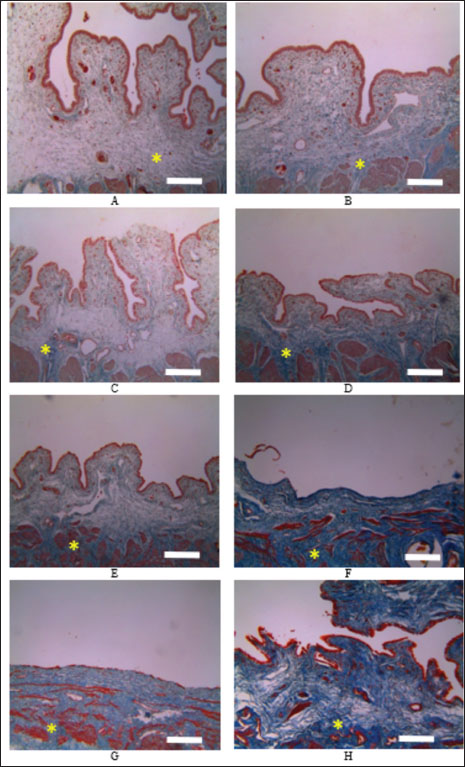
Table 2. Average area of collagen deposition between the control and secretome groups during observation. Measurement of collagen depositionThe results of collagen deposition are presented in Table 2. The results of collagen deposition measurements showed that collagen deposition increased in both research groups as the research observation time progressed; however, the area number of collagen deposition in the secretome group was significantly higher than that in the control group at all observation days (p < 0.05) (Fig. 3). The observed increase in collagen deposition suggests that secretome administration enhances tissue stability. Collagen is a fundamental component of the ECM and plays a critical role in the healing process by providing structural support and contributing to the maintenance of tissue stability and elasticity. DiscussionThe evaluation of secretome administration during mesh implantation for pelvic organ prolapse in this study yielded favorable outcomes. The assessment focused on tissue healing and integrity using the parameter of increased neovascularization (angiogenesis). This parameter is an indicator of ongoing tissue repair. The study found that the secretome treatment group exhibited a significantly higher number of angiogenesis events compared with the control group. The formation of new blood vessels (angiogenesis) in the secretome group demonstrated a mean value of 9.81 ± 2.2 compared to 0.37 ± 0.03 in the control. These findings demonstrate markedly improved outcomes compared with those reported by Mancuso et al. (2019). This effect is attributed to the mesenchymal stem cell secretome containing a number of chemokine and cytokine growth factors such as IL-6, IL-8, proteases and protease inhibitors such as MMP -1 and 2, and ECM molecules that will assist in cell regeneration so as to improve tissue integration, prevent excess tissue inflammatory response, and accelerate the wound-healing process (Dilogo et al., 2020). Based on the refference, it is necessary to test and measure in living tissue the ability of stem cell secretome tolerance when applied as a medicinal preparation. A previous study proved that secretomes have therapeutic potential (Hacker et al., 2021). The study of secretomes, or secretomes, has significant implications for diagnostics and therapeutics. The secretome reflects the state of cells and tissues and can serve as a source of biomarkers for diseases such as cancer, cardiovascular diseases, and neurodegenerative disorders (Jerard et al., 2023). Secretomes play a significant role in the healing process because they comprise various signaling molecules, enzymes, growth factors, and ECM components that facilitate tissue repair and regeneration. The healing process generally involves several stages: hemostasis, inflammation, proliferation, and remodeling (Damayanti et al., 2021; Hacker et al., 2021; Jammes et al., 2023; Tilotta et al ., 2023). The secretome effect is observed throughout these stages, with different cell types contributing to the secreted factors that drive healing. In the wound-healing process, the administration of secretome helps accelerate healing because secretome plays a role in stimulating the migration of the number of white blood cells, especially macrophages, to the wounded area, so that the number of macrophages increases, the secretion of growth factors, such as epidermal growth factor (EGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF), also increases. These growth factors stimulate the proliferation (cell division) and migration (cell movement) of cells involved in the healing process. The proliferation and migration of these cells are important for the formation of new tissue and wound closure (Shi et al., 2018; Porro et al., 2020).
Fig. 3. Microscopic observation of collagen area deposition using Masson’s trichrome staining and observed using microscope with 100× magnify. A. Control groups on day 7, B. Secretome groups on day 7, C. Control group on day 14, D. Secretome groups on day 14, E. Control groups on day 30, F. Secretome groups on day 30, G. Control groups on day 90, H. Secretome groups on day 90. Asterisk (*) refers to the area of collagen deposition. The scale bar equals with the 100 μm. Tissue integrity refers to the structural and functional stability of tissues, ensuring that they can perform their physiological roles effectively (Hacker et al., 2021). The secretome influences tissue integrity through various mechanisms, including promoting cellular communication, supporting the ECM, modulating immune responses, and facilitating repair and regeneration processes. The components of the secretome involved in tissue integrity are ECM proteins (Maacha et al., 2020; Sandonà et al., 2021). The secretome treatment group exhibited a significantly higher fibroblast proliferation rate compared with the control group. This difference is attributed to the secretion of proteins such as collagen, fibronectin, laminin, and elastin, which form the structural framework of the ECM. These components provide mechanical support and elasticity to tissues and are crucial for maintaining tissue architecture. Growth factor molecules such as transforming growth factor-beta (TGF-β), FGFs, and EGF are involved in cell proliferation, differentiation, and migration (Maacha et al., 2020). These growth factors are vital for tissue maintenance and repair. Enzymes such as MMPs degrade ECM components, which are crucial for tissue remodeling and repair. The activity of these enzymes is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs) to prevent excessive degradation and maintain tissue integrity (Ahangar et al., 2020; Maacha et al., 2020; Gwam et al., 2021; Jammes et al., 2023). The mechanisms by which the secretome maintains tissue integrity include ECM maintenance and remodeling (Cases-Perera et al., 2022). The secretome contributes to the dynamic balance of ECM synthesis and degradation, ensuring that tissues maintain their structural properties. For instance, collagen and other matrix proteins strengthen the ECM, whereas MMPs and TIMPs regulate the remodeling and turnover of ECM components (Maacha et al., 2020). Cell communication and coordination, growth factors, and cytokines in the secretome facilitate communication between cells, coordinating responses to physiological and pathological stimuli. This communication is essential for processes such as tissue development, repair, and immune responses (Liu et al., 2020; Maacha et al., 2020; Jammes et al., 2023). Angiogenesis, the formation of new blood vessels, is crucial for supplying nutrients and oxygen to tissues, especially during repair and regeneration. The secretome promotes angiogenesis through factors such as VEGF and angiogenesis, ensuring adequate blood supply and supporting tissue health (Ahangar et al., 2020; Maacha et al., 2020; Porro et al., 2020; Sears et al., 2020). Secretome plays many roles in collagen deposition: the first role in collagen synthesis. Secretome can stimulate cells to produce TGF-β to stimulate collagen synthesis by activating fibroblasts and promoting the expression of collagen genes (Cifuentes et al ., 2021; Reyes-Ramos et al., 2021). In addition, it enhances the production of other ECM components. FGFs can promote fibroblast proliferation and collagen production, thereby aiding tissue repair and ECM maintenance (Cifuentes et al., 2021). The platelet-derived growth factor (PDGF) function enhances fibroblast activity and collagen production, particularly in wound healing. Certain interleukins (ILs), such as IL-6 and IL-10, can influence collagen production by modulating fibroblast activity and ECM composition (Basalova et al., 2020; Zhou et al., 2021). In collagen maturation, the secretome plays a role in many stages of maturation. In post-translational modifications, secretomes play a role in hydroxylation and glycosylation, and they are essential for collagen stability and functionality (García de Vinuesa et al., 2016; Ahangar et al., 2020; Sandonà et al., 2021). The secretome provides enzymes and cofactors necessary for these modifications, such as prolyl, lysyl hydroxylase, and proteolytic enzymes. The secretome also plays a role in MMPs and is involved in the degradation of ECM components, including collagen. MMPs such as MMP-1 (collagenase) facilitate the breakdown of mature collagen fibers, which is crucial for tissue remodeling and repair. The secretome also participates in collagenase enzyme synthesis. Collagenases are specific MMPs that target and degrade collagen, allowing for remodeling and the replacement of old or damaged collagen. In TIMPs, collagen can regulate MMP activity, ensuring a balance between collagen degradation and synthesis. Proper regulation is essential for maintaining ECM homeostasis and preventing excessive tissue damage or fibrosis (Sears et al., 2020; Damayanti et al., 2021; Zhou et al ., 2021). The secretome stimulates the formation of growth factors. Many growth factors during wound healing are closely related to wound repair, one of which is FGF (Wilkinson et al., 2017). FGF is a polypeptide growth factor with diverse biological functions. Secreted FGF can be classified into two categories: classical FGF (also known as paracrine FGF) and endocrine FGF. Apart from their conventional role in regulating cell proliferation and differentiation, fibroblasts serve as more potent angiogenesis factors than PDGF and VEGF (Porro et al., 2020). These growth factors may be related to angiogenesis and fibroblast stimulation in secretome-treated wounds. The results showed that the stem cell secretome was able to significantly induce collagen formation compared with the control group. In line with other studies, collagen levels were significantly higher in the secretome-treated group than in the control group (Wilkinson et al., 2017; Cifuentes et al., 2021; Ajit et al., 2023). The secretome contains various growth factors and cytokines that stimulate collagen production by cells such as fibroblasts. Tissue healing requires collagen synthesis, which is an important part of cell growth (Ravishankar et al., 2018). The secretome may also contain transcription factors or regulatory signals, such as specificity protein-1 and activator protein-1 can bind to the promoters of collagen genes and regulate their expression (Reyes-Ramos et al., 2021). When a wound occurs, fibroblasts produce new collagen to help repair the tissue. During the early phase of wound healing, the synthesis of Type III collagen is most prominent, accompanied by the presence of inflammatory cells. Type III collagen is replaced by type I collagen, which has a stronger and more stable linking ability that increases tensile strength after implantation (Gonzalez et al., 2016). ConclusionThe administration of an UC-MSC secretome has shown promising potential to enhance tissue integrity and accelerate wound healing. The regenerative properties observed in this study suggest that UC-MSC secretomes could serve as valuable therapeutic tools, particularly in conditions characterized by compromised tissue repair, such as pelvic organ prolapse, chronic wounds, and tissue injuries resulting from surgery. Further research is warranted to refine and optimize secretome formulations, including the identification of specific growth factors and cytokines responsible for these regenerative effects. Advanced studies involving larger clinical trials are essential to confirm the safety, efficacy, and long-term benefits. Additionally, exploring delivery methods such as injectable hydrogels, biocompatible scaffolds, and targeted release systems could enhance the therapeutic reach of the UC-MSC secretome, making it adaptable to diverse clinical needs. AcknowledgmentsWe are grateful to the Stem Cell and Tissue Engineering Research Cluster Indonesian Medical Education and Research Institute (SCTE IMERI), University of Indonesia, for providing the secretome. Conflicts of interestThe authors declare that they have no conflicts of interest. FundingThis research was not funded by any funding organization. Authors’ contributionsTITD: Study design, data collection during research, data interpretation, and manuscript preparation. DN: supervised the research, interpreted the data, drafted, and revised the manuscript. BPP: interpreted the data of histopathology, drafted, and revised the manuscript. GNT: supervised the research, drafted, and revised the manuscript. MSB: data analyzed, drafted, and revised the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data were provided in the manuscript. References Ahangar, P., Mills, S.J. and Cowin, A.J. 2020. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int. J. Mol. Sci. 21(19), 1–15. Ajit, A., Kumar, T.R.S., Harikrishnan, V.S., Anil, A., Sabareeswaran, A. and Krishnan, L.K. 2023. Enriched adipose stem cell secretome as an effective therapeutic strategy for in vivo wound repair and angiogenesis. 3 Biotech. 13(3), 83. Amani, S., Shahrooz, R., Mortaz, E., Hobbenaghi, R., Mohammadi, R. and Khoshfetrat, A.B. 2019. Histomorphometric and immunohistochemical evaluation of angiogenesis in ischemia by tissue engineering in rats: Role of mast cells. Vet. Res. Forum. 10(1), 23–30. AVMA [American Veterinary Medical Assosioation]. 2020. AVMA Guidelines for Animal Euthanasia: 2020 Edition. AVMA.org. Bakhtiary, Z., Shahrooz, R., Hobbenaghi, R., Azizi, S., Soltanalinejad, F. and Khoshfetrat, A.B. 2021. Histomorphometrical evaluation of the extensor digitorum longus muscle for sciatic nerve regeneration using tissue engineering in rats. Vet. Res. Forum. 12(4), 451–457. Basalova, N., Sagaradze, G., Arbatskiy, M., Evtushenko, E., Kulebyakin, K., Grigorieva, O., Akopyan, Z., Kalinina, N. and Efimenko, A. 2020. Secretome of mesenchymal stromal cells prevents myofibroblasts differentiation by transferring fibrosis-associated microRNAs within extracellular vesicles. Cells 9(5), 1272. Cases-Perera, O., Blanco-Elices, C., Chato-Astrain, J., Miranda-Fernández, C., Campos, F., Crespo, P.V., Sánchez-Montesinos, I., Alaminos, M., Martín-Piedra, M.A. and Garzón, I. 2022. Development of secretome-based strategies to improve cell culture protocols in tissue engineering. Sci. Rep. 12(1), 10003. Cifuentes, S.J., Priyadarshani, P., Castilla-Casadiego, D.A., Mortensen, L.J., Almodóvar, J. and Domenech, M. 2021. Heparin/collagen surface coatings modulate the growth, secretome, and morphology of human mesenchymal stromal cell response to interferon-gamma. J. Biomed. Mater. Res. A. 109(6), 951–965. Damayanti, R.H., Rusdiana, T. and Wathoni, N. 2021. Mesenchymal stem cell secretome for dermatology application: a review. Clin. Cosmet. Investig. Dermatol. 14, 1401–1412. Dewi, T.I.T., Noviana, D., Priosoeryanto, B.P. and Gunanti. 2024. Sitologi Vagina dan Kadar Estradiol pada Model Hewan Hipoestrogenik Kelinci (Oryctolagus cuniculus). Acta Vet Indo. 12(1), 40–46. Dilogo, I.H., Canintika, A.F., Hanitya, A.L., Pawitan, J.A., Liem, I.K. and Pandelaki, J. 2020. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur. J. Ortho. Surg. Traumatol. 30(5), 799–807. García de Vinuesa, A.A., Abdelilah-Seyfried, S., Knaus, P., Zwijsen, A. and Bailly, S. 2016. BMP signaling in vascular biology and dysfunction. Cyto. Gro. Fact. Rev. 27, 65–79. Gonzalez, A.C.D.O., Andrade, Z.D.A., Costa, T.F. and Medrado, A.R.A.P. 2016. Wound healing: a literature review. An. Bras. Dermatol. 91(5), 614–620. Gwam, C., Mohammed, N. and Ma, X. 2021. Stem cell secretome, regeneration, and clinical translation: a narrative review. Ann. Transl. Med. 9(1), 70. Hacker, S., Mittermayr, R., Traxler, D., Keibl, C., Resch, A., Salminger, S., Leiss, H., Hacker, P., Gabriel, C., Golabi, B., Pauzenberger, R., Slezak, P., Laggner, M., Mildner, M., Michlits, W., Ankersmit, H.J. 2021. The secretome of stressed peripheral blood mononuclear cells increases tissue survival in a rodent epigastric flap model. Bioeng. Transl. Med. 6(1), e10186. Jammes, M., Contentin, R., Audigié, F., Cassé, F. and Galéra, P. 2023. Effect of proinflammatory cytokine priming and storage temperature of the mesenchymal stromal cell (MSC) secretome on equine articular chondrocytes. Front. Bioeng. Biotechnol. 11, 1204737. Jerard, C., Madhusudanan, P., Swamy, A., Ravikumar, K. and Shankarappa, S.A. 2023. Secretome-mediated interactions between sensory neurons and breast cancer cells. Int. J. Cancer. 153(2), 427–436. Liu, K., Veenendaal, T., Wiendels, M., Ruiz-Zapata, A.M., Van Laar, J., Kyranas, R., Enting, H., Van Cranenbroek, B., Koenenen, H.J.P.M., Mihaila, S.M. 2020. Synthetic extracellular matrices as a toolbox to tune stem cell secretome. ACS Appl. Mater. Interfaces. 12(51), 56723–56730. Maacha, S., Sidahmed, H., Jacob, S., Gentilcore, G., Calzone, R., Grivel, J.C. and Cugno, C. 2020. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020(1), 4356359. Mancuso, P., Raman, S., Glynn, A., Barry, F. and Murphy, J.M. 2019. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 7, 9. Moen, M., Noone, M. and Vassallo, B. 2014. Anterior colporrhaphy: Why surgeon performance is paramount. Int. Urogynecol. J. 25(7), 857–862. Porro, C., Cianciulli, A. and Panaro, M.A. 2020. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules 10(7), 1–15. Ravishankar, K., Kiranmayi, G.V.N., Prasad, Y.R. and Devi, L. 2018. Wound-healing activity in rabbits and antimicrobial activity of Hibiscus hirtus ethanolic extract. Braz. J. Pharm. Sci. 54(4), e17075. Reyes-Ramos, A.M., Álvarez-García, Y.R., Solodin, N., Almodovar, J., Alarid, E.T., Torres-Garcia, W. and Domenech, M. 2021. Collagen i fibrous substrates modulate the proliferation and secretome of estrogen receptor-positive breast tumor cells in a hormone-restricted microenvironment. ACS Biomater. Sci. Eng. 7(6), 2430–2443. Sandonà, M., Di Pietro, L., Esposito, F., Ventura, A., Silini, A.R., Parolini, O. and Saccone, V. 2021. Mesenchymal stromal cells and their secretome: new therapeutic perspectives for skeletal muscle regeneration. Front. Bioeng. Biotechnol. 9, 652970. Sears, V. and Ghosh, G. 2020. Harnessing mesenchymal stem cell secretome: Effect of extracellular matrices on proangiogenic signaling. Biotechnol. Bioeng. 117(4), 1159–1171. Shi, G.J., Shi, G.R., Zhou, J.Y., Zhang, W.J., Gao, C.Y., Jiang, Y.P., Zi, Z.G., Zhao, H.H., Yang, Y., Yu, J.Q. 2018. Involvement of growth factors in diabetes mellitus and its complications: a general review. Biomed. Pharmacother. 101, 510–527. Skala, C., Renezeder, K., Albrich, S., Puhl, A., Laterza, R.M., Naumann, G. and Koelbl, H. 2011. The IUGA/ICS classification of complications of prosthesis and graft insertion: A comparative experience in incontinence and prolapse surgery. Int. Urogynecol. Jol. 22, 1429–1435. Tilotta, V., Vadalà, G., Ambrosio, L., Cicione, C., Di Giacomo, G., Russo, F., Papalia, R., and Denaro, V. 2023. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 11, 1152207. Van Geelen, J.M. and Dwyer, P.L. 2013. Where to for pelvic organ prolapse treatment after the FDA pronouncements?: a systematic review of the recent literature. Int. Urogynecol. J. Pelvic Floor Dysfunct. 24(5), 707–718. Wilkinson, H. N. and Hardman, M. J. 2017. The role of estrogen in cutaneous ageing and repair. Maturitas 103, 60–64. Zhou, X., Li, J., Giannopoulos, A., Kingham, P.J. and Backman, L.J. 2021. Secretome from in vitro mechanically loaded myoblasts induces tenocyte migration, transition to a fibroblastic phenotype and suppression of collagen production. J. Mol. Sci. 22(23), 13089. | ||
| How to Cite this Article |
| Pubmed Style Dewi TIT, Noviana D, Priosoeryanto BP, Gunanti G, Subangkit M. Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Vet. J.. 2025; 15(1): 162-170. doi:10.5455/OVJ.2025.v15.i1.15 Web Style Dewi TIT, Noviana D, Priosoeryanto BP, Gunanti G, Subangkit M. Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. https://www.openveterinaryjournal.com/?mno=213819 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.15 AMA (American Medical Association) Style Dewi TIT, Noviana D, Priosoeryanto BP, Gunanti G, Subangkit M. Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Vet. J.. 2025; 15(1): 162-170. doi:10.5455/OVJ.2025.v15.i1.15 Vancouver/ICMJE Style Dewi TIT, Noviana D, Priosoeryanto BP, Gunanti G, Subangkit M. Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 162-170. doi:10.5455/OVJ.2025.v15.i1.15 Harvard Style Dewi, T. I. T., Noviana, . D., Priosoeryanto, . B. P., Gunanti, . G. & Subangkit, . M. (2025) Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Vet. J., 15 (1), 162-170. doi:10.5455/OVJ.2025.v15.i1.15 Turabian Style Dewi, Tri Isyani Tungga, Deni Noviana, Bambang Pontjo Priosoeryanto, Gunanti Gunanti, and Mawar Subangkit. 2025. Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Veterinary Journal, 15 (1), 162-170. doi:10.5455/OVJ.2025.v15.i1.15 Chicago Style Dewi, Tri Isyani Tungga, Deni Noviana, Bambang Pontjo Priosoeryanto, Gunanti Gunanti, and Mawar Subangkit. "Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome." Open Veterinary Journal 15 (2025), 162-170. doi:10.5455/OVJ.2025.v15.i1.15 MLA (The Modern Language Association) Style Dewi, Tri Isyani Tungga, Deni Noviana, Bambang Pontjo Priosoeryanto, Gunanti Gunanti, and Mawar Subangkit. "Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome." Open Veterinary Journal 15.1 (2025), 162-170. Print. doi:10.5455/OVJ.2025.v15.i1.15 APA (American Psychological Association) Style Dewi, T. I. T., Noviana, . D., Priosoeryanto, . B. P., Gunanti, . G. & Subangkit, . M. (2025) Tissue integrity and healing response in hypoestrogenic animal model treated by mesh implantation with addition of mesenchymal stem cell secretome. Open Veterinary Journal, 15 (1), 162-170. doi:10.5455/OVJ.2025.v15.i1.15 |