| Review Article | ||
Open Vet. J.. 2025; 15(1): 8-17 Open Veterinary Journal, (2025), Vol. 15(1): 8-17 Review Article Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A reviewMohamed Tharwat1*, Haytham Ali2,3 and Abdulrahman A. Alkheraif41Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Animal and Veterinary Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Oman 3Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email:atieh [at] qu.edu.sa Submitted: 15/07/2024 Accepted: 13/10/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractParatuberculosis (PTB) is a long-standing granulomatous infectious disease of both domesticated and wild ruminants. It is caused by Mycobacterium avium subsp. paratuberculosis (MAP). This review emphasizes the clinical, hematobiochemical, sonographic, and pathologic findings as well as therapeutic and control measures in dromedary camels infected with PTB. The clinical signs include intermittent and/or chronic diarrhea, decreased milk yield, emaciation, submandibular edema, dehydration, irregular and weak rumen contractions, and abdominal pain. Hematological changes include leukocytosis, neutrophilia, and decreased erythrocytes, hematocrit percent, and hemoglobin concentration. Biochemical alterations included hypoalbuminemia, hypoproteinemia, hyperglobulinemia, hypomagnesemia, hypoglycemia, increased alanine aminotransferase and aspartate aminotransferase activity, and increased concentration of magnesium and calcium. Sonographically, the intestinal wall is either mildly, moderately, or severely thickened along with mesenteric lymph nodes (LNs) enlargement. The LN capsule is either anechoic or echoic and the contents are either echogenic, anechoic, or heterogenous. Other sonographic findings include bright hepatic parenchyma, aggregation of echogenic materials separated with fluids among the intestines, and pleural and pericardial effusions. The typical pathological lesions are corrugation of the small intestinal mucosa, especially that of the ileum, and the colonic mucosa is folded. Mesenteric and ileocecal LNs are edematous, congested, and granular. Other necropsy findings include fatty liver and peritoneal, pericardial, and pleural effusions. Histologically, proliferative enteritis and lepromatous granulomas are detected. Clusters of acid-fast bacilli are usually found in the intestinal mucosa and lamina propria. Accurate diagnosis of PTB depends on the culture and identification of the causative organism MAP from tissue or feces. Herd screening is also performed through complement fixation, agar gel immunodiffusion, competitive-enzyme linked immunosorbent assay, Ziehl–Neelsen staining of tissue or feces, histologic pattern of a granulomatous reaction, DNA probes, and polymerase chain reaction. A trial for the treatment of PTB in dromedary camels was carried out through IM injection of rifampin and streptomycin for 10 weeks. The diarrhea resolved within 1 week of treatment, and MAP disappeared from rectal scraping 5–9 weeks after treatment. In conclusion, early detection and eradication procedures of PTB should be more implemented for the control and prevention of PTB in dromedary camels. More research should be directed toward vaccination programs in those species. Keywords: Camel, Johne’s disease, Pathology, Paratuberculosis, Ultrasound. IntroductionParatuberculosis (PTB), known as Johne’s disease (JD), is a long-standing granulomatous infectious disease affecting both domesticated and wild ruminants. It is caused by Mycobacterium avium subsp. paratuberculosis (MAP), a gram-positive, slowly growing, rod-shaped, and acid-fast mycobacteria (Corbett et al., 2019; Whittington et al., 2000). The disease is characterized by clinical manifestations of chronic watery diarrhea, emaciation, severe loss of body condition, and abrupt decline in wool, milk, and meat production (Harris and Barletta 2001; Tharwat et al., 2012a,b; Tharwat et al., 2013). The bacilli can be excreted in milk and feces for a period extending up to 24 months before clinical symptoms (Mitchell et al., 2015), therefore potentially transmitting the disease to susceptible animals through the fecal–oral route (Rathnaiah et al., 2017). Globally, PTB has a financial impact on the animal industry. For example, in the United States, an estimated loss of 198 million US$ due to JD was found, while in Germany, France, New Zealand, Canada, economic losses were 75 million US$, 56 million US$, 54 million US$, and 17 million US$, respectively (Rasmussen et al., 2021). It was found that cattle with PTB experience a drop in milk yield, fertility, milk quality, and longevity (Ozsvari et al., 2020). It was also reported that PTB increases culling rates, decreases milk production, increases replacement costs, decreases diet conversion efficacy, increases fertility disorders, decreases slaughter benefits, and increases capability for other diseases or disorders (Garcia and Shalloo, 2015). In camel medicine, huge economic losses are also detected due to infection with PTB (Salem et al., 2019; Selim et al., 2022; Al Naeem et al., 2024). For a prolonged period, screening of MAP depends mainly on three main methods: light microscopy, MAP culture, and serology and polymerase chain reaction (PCR). Moreover, despite the enzyme-linked immunosorbent assay (ELISA) serological tests being able to easily detect antibodies for MAP, it cannot distinguish between infected and vaccinated animals (Chaubey et al., 2016). Unique markers for the detection of PTB have been developed using recombinant ELISA (rELISA) (Jain et al., 2021). In addition, an earlier detection could be made through fecal shedding and ELISA in young animals (Mortier et al., 2015). In subclinically affected animals, the detection of MAP can be made by using an interferon-gamma assay to manage infected animals or those at risk to MAP (Corneli et al., 2021). The discovery of seroreactive antigens and serodiagnostic antigens for the early detection of PTB has been developed (Li et al., 2017; Li et al., 2019). The control of JD is difficult due to the lack of efficacious, sensitive, and cost-effective diagnostic techniques as well as marker vaccines (Chaubey et al., 2016). An individual-based modeling approach was developed for the control of PTB in cattle. In this model, the most effective control measure was to reduce neonatal exposure, followed by testing and culling infected animals (Camanes et al., 2018). A bioeconomic model was developed for the control of MAP spreading within dairy cows. This model is based on four control measures (Kirkeby et al., 2016). These measures include age-dependent susceptibility for infection; age-dependent sensitivity for detection; environmental MAP builds up in five separate areas of the farm; in utero infection; and infection via colostrum and waste milk, and it allows for realistic culling (i.e., due to other diseases) by including a ranking system. It was reported that there is a powerful association between MAP and Crohn’s disease (CD) in humans, but this link is still controversial (Behr and Kapur, 2008; Pierce, 2018). If the suspected association between JD and CD is confirmed, huge financial crises may be expected, especially for the dairy farming industry. Consequently, the control strategies of PTB should be a top priority for the global dairy animal industry (Garcia and Shalloo, 2015). It should be stressed that successful PTB control measures are dependent on a parallel country and herd level on the condition that critical prerequisites are assembled (Weber et al., 2024). The camel has important values particularly in rural and desert areas, because of its several functions and its ability to adapt to tough environments. Worldwide, about 29 million camels are found, 95% of them are one-humped (Sikkema et al., 2019). However, based on an authorized report released by the FAO, 35 million dromedary camels exist all over the world (FAO, 2018). They are used for human traveling and transport of goods through the desert. In addition, camels provide people with meat and milk especially in poor countries (Ho et al., 2022), and therefore, diseases affecting this species will subsequently affect poor societies in developing countries. Recently, the usage of camels in several racing and other cultural events are widely spread (Tharwat et al., 2013; Tharwat and Al-Sobayil, 2015; Tharwat and Al-Sobayil, 2018; Tharwat and Al-Hawas, 2021; Tharwat and Al-Hawas, 2024; Tharwat et al., 2024a). This review aims to emphasize the clinical, hematobiochemical, sonographic, and pathologic findings as well as control measures in infected dromedary camels with PTB. Clinical presentationsIn a study conducted by Hereba et al. (2015), eight dromedary camels with PTB suffered from intermittent and/or chronic diarrhea for periods ranging from 1 to 4 weeks, decreased milk yield, emaciation, and submandibular edema. In another study conducted by Elsohaby et al. (2021), clinical signs included progressive loss of body weight and chronic diarrhea. In a third study conducted by Alluwaimi (2015), clinical manifestations included also drop in milk production, intermittent diarrhea, emaciation, dehydration, and submandibular edema. Described as nonresponsive diarrhea, either mild, severe, intermittent or chronic, was also observed in 30 dromedary camels with PTB (Salem et al., 2019). In a study with a dromedary camel with JD, clinical symptoms included long-standing progressive intermittent watery diarrhea, dehydration, severe emaciation, ventral body edema, and pica or depraved appetite (El Tigani-Asil et al., 2023). Our previous research revealed that the most evident clinical symptoms included inappetence, severe weakness, irregular and weak rumen contractions, abdominal pain, and chronic and intermittent diarrhea (Fig. 1) (Tharwat et al., 2012a; Tharwat et al., 2013). Hematological and biochemical alterationsIn a study conducted by Alharbi et al.,(2012) laboratory changes in camels with PTB included decrease in both hemoglobin concentration (HGB) and hematocrit (HCT) values and increase in serum activity of alanine aminotransferase. Significant decreases in erythrocytes, HGB and albumin concentration, and significant increases in HCT, neutrophils, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase were also reported (El-Deeb et al., 2014). In a study conducted by our group, hematological changes in dromedary camels with PTB compared to healthy camels included leukocytosis and decreased HCT percent and HGB concentration (Tharwat et al., 2012a). The leukocytosis in the latter study was referred to as the chronic nature of the disease in camels.
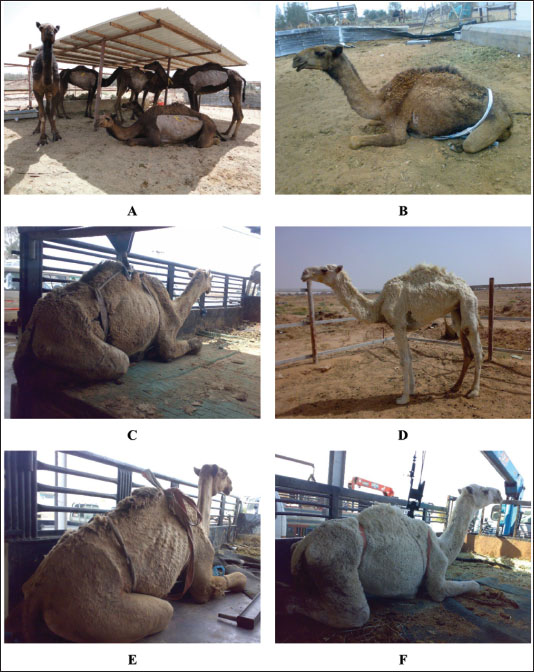
Fig. 1. Clinical presentation of 6 dromedary camels with paratuberculosis in a group (A) and separate cases (B, C, D, E, and F). Progressive weakness, severe loss of body conditions, chronic and/or intermittent watery diarrhea, abdominal pain, decreased body condition score, and anorexia were the admission complaints. In camels with PTB, biochemical changes included hypoalbuminemia, hypoproteinemia, hypoglobulinemia, hypomagnesemia, hypoglycemia, and increased AST activity. However, γ-glutamyl transferase activity and the serum concentrations of blood urea nitrogen, total bilirubin, calcium, phosphorus, and creatinine did not alter significantly versus healthy controls (Tharwat et al., 2012a). In another study carried out also by our group (Tharwat et al., 2013), the same hematological alterations were recorded and included decreased HCT, leukocytosis, and lower HGB concentration. In addition, biochemical variables change include hypoalbuminemia, hypoproteinemia, hyperglobulinemia, and increased concentration of magnesium and calcium (Tharwat et al., 2012a). The hyperglobulinemia in the latter study was referred to as the chronic nature of the disease in camels. The hypoproteinemia and hypoalbuminemia could all be attributed to the malabsorption caused by diarrhea (Tharwat et al., 2012a). Sonographic findingsUltrasonography has been confirmed as a safe, very beneficial for diagnosis of several diseases and disorders of the gastrointestinal tract in dromedary camels (Tharwat et al., 2012a,b,c; Tharwat et al., 2013; Tharwat and Al-Sobayil, 2016; Tharwat et al., 2018; Tharwat, 2019; Tharwat, 2020; Sadan et al., 2024; Tharwat, 2024; Tharwat et al., 2024b,c,d,e; Tharwat and Tsuka, 2024). As a rapid screening test for dromedary camels with suspicion to be affected with PTB, clinicians may use sonography to image the abdominal viscera, especially the intestines and mesenteric lymph nodes (LNs) (Tharwat et al., 2012a). Usually, the intestinal wall becomes either mildly, moderately, or severely thickened (6.8 ± 1.9 mm; 12.8 ± 4.6 mm, and 17.5 ± 3.6 mm, respectively) versus a thickness of 3.6 ± 1.2 mm in healthy non-affected camels. Visualizing of enlarged mesenteric LNs was also reported (Tharwat et al., 2012a). The LN capsule was either anechoic or echoic, and the contents were either echogenic, anechoic, or heterogenous. Other sonographic findings include bright hepatic parenchyma, aggregation of echogenic materials separated with fluids among the intestines, and pleural and pericardial effusions (Fig. 2) (Tharwat et al., 2012a). Pathologic findingsAt postmortem examination, camels with PTB are usually emaciated. The typical pathological lesions are corrugation of the small intestinal mucosa especially that of the ileum that reaches 3–4 times than in normal camels, and the colonic mucosa is folded. Mesenteric and ileocecal LNs are edematous, congested, and granular (El Tigani-Asil et al., 2023). Over the hepatic tissue, dispersed varied sizes and shapes of whitish granulomatous lesions are found (Hereba et al., 2015). It was reported in another study that necropsy findings in a camel with PTB were corrugation and thickening of the ileocecal part of the intestines and the cut section showed corrugation and folding of the mucosa. In addition, the mesenteric LNs were enlarged that showed mottled appearance, firm consistency, and caseation, and the liver showed fatty degeneration, nutmeg appearance, hepatic necrosis, passive congestion, atrophy, and fatty change (Tigani-Asil et al., 2023). In a study conducted by Alharbi and colleagues (2012), postmortem findings in camels with PTB included corrugated and thickened intestinal mucosa, granulomatous and enlarged mesenteric LNs, diffuse and miliary granulomas in the liver, generalized LN granulomas, and splenic granuloma and mediastinal LN granuloma. In a large study conducted by our group (Tharwat et al., 2012a), camels with PTB showed diffuse corrugation and thickening of the small intestinal mucosa. In addition, the large intestine mucosa is ulcerated, hemorrhagic, and folded (Fig. 3). The mesenteric and hepatic LNs may be enlarged, granulomatous and the surface was hemorrhagic on the cut section. Other necropsy findings include fatty liver and peritoneal, pericardial, and pleural effusions (Tharwat et al., 2012a). In another report, a necropsy of camels with PTB revealed thickened and folded intestinal mucosa with a granulomatous appearance seen from the serosa. The granulomas were also observed in the hepatic, mesenteric, and mediastinal LNs. Other findings included flabby heart, enlarged kidneys, and hydroperitoneum (Tharwat et al., 2013).
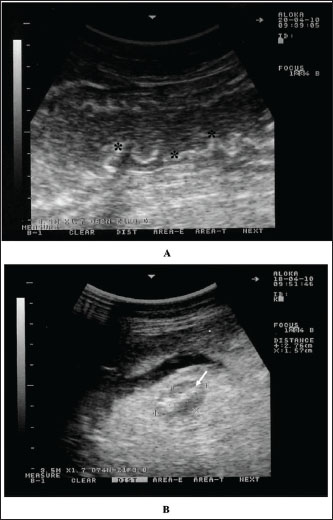
Fig. 2. Intestinal imaging in a dromedary camel with paratuberculosis. The intestinal walls were thickened and corrugated (stars) (A). Image B shows an enlarged mesenteric lymph node (white arrow) having an echogenic core. Scanning was performed at the right ventral abdomen using a 3.5 MH sector transducer.
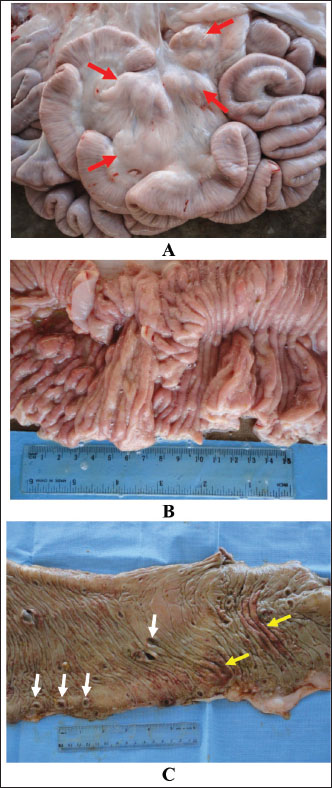
Fig. 3. Intestinal mucosa in a camel with paratuberculosis showing enlarged mesenteric lymph nodes (A, red arrows), thickening and corrugation of small intestines (B) and ulcerated and hemorrhagic large intestine (C, white arrows, ulceration; yellow arrows, hemorrhage). Histologically, proliferative enteritis and lepromatous granulomas were detected. Clusters of acid-fast bacilli were found also in the mucosa and lamina propria. Sectioning of the enlarged mesenteric LNs showed a diffuse lepromatous reaction with a proliferation of epithelioid cells and macrophages mixed with mononuclear cells and lymphocytes without proof of calcification and caseation (Tigani-Asil et al., 2023). In another study, histopathological examination showed intense invasion of epithelioid cells in the mucosa as well as the submucosa and the enlarged LNs, showing liquefactive necrosis and abscess formation (Tharwat et al., 2013). In a third study, histopathological findings included diffuse infiltration of macrophages in organs revealing lesions and staining of tissue scraping and tissue sections by Ziehl–Neelsen showed clusters of acid-fast bacilli (Alharbi et al., 2012).
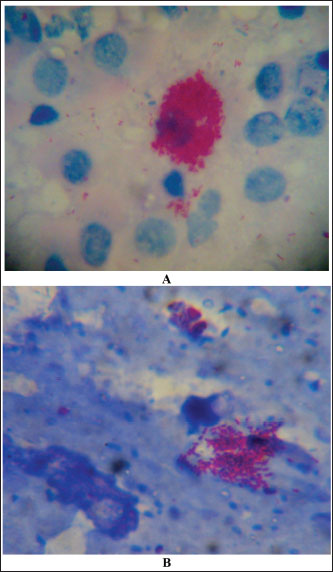
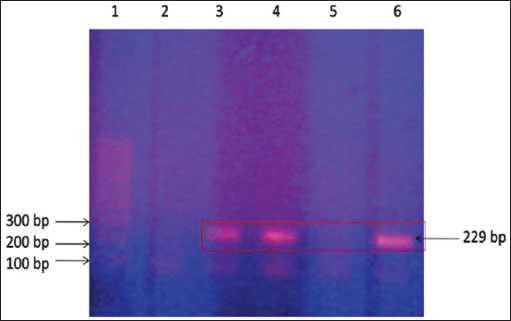
Fig. 4. Ziehl–Neelsen stain showing acid-fast bacilli obtained from a camel with paratuberculosis. Clusters of bacilli were identified from an enlarged and hemorrhagic lymph node at postmortem examination (A) while the bacilli were identified from feces antemortem from the same animal (B). Diagnostic proceduresCurrently, there are several techniques available for direct (i.e., PCR and bacterial culture) and indirect (i.e., interferon-γ and ELISA) detection of MAP infection. Bacterial culture is slow, expensive, and it takes up to 42 days or longer and is not available in all diagnostic laboratories (Prendergast et al., 2018). In the contrary, PCR is frequently used and more rapid to segregate high shedders from infected herds (Hereba et al., 2015). Additionally, PCR is used to immediately diagnose MAP from fecal samples with sensitivity ranges from 70% to 100% and specificity reaching 100% (Logar et al., 2012). Feces may also be tested by PCR for the verification of the IS900 gene and the identification of strains of MAP (Elsohaby et al., 2021). Real-time quantitative PCR is also currently used to confirm the diagnosis (Tigani-Asil et al., 2023). Herd screening is usually performed through complement fixation, agar gel immunodiffusion, ELISA, Ziehl–Neelsen staining of tissue or feces (Fig. 4), histologic pattern of a granulomatous reaction, DNA probes, and PCR techniques (Fig. 5) (Ellingson et al., 2004; Stott et al., 2005; Al-Swailem et al., 2011; Tharwat et al., 2012a,b; Al Naeem et al., 2024).
Fig. 5. Polymerase chain reaction on camel fecal samples using Mycobacterium avium subsp. paratuberculosis-specific primers. Lane 1: 100 bp DNA size ladder, lane 2: negative test control (no DNA template), lanes 3 and 4: DNA extracted from fecal samples and lymph nodes, lane 5: DNA extracted from fecal samples of controls, and lane 6: control positive: DNA from a Mycobacterium avium subsp. paratuberculosis isolate. Positive results are indicated by obtaining a 229-bp product. Therapeutic and control trialsAl-Swailem et al. (2011) conducted a trial for the treatment of PTB in dromedary camels through intramuscular injection of rifampin (5 mg/kg BW) and streptomycin (5 mg/kg BW) into seven diseased animals for 10 weeks, where three diseased camels left without treatment and kept as positive controls. Diarrhea disappeared 1 week after therapy, and the animals began to acquire vitality. Improvement in body condition was detected after 3 weeks of treatment. Mycobacterium avium subsp. paratuberculosis disappeared from rectal scraping 5–9 weeks after rifampin and streptomycin therapy, while the untreated camels remained passing MAP in feces until the end of the experiment. The treated camels remained free of MAP excretion until the end of the 10th week, thus indicating good sensitivity of rifampin and streptomycin against MAP. In a study conducted by Elmoslemany et al., (2022), it was reported that the herd size affects the seropositivity of PTB where a greater risk seropositivity was found if the herd is larger than 75 animals. Moreover, seropositivity of MAP decreased in groups with calving pens versus herds without. The latter report also pointed out that the risk of MAP seroprevalence decreased in older camels. Camel breeds used for beauty shows and racing are reported to increase the risk of transmission of MAP as these animals travel regularly across different areas (Al Naeem et al., 2024). The seropositivity of PTB was also reported in camels to increase with age, with the highest prevalence in camels over 10 years of age. Medium- and large-sized herds where animals were kept as single species and confined were found more likely to test positive (Hussain et al., 2015). Selim et al., (2022) stated that MAP risk for infection in camels increases in elderly animals especially those over 10 years; however, sex, geographical location, and contact with cattle did not affect the MAP seroprevalence in camels. The control of PTB is also attempted in farm animals through vaccination programs (Patton, 2011; Davis et al., 2021; Juste et al., 2021; Lianou et al., 2022). However, in camels, no reports of vaccination against PTB were found in veterinary literature. In conclusion, PTB is a fatal life-threatening, grave prognostic, and possible zoonotic disease in dromedary camels. Up until now, no effective cure methods have been documented for the treatment of such global disease. Therefore, early detection and eradication procedures should be implemented for the control and prevention of such diseases in dromedary camels. Initially, camels suspected to be infected with PTB should be clinically evaluated such as scanning by abdominal sonography and directly must be isolated if intestinal sonography results point to infection by PTB until confirmatory laboratory results such as ELISA or PCR arrive. Finally, more research should be directed toward vaccination programs worldwide, especially in food-producing animals including dromedary camels. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Author contributionMT: conceptualization, data collection, and writing the original manuscript draft. HA: sharing in writing of manuscript draft and editing. AA: review and editing. All authors approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. References Al Naeem, A., Salem, M., Housawi, F., Al-Mohammed Salem, K., Hussen, J., Fayez, M., Zaghawa, A. and Kostoulas P. 2024. Epidemiological insights into paratuberculosis in camels in Saudi Arabia: Bayesian estimation of true prevalence and identification of risk factors. PLoS One 19, e0299881. Alharbi, K.B., Al-Swailem, A., Al-Dubaib, M.A., Al-Yamani, E., Al-Naeem, A., Shehata, M., Hashad, M.E., Albusadah, K.A. and Mahmoud, O.M. 2012. Pathology and molecular diagnosis of paratuberculosis of camels. Trop. Anim. Health Prod. 44, 173–177. Al-Swailem, A., Al-Dubaib, M.A., Abo El-Hassan, D.G., Tharwat, M., Al-Yamani, E., Shehata, M., Hashad, M. and Mahmoud, O.M. 2011. Treatment of paratuberculosis in camels by rifampin and streptomycin. J. Camel Pract. Res. 18, 225–229. Alluwaimi, A.M. 2015. Paratuberculosis infection in camel (Camelus dromidarius): current and prospective overview. Open J. Vet. Med. 5, 153–160. Behr, M.A. and Kapur, V. 2008. The evidence for Mycobacterium paratuberculosis in Crohn’s disease. Curr. Opin. Gastroenterol. 24, 17–21. Camanes, G., Joly, A., Fourichon, C., Ben Romdhane, R. and Ezanno, P. 2018. Control measures to prevent the increase of paratuberculosis prevalence in dairy cattle herds: an individual-based modelling approach. Vet. Res. 49, 60. Chaubey, K.K., Gupta, R.D., Gupta, S., Singh, S.V., Bhatia, A.K., Jayaraman, S., Kumar, N., Goel, A., Rathore, A.S., Sahzad, Sohal, J.S., Stephen, B.J., Singh, M., Goyal, M., Dhama, K. and Derakhshandeh A. 2016. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet. Q. 2016 36, 203–227. Corbett, C.S., de Jong, M.C.M., Orsel, K., De Buck, J. and Barkema, H.W. 2019. Quantifying transmission of Mycobacterium avium subsp. paratuberculosis among group-housed dairy calves. Vet. Res. 50, 60. Corneli, S., Di Paolo, A., Vitale, N., Torricelli, M., Petrucci, L., Sebastiani, C., Ciullo, M., Curcio, L., Biagetti, M., Papa, P., Costarelli, S., Cagiola, M., Dondo, A. and Mazzone, P. 2021. Early detection of Mycobacterium avium subsp. paratuberculosis infected cattle: use of experimental Johnins and innovative interferon-gamma test interpretative criteria. Front. Vet. Sci. 8, 638890. Davis, W.C., Abdellrazeq, G.S., Mahmoud, A.H., Park, K.T., Elnaggar, M.M., Donofrio, G., Hulubei, V. and Fry, L.M. 2021. Advances in understanding of the immune response to mycobacterial pathogens and vaccines through use of cattle and Mycobacterium avium subsp. paratuberculosis as a prototypic mycobacterial pathogen. Vaccines 9, 1085. El-Deeb, W., Fouda, T. and El-Bahr, S. 2014. Clinico-biochemical investigation of paratuberculosis of dromedary camels in Saudi Arabia: proinflammatory cytokines, acute phase proteins and oxidative stress biomarkers. Pak. Vet. J. 34, 484–488. Ellingson, J.L.E., Koziczkowski, J.J. and Anderson, J.L. 2004. Comparison of PCR prescreening to two cultivation procedures with PCR confirmation for detection of Mycobacterium avium subsp. paratuberculosis in U.S. Department of Agriculture fecal check test samples. J. Food Protect. 67, 2310–2314. Elmoslemany, A., Alanazi, F., Elsohaby, I., Fayez, M. and Alnaeem A. 2022. Associations between management factors and seroprevalence of Mycobacterium avium subsp. paratuberculosis in dromedary camels. Comp. Immunol. Microbiol. Infect. Dis. 83, 101780. Elsohaby, I., Fayez, M., Alkafafy, M., Refaat, M., Al-Marri, T., Alaql, F.A., Al Amer, A.S., Abdallah, A. and Elmoslemany, A. 2021. Serological and molecular characterization of Mycobacterium avium Subsp. paratuberculosis (MAP) from sheep, goats, cattle and camels in the Eastern Province, Saudi Arabia. Animals 11, 323. FAO. 2018. ‘Food and Agricultural Organization, United Nations. Rome, Italy: FAO. Available via http://www.fao.org/faostat/en/#data Garcia, A.B. and Shalloo, L. 2015. Invited review: the economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 98, 5019–39. Harris, N.B. and Barletta, R.G. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14, 489–512. Hereba, A.M., Hamouda, M.A. and Al-Hizab, F.A. 2015. Pathological and molecular diagnosis of paratuberculosis among dromedary camels in Saudi Arabia. J. Anim. Plant Sci. 25, 997–1002. Ho, T.M., Zou, Z. and Bansal N. 2022. Camel milk: a review of its nutritional value, heat stability, and potential food products. Food Res. Int. 153, 110870. Hussain, M.H., Saqib, M., Al-Maawali, M.G., Al-Makhladi, S., Al-Zadjali, M.S., Al-Sidairi, T., Asubaihi, S., Al-Rawahi, A. and Mansoor, M.K. 2015. Seroprevalence of Mycobacterium avium subspecies paratuberculosis (MAP) and evaluation of risk factors in camels of the Sultanate of Oman. Trop. Anim. Health Prod. 47, 383–389. Jain, M., Kumar, A., Polavarapu, R., Gupta, S., Aseri, G.K., Sharma, D. and Sohal, J.S. 2021. Development of rELISA using novel markers for the diagnosis of paratuberculosis. J. Immunol. Methods. 497, 113105. Juste, R.A., Geijo, M.V., Elguezabal, N., Sevilla, I.A., Alonso-Hearn, M. and Garrido, J.M. 2021. Paratuberculosis vaccination specific and non-specific effects on cattle lifespan. Vaccine 39, 1631–1641. Kirkeby, C., Græsbøll, K., Nielsen, S.S., Christiansen, L.E., Toft, N., Rattenborg, E. and Halasa, T. 2016. Simulating the epidemiological and economic impact of paratuberculosis control actions in dairy cattle. Front. Vet. Sci. 3, 90. Li, L., Bannantine, J.P., Campo, J.J., Randall, A., Grohn, Y.T., Katani, R., Schilling, M., Radzio-Basu, J. and Kapur V. 2017. Identification of sero-reactive antigens for the early diagnosis of Johne’s disease in cattle. PLoS One 12, e0184373. Li, L., Bannantine, J.P., Campo, J.J., Randall, A., Grohn, Y.T., Schilling, M.A., Katani, R., Radzio-Basu, J., Easterling, L. and Kapur, V. 2019. Identification of sero-diagnostic antigens for the early diagnosis of Johne’s disease using MAP protein microarrays. Sci. Rep. 9, 17573. Lianou, D.T., Michael, C.K., Petinaki, E., Mavrogianni, V.S. and Fthenakis, G.C. 2022. Administration of vaccines in dairy sheep and goat farms: patterns of vaccination, associations with health and production parameters, predictors. Vaccines 10, 1372. Logar, K., Kopinč, R., Bandelj, P., Starič, J., Lapanje, A. and Ocepek, M. 2012. Evaluation of combined high-efficiency DNA extraction and real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in subclinically infected dairy cattle: comparison with faecal culture, milk real-time PCR and milk ELISA. BMC Vet. Res. 8, 49. Mitchell, R.M., Schukken, Y., Koets, A., Weber, M., Bakker, D., Stabel, J., Whitlock, R.H. and Louzoun, Y. 2015. Differences in intermittent and continuous fecal shedding patterns between natural and experimental Mycobacterium avium subspecies paratuberculosis infections in cattle. Vet. Res. 46, 66. Mortier, R.A., Barkema, H.W. and De Buck, J. 2015. Susceptibility to and diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy calves: a review. Prev. Vet. Med. 121, 189–198. Ozsvari, L., Harnos, A., Lang, Z., Monostori, A., Strain, S. and Fodor, I. 2020. The impact of paratuberculosis on milk production, fertility, and culling in large commercial hungarian dairy herds. Front. Vet. Sci. 7, 565324. Patton, E.A. 2011. Paratuberculosis vaccination. Vet. Clin. North Am. Food Anim. Pract. 27, 573–580. Pierce, E.S. 2018. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis…and colorectal cancer? Infect. Agent. Cancer 13, 1. Prendergast, D.M., Pearce, R.A., Yearsley, D., Ramovic, E. and Egan, J. 2018. Evaluation of three commercial PCR kits for the direct detection of Mycobacterium avium subsp. paratuberculosis (MAP) in bovine faeces. Vet. J. 241, 52–57. Rasmussen, P., Barkema, H.W., Mason, S., Beaulieu, E. and Hall, D.C. 2021. Economic losses due to Johne’s disease (paratuberculosis) in dairy cattle. J. Dairy Sci. 104, 3123–3143. Rathnaiah, G., Zinniel, D.K., Bannantine, J.P., Stabel, J.R., Gröhn, Y.T., Collins, M.T. and Barletta, R.G. 2017. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne’s disease. Front. Vet. Sci. 4, 187. Sadan, M., Tharwat, M., Alkhedhairi, S., Refaai, W., Moghazy, HMEL., Khodier, M.M., Alkhamiss, A.S. and Ghallab, A. 2024. Abdominal pedunculated leiomyoma in a male dromedary camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 458–462. Salem, M.A., El-Deeb, W.M., Zaghawa, A.A., Housawi, F.M. and Alluwaimi, A.M. 2019. Investigation of Mycobacterium paratuberculosis in Arabian dromedary camels (Camelus dromedarius). Vet. World. 12, 218–223. Selim, A., Attia, K.A., Alsubki, R.A., Kimiko, I. and Sayed-Ahmed, M.Z. 2022. Cross-sectional survey on Mycobacterium avium Subsp. paratuberculosis in dromedary camels: seroprevalence and risk factors. Acta Trop. 226, 106261. Shabana, I.I. and Aljohani, A.A. 2019. Sero-surveillance of Mycobacterium avium subspecies paratuberculosis infection in ruminants in Medina. J. Adv. Vet. Anim. Res. 7, 69–76. Sikkema, R.S., Farag, E.A.B.A., Islam, M., Atta, M., Reusken, C.B.E. M., Al-Hajri, M.M. and Koopmans, M.P.G. 2019. Global status of middle east respiratory syndrome coronavirus in dromedary camels: a systematic review. Epidemiol. Infect. 147, e84–e84. Stott, A.W., Jones, G.M., Humphry, R.W. and Gunn, G.J. 2005. Financial incentive to control paratuberculosis (Johne’s disease) on dairy farms in the United Kingdom. Vet. Rec. 156:825–831. Tharwat, M. 2019. Chronic peritonitis in dromedary camels: clinical, ultrasonographic and pathologic findings. J. Camel Pract. Res. 26, 169–172. Tharwat, M. 2020. Ultrasonography of the abdomen in healthy and diseased camels (Camelus dromedaries)—a review. J. Appl. Anim. Res. 48, 300–312. Tharwat, M. 2024. Fundamentals of diagnostic ultrasound in dromedary camel medicine. Int. J. Vet. Sci. 13, 1–6. Tharwat, M. and Al-Hawas A. 2021. Ultrasound detection of cosmic fillers injection of lips in camel beauty pageants: first report in veterinary medicine. Trop. Anim. Health Prod. 53, 53. Tharwat, M. and Al-Hawas, A. 2024. The emerging topic of cosmetic medicine in dromedary camels. Int. J. Vet. Sci. 13, 139–146. Tharwat, M., Alkhedhairi, S. and Marzok, M. 2024d. Intestinal obstruction in dromedary camels: clinical and ultrasonographic findings as well as variations in acid-base balance, blood gases and hematobiochemical profiles. I.J.A.B. 13, 500–504. Tharwat, M. and Al-Sobayil, F. 2015. The impact of racing on the serum concentrations of acute phase proteins in racing dromedary camels. Comp. Clin. Pathol. 24, 575–579. Tharwat, M. and Al-Sobayil, F. 2016. Ultrasonographic findings in camels (Camelus dromedarius) with abdominal disorders. J. Camel Pract. Res. 23, 291–299. Tharwat, M. and Al-Sobayil, F. 2018. The impact of racing on serum concentrations of bone metabolism biomarkers in racing Arabian camels. J. Camel Pract. Res. 25, 59–63. Tharwat, M., Al-Sobayil, F and Buczinski S. 2013. Effect of racing on the serum concentrations of cardiac troponin I and CK-MB in racing camels (Camelus dromedarius). Vet. Res. Commun. 37, 139–144. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012c. Ultrasonographic evaluation of abdominal distension in 52 camels (Camelus dromedarius). Res. Vet. Sci. 93, 448–456. Tharwat, M., Al-Sobayil, F., Ali, A., Hashad, M. and Buczinski, S. 2012a. Clinical, ultrasonographic, and pathologic findings in 70 camels (Camelus dromedarius) with Johne’s disease. Can. Vet. J. 53, 543–548. Tharwat, M., Al-Sobayil, F. and El-Magawry, S. Mitchel. 2013. Clinicobiochemical and postmortem investigations in 60 camels (Camelus dromedarius) with Johne’s disease. Camel Pract. Res. 20, 145–149. Tharwat, M., Al-Sobayil, F., Hashad, M. and Buczinski, S. 2012b. Transabdominal ultrasonographic findings in goats with paratuberculosis. Can. Vet. J. 53, 1063–1070. Tharwat, M., El-Shafaey, E. and Alkheraif. 2024c. Ultrasonographic evaluation of thoracic and abdominal neoplasia in domestic ruminants: a systemic review. Open Vet. J. 14, 1751–1760. Tharwat, M., El-Ghareeb, W.R. and Almundarij, T.I. 2024e. Depraved appetite in dromedary camels: clinical, ultrasonographic, and postmortem findings. Open Vet. J. 14, 652–663. Tharwat, M., El-Shafaey, E., Sadan, M., Ali, A., Al-Sobayil, F. and Al-Hawas, A. 2018. Omaso-abomasal adenocarcinoma in a female Arabian camel (Camelus dromedarius). J. Appl. Anim. Res. 46, 1268–1271. Tharwat, M., Haridy, M., Elmoghazy, H.M.M., Elnahas, A. and Alkheraif, A.A. 2024b. Abdominal fat necrosis in a female dromedary camel: clinical, hematobiochemical, sonographic and pathologic findings. Open Vet. J. 14, 1726–1732. Tharwat, M., Sadan, M. and Al-Hawas, A. 2024a. The emerging topic of injected cosmetic fillers in the perinasal region of dromedary camels: ultrasonographic and radiographic verification. Open Vet. J. 14, 840–845. Tharwat, M. and Tsuka, T. 2024. Diagnostic utility of ultrasonography for thoracic and abdominal bacterial and parasitic diseases in ruminants: a comprehensive overview. Front. Vet. Sci. 11, 1435395. Tigani-Asil, E.T.A.E., Abdelwahab, G.E.D., Abdu E.H.A.M., Terab, A.M.A., Khalil, N.A.H., Marri, Z.J.M.A., Yuosf, M.F., Shah, A.A.M., Khalafalla, A.I. and Ishag, H.Z.A. 2023. Pathological, microscopic, and molecular diagnosis of paratuberculosis/John’s disease in naturally infected dromedary camel (Camelus dromedarius). Vet. World. 16, 1277–1283. Tigani-Asil, E.T.A.E., Abdelwahab, G.E.D., Abdu, E.H.A.M., Terab, A.M.A., Khalil, N.A.H., Marri, Z.J.M.A., Yuosf, M.F., Shah, A.A.M., Khalafalla, A.I. and Ishag, H.Z.A. 2023. Pathological, microscopic, and molecular diagnosis of paratuberculosis/John’s disease in naturally infected dromedary camel (Camelus dromedarius). Vet. World. 16, 1277–1283. Weber, M.F., Kelton, D., Eisenberg, S.W.F. and Donat, K. 2024. Progress in paratuberculosis control programmes for dairy herds. Animals 14, 1127. Whittington, R.J., Hope, A.F., Marshall, D.J., Taragel, C.A. and Marsh, I. 2000. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J. Clin. Microbiol. 38, 3240–3248. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Ali H, Alkheraif AA. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Vet. J.. 2025; 15(1): 8-17. doi:10.5455/OVJ.2025.v15.i1.2 Web Style Tharwat M, Ali H, Alkheraif AA. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. https://www.openveterinaryjournal.com/?mno=209936 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i1.2 AMA (American Medical Association) Style Tharwat M, Ali H, Alkheraif AA. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Vet. J.. 2025; 15(1): 8-17. doi:10.5455/OVJ.2025.v15.i1.2 Vancouver/ICMJE Style Tharwat M, Ali H, Alkheraif AA. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Vet. J.. (2025), [cited January 25, 2026]; 15(1): 8-17. doi:10.5455/OVJ.2025.v15.i1.2 Harvard Style Tharwat, M., Ali, . H. & Alkheraif, . A. A. (2025) Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Vet. J., 15 (1), 8-17. doi:10.5455/OVJ.2025.v15.i1.2 Turabian Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. 2025. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Veterinary Journal, 15 (1), 8-17. doi:10.5455/OVJ.2025.v15.i1.2 Chicago Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. "Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review." Open Veterinary Journal 15 (2025), 8-17. doi:10.5455/OVJ.2025.v15.i1.2 MLA (The Modern Language Association) Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. "Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review." Open Veterinary Journal 15.1 (2025), 8-17. Print. doi:10.5455/OVJ.2025.v15.i1.2 APA (American Psychological Association) Style Tharwat, M., Ali, . H. & Alkheraif, . A. A. (2025) Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): A review. Open Veterinary Journal, 15 (1), 8-17. doi:10.5455/OVJ.2025.v15.i1.2 |