| Research Article | ||
Open Vet. J.. 2024; 14(4): 1012-1018 Open Veterinary Journal, (2024), Vol. 14(4): 1012–1018 Original Research Evaluation of recombinant human erythropoietin as a promoter of bone healing in cats with femoral fracturesRadina Vasileva* and Tsvetan ChaprazovDepartment of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria *Corresponding Author: Radina Vasileva. Department of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria. Email: radina.vasileva [at] trakia-uni.bg Submitted: 19/12/2023 Accepted: 12/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
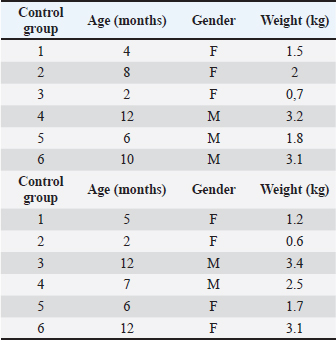
AbstractBackground: The bone regeneration potential of erythropoietin (EPO) is not yet fully investigated, but some previous experimental studies demonstrated that its application activated the differentiation of osteoblasts and promoted bone formation. Aim: The aim of the present study was to evaluate the effects of recombinant human erythropoietin (rhEpo) on bone healing in cats with fragmented long bone fractures. Methods: Twelve cats were divided into two groups—control (n=6) in which physiological saline was applied at the fracture gap site and EPO (n=6) with the application of 1,000 IU rhEpo. The effects of EPO on blood erythrocyte counts, hemoglobin content, and hematocrit were monitored by serial complete blood cell tests, whereas bone formation was evaluated by clinical and radiographic examinations on post-operative weeks 1, 2, 3, 4, 6, and 8. Results: All tested blood parameters were within the reference range. A faster fracture healing and full limb weight-bearing were observed in the EPO group, with statistically significant differences with respect to the control group. Conclusion: The obtained results confirmed that the local application of rhEpo promoted bone healing in cats with fragmented femoral fractures and increased bone callus strength without having significant systemic effects. Keywords: Bone healing, Cat, DEXA, Erythropoietin, Fracture. IntroductionErythropoietin (ЕPO)—a hormone produced by peritubular fibroblasts of the renal cortex, stimulates the production of red blood cells (RBCs) in animal and human bodies (Jelkmann, 2016). EPO-stimulating agents are recombinant versions of EPO synthesized pharmacologically in cell cultures (Schoener and Borger, 2023). Their use is indicated mainly in chronic renal failure patients with anemia syndrome or during chemotherapy of cancer patients (Bruce et al., 2022; Chung et al., 2023). The additional (pleiotropic) functions of EPO are of interest to regenerative medicine. EPO possesses an angiogenic, anti-inflammatory, cytoprotective, and anti-apoptotic effect (Trassanidou-Chatzifotiadou, 2014). Studies in the field of bone pathology demonstrated that the recombinant human erythropoietin (rhEpo) improved bone healing and may serve as a therapeutic agent promoting bone regeneration (Wan et al., 2014). There is evidence that EPO stimulates bone healing and improves bone mechanical strength (McGee et al., 2012). Its direct influence consists in the activation of the differentiation of osteoblasts, whereas the indirect effect involves stimulation of the expression of vascular endothelial growth factor (Holstein et al., 2011; Singbrant et al., 2011). Previous experimental studies on calvarial and long bones in rats showed that EPO had an osteoinductive potential and that its local application resulted in healing of critical bone defects (Chaprazov et al., 2022; Vasileva and Chaprazov, 2023). Similar studies were performed also in mice and rabbits with promising results regarding the regenerative potential of EPO (Holstein et al., 2011; Rölfing et al., 2012). Data from clinical studies are however scarce. The first research in humans with tibial and fibular fractures has analyzed the effect of rhEpo applied at the fracture site 2 weeks after osteosynthesis. The results confirmed that EPO enhanced bone healing and shortened the restoration period (Bakhshi et al., 2013). A recent preliminary study in men with periodontitis demonstrated that local EPO gel application reduced inflammation and improved the strength of the periodontal ligament (Aslroosta et al., 2021). To the best of our knowledge, there are no veterinary scientific reports on the effect of rhEpo on bone regeneration in patients. The aim of the present study was to evaluate the effects of rhEpo on bone healing in cats with fragmented long bone fractures. Materials and MethodsErythropoietinIn this study, 2,000 IU Binocrit injection solution containing 2,000 IU/ml epoietin alpha equivalent to 16.8 μg/ml (Sandoz, Kundl) was used. It is produced by re-combinant DNA technology in the Chinese Hamster Ovary cell line expression system. AnimalsTwelve cats and patients of the Small Animal Clinic, University Veterinary Hospital were included. The inclusion criterion was a recent fragmented fracture of the femoral diaphysis. Exclusion criteria were animals with systemic diseases, animals with multiple fractures, and animals, which due to the type of fracture, needed a combination of different techniques of bone fixation at the same time. The study included 12 mixed-breed cats. The average body weight of patients was 2.05 ± 0.96 kg (control group) and 2.17 ± 1.2 kg (EPO group). The respective average age of both groups was 7.0 ± 3.7 and 7.3 ± 4.3 months. There were no statistically significant between-group differences in body weight and age. The signalment data of patients are listed in Table 1. The cats were assigned to one of two groups depending on the applied treatment.
Anesthesiological and operative protocolThe cats were premedicated with atropine sulfate (0.1% Atropin, Sopharma) at a dose of 0.02 mg/kg, and applied subcutaneously. The induction of anesthesia was done by intravenous application of the combination zolazepam/tiletamine (Zoletil 50, Virbac) at a dose of 15 mg/kg, and the maintenance through inhalational anesthesia with 2.5% isoflurane with oxygen (AErrane, Baxter). Table 1. Signalment data of cats from the control and EPO groups.
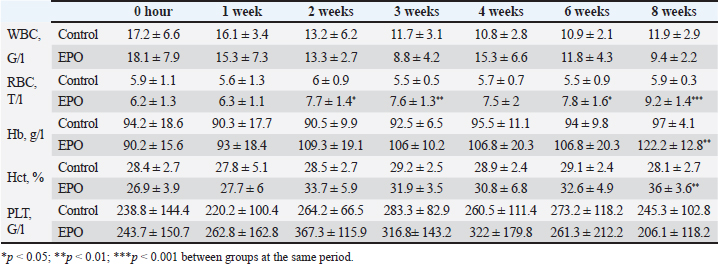
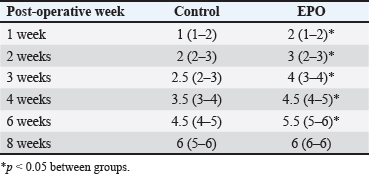
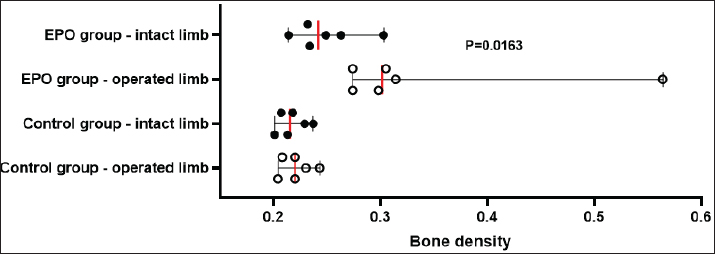
After hair clipping, the field was disinfected with 4% chlorhexidine digluconate (HMI Scrub Classic) and 10% povidone-iodine (Braunol, B. Braun Melsungen). After a routine lateral approach to the femur, bone fragments were aligned and fixed with a metal plate and screws. Fixation elements were selected according to the patient’s size. The skin incision was closed in layers through a simple continuous suture. Fasciae and subcutaneous tissue were closed with absorbable suture material (PGA 2-0, Kruuse), and the skin—with nonabsorbable material (Nylon 3-0, Kruuse). During the post-operative period, pain was controlled with meloxicam (Meloxidolor, Produlab Pharma) at a dose of 0.3 mg/kg, applied subcutaneously. Amoxicillin/clavulanic acid (Synulox RTU, Pfizer) at 25 mg/kg was also subcutaneously applied for 6 days. Complete blood count testsComplete blood count analyses were conducted before the surgery and at post-operative weeks 1, 2, 3, 4, 6, and 8. The following parameters were determined: total white blood cell count (WBC, G/l), RBC, T/l, hemoglobin (Hb, g/l), hematocrit (Hct, %), and platelet counts (PLT, G/l) on an automated hematological analyzer BS-5000 Vet (Mindray). Clinical examinationsThe operated limb function was evaluated before the surgery and at post-operative weeks 1, 2, 3, 4, 6, and 8. The day of limb loading and lameness disappearance was recorded by inspection, and the day without swelling and pain was by palpation. The day of removal of metal implants was also registered. Radiological examinationsSerial craniocaudal radiographs of the femoral region were obtained before the surgery and at post-operative weeks 1, 2, 3, 4, 6, and 8 with PHILIPS SUPER 50 CP-D equipment (Hamburg, Germany) and exposure data 50 kV/10 mAs for monitoring of early bone healing stages. Radiographs were processed by the iQ-VIEW/PRO version 2.7.0 software. The researcher who scored all radiographs with 1–6 points (0: Presence of fracture line with no bone formation; 1: Visible fracture line, no callus formation; 2: Visible fracture line with initial callus formation; 3: Visible fracture line with bridging callus formation; 4: Exuberant osseous callus in evolution and discrete radiolucent line at the gap between the fracture fragments; 5: Exuberant osseous callus and absence of radiolucent line; 6: Bone remodeling) was blind to the given treatment (EPO or control). OsteodensitometryThe femoral bone density of all cats was measured after the removal of metal implants by means of dual-energy X-ray absorptiometry (DEXA). During the procedure, cats were anesthetized with intravenous application of 7 mg/kg 1% Propofol (Propofol, Fresenius Kabi Deutschland). Scans were done with OsteoSys SMART FAN-BEAM densitometer (OsteoSys, Seoul, Korea). Bone mineral density data were generated by the Excellus software (OsteoSys, Seoul, Korea) and presented as g/cm2. Statistical analysisData from clinical and radiographic examinations were presented as median (minimum-maximum), and those from complete blood count tests: as mean and standard deviation (SD). The differences between the groups were evaluated by the Mann-Whitney test or one-way ANOVA. For all analyses, the MedCalc v.15.8 (Belgium) statistical software was used. Ethical approvalAll owners were informed about the purpose of the experiments and gave informed written consent for the inclusion of their pets. ResultsControl cats began to bear weight on the operated limb 3 (2–4) days after the surgery, whereas those with local EPO application—significantly earlier: 1.5 (1–2) days after the osteosynthesis (p < 0.01). The swelling at the fracture region was visible until post-operative day 5 (4–7) in control cats and day 3 (2–5) in cats from the EPO group (p < 0.05). In cats from the control group, the lameness disappeared as late as the 10th day after the surgery (8–10) which was statistically significantly different (p < 0.01) from the EPO group: 6.5 (5–7) day. The pain upon palpation of the limb disappeared on post-operative day 3 (2–4) in the EPO-treated group compared to day 7 (6–9) in the control group (p < 0.01). The time until removal of the implant in cats from the EPO group was significantly shorter (median 61; range 60–70 days) than in cats from the control group (median 72.5; range 65–80) (p < 0.05). The results from hematological analysis confirmed that all studied parameters were within the reference ranges for the species. The RBC counts, Hb content, and Hct increased during the different post-operative periods only in patients from the EPO group. By the end of the 8th post-operative week, they were statistically significantly higher than the respective average values of control cats (Table 2). During the first post-operative week, the fracture line was visible and both groups exhibited decreased radiopacity at the fracture site corresponding to bone resorption occurring at the ends of bone fragments. Initial bone callus formation was visualized on radiographs during the second week after the osteosynthesis at the time of calcification, which was more evident in the EPO group. Bridging callus formation was manifested during the 3rd and the 4th week, and again, was more expressed in the EPO group. Signs of bone remodeling were observed during the 8th week when the bone regained its initial shape. The radiological scores of both groups are presented in Table 3. The results from DEXA scans confirmed that the bone mineral density of operated femurs exceeds that of healthy contralateral limbs, with a statistically significant difference in the EPO group (Fig. 1). DiscussionIn cats, the proportion of femoral bone fractures is reported to be 20%–25% (Beale, 2004; DeCAMP et al., 2016). According to a more recent study, the percentage of femoral fractures from all long tubular fractures was 52% (Vasileva and Chaprazov, 2022). The commonest causes are road accidents and high-rise syndrome injuries (Denny and Butterworth, 2000; Piermattei et al., 2006; Scott and McLaughlin, 2007). The patients included in this study were with fragmented femoral fractures, associated with bone defects requiring a longer healing period. The effects of the local application of EPO on the healing of fragmented fractures were assessed by clinical, laboratory, and radiological methods, familiar to every orthopaedist. Table 2. Results from studied hematological parameters (mean ± SD, n=6).
Table 3. Radiological healing scores in cats from control (n=6) and EPO-treated (n=6) groups. Data are presented as median (min-max).
Fig. 1. Bone mineral density (g/cm2) of the femur in control and EPO-treated cats. The red line corresponds to the median value. The pain from limb loading, pain after palpation at the fracture site, and weight bearing on the operated limb are among the most frequently used clinical criteria for the evaluation of bone healing (Corrales et al., 2008). The last parameter was found to increase with time after the surgery and correlated with fractured bone strength (Joslin et al., 2008). The cats from the EPO group began bearing weight on the operated limb as early as the first post-operative day, whereas control cats—on the third day. This finding indicates stable fixation of bone fragments from one hand and dull pain sensation from the other. EPO pain control (Derakhshan, 2018) is mediated through its regulatory effect on inflammation, as it inhibits the expression of pro-inflammatory factors by reducing of activity of macrophageal nuclear factor kappa B (Nairz et al., 2011). Complete blood count tests are used for monitoring the clinical status of the patients and occurred post-traumatic changes for the prevention of possible complications. On the 7th day after the osteosynthesis, Kumar et al. (2016) reported an insignificant decrease in RBC counts and Hb content, followed by variations within the reference range for the species, a finding supported by our results in control animals (Kumar et al., 2016). Comparable results were also reported by Tembhurne et al. (2010) and Rajhans (2013) in dogs (Tembhurne et al., 2010; Rajhans, 2013), Kumar et al. (1999) in calves (Kumar et al., 1999), and Gabriel et al. (2014) and Gupta (2015) in goats (Gabriel et al., 2014; Gupta, 2015). The transient reduction of RBC counts in the above-mentioned studies may be attributed to fracture trauma and preoperative and intraoperative blood loss. In the EPO group, the opposite tendency was observed: RBCs, Hb, and Hct increased gradually than the initial levels, with a statistically significant difference versus baseline on the 8th post-operative week. This is attributed to the hematopoietic effect of EPO, which was also observed in experimental studies in rats (Diker et al., 2018). In humans subjected to orthopedic surgery, the perioperative application of EPO reduced the need for blood transfusion (Tamir et al., 2000). Radiographic changes are characterized by fracture gap widening during the first post-operative week due to the enhanced osteoclastic activity, which usually occurs within 2–4 weeks (Langley-Hobbs, 2003). After the second week, bone formation events were initiated, which in general are not clearly obvious in primary bone healing and may worry the inexperienced clinician. After the application of EPO, bone healing occurred within a shorter period and even earlier than is usually observed for secondary bone healing. In a feline model of closed femoral fracture fixed with intramedullary osteosynthesis, Emery and Murakami (1967) found no radiological signs of bone formation 2 weeks post surgery, periosteal thickening was observed only by the third week, and during the fourth week—first callus appearance (Emery and Murakami, 1967). For the cats from the EPO group, these processes occurred about 1 week earlier than in the control group: periosteal reaction was visualized by the second week and bone formation—in the third post-operative week. The obtained results confirmed that the radiological assessment of fracture healing was based on the loss of bone fragment sharpness 1–2 weeks after the traumatic injury and the visualization of periosteal callus 3–6 weeks after the fracture (Jeon et al., 2020). The bone healing rate depends on several factors: the type of fractured bone, the fracture type, the patient’s age, the treatment technique, and the presence of other systemic diseases (Claes et al., 2012). Fragmented fractures heal more slowly because of the relative instability of bone fragments and decreased angiogenesis and blood supply of fragments (Dhillon and Dhatt, 2012). In skeletally immature dogs and cats and in ideal conditions with appropriate vascularisation and stable fixation with plates and screws, the expected time for bone union of a simple diaphyseal fracture is about 4–12 weeks (Menghini et al., 2023). According to Drosos et al. (2006), human patients with fragmented fractures needed more time for defect healing whereas out results in the EPO group are entirely close to the normal time for bone healing regardless of the fracture type (Drosos et al., 2006). The DEXA scans performed after the bone union provided proof for a higher bone mineral density of the operated limb compared to the contralateral intact limb. These results are in line with the only similar study, performed more than 20 years ago in men. Janes et al. (1993) affirmed that the increased mineral content 2 weeks after the removal of the metal osteosynthesis plate was due to the amount of formed mineralized bone callus and the still incomplete bone remodeling events (Janes et al., 1993). The authors did not associate these changes with fractured bone strength, whereas a more recent study proved a positive correlation between bone strength and bone mineral density measured by means of DEXA (Seo et al., 2014). It should be, however, remembered that bone strength depends not only on bone mineral content but also on structural and biochemical bone features (Hart et al., 2017). The observed statistically significant differences in bone mineral density between the control and EPO groups supported the data of Holstein et al. (2007) that local EPO application increased the strength of the newly formed bone callus (Holstein et al., 2007). ConclusionOn the basis of the results, it may be concluded that the local application of EPO in cats with fragmented femoral fractures enhanced bone healing and increased bone callus strength without any significant systemic effects. AcknowledgmentsThe authors are grateful to all colleagues from the Faculty of Veterinary Medicine, Trakia University, who made this study possible. Conflicts of interestThe authors declare no conflict of interest. FundingThis research was funded by the Faculty of Veterinary Medicine, Trakia University, Stara Zagora, 6000, Bulgaria, scientific project number 02/2021. Authors’ contributionsBoth authors contributed to this study. Both authors read and approved the final manuscript. Data availabilityAll data from this study are provided in the manuscript. ReferencesAslroosta, H., Yaghobee, S., Akbari, S. and Kanounisabet, N. 2021. The effects of topical erythropoietin on non-surgical treatment of periodontitis: a preliminary study. BMC Oral Health. 21(1), 240. Bakhshi, H., Kazemian, G., Emami, M., Nemati, A., Karimi, Yarandi, H. and Safdari, F. 2013. Local erythropoietin injection in tibiofibular fracture healing. Trauma Mon. 17(4), 386–388. Beale, B. 2004. Orthopedic clinical techniques femur fracture repair. Clin. Tech. Small Anim. Pract. 19(3), 134–150. Bruce, G., Schulga, P. and Reynolds, B.C. 2022. Use of erythropoiesis-stimulating agents in children with chronic kidney disease: a systematic review. Clin. Kidney J. 15(8), 1483–1505. Chaprazov, T., Vasileva, R., Atliev, K. and Firkova, E. 2022. Diagnostic imaging studies on local and systemic erythropoietin application for promoting bone regeneration in rat calvarial defects. Vet. Sci. 9(10), 578. Chung, E.Y., Palmer, S.C., Saglimbene, V.M., Craig, J.C., Tonelli, M. and Strippoli, G.F. 2023. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst. Rev. 2(2), CD010590. Claes, L., Recknagel, S. and Ignatius, A. 2012. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8(3), 133–143. Corrales, L.A., Morshed, S., Bhandari, M. and Miclaus, T. 2008. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Joint Surg. Am. 90, 1862–1868 DeCAMP, C.E., Johnston, S.A., Dejardin, L.M. and Schaefer, S.L. 2016. Brinker, Piermattei and Flo’s handbook of small animal orthopedics and fracture repair, 5th ed. St. Louis, MO: Saunders Elsevier. Denny, H.R. and Butterworth, S.J. 2000. Classification of fractures, 83-86. In A guide to canine and feline orthopaedic surgery. Ed., Ibid. Oxford, UK: WileyBlackwell. Derakhshan, P. 2018. What is the role of erythropoietin prolotherapy on pain relief of knee osteoarthritis? J. Res. Med. Sci. 23, 50. Dhillon, M. and Dhatt, S. 2012. Introduction to fractures and dislocation. In First aid and emergency management in orthopedic injuries, 1 ed. Eds., Dhillon, M., Dhatt, S. and De Boer, P. New Delhi, India: JP Medical Ltd, pp: 2–4. Diker, N., Sarican, H., Cumbul, A. and Kilic, E. 2018 Effects of systemic erythropoietin treatment and heterogeneous xenograft in combination on bone regeneration of a critical-size defect in an experimental model. J. Craniomaxillofac. Surg. 46, 1919–1923. Drosos, G.I., Bishay, M. and Karnezis, I.A. 2006. Factors affecting fracture healing after intramedullary nailing of the tibial diaphysis for closed and grade I open fractures. J. Bone Joint Surg. 88, 227–231. Emery, M.A. and Murakami, H. 1967. The features of fracture healing in cats after immediate and delayed open reduction. J. Bone Joint Surg. 49(3), 571–579. Gabriel, O., Hassan, A.Z., Abdullahi, U.S. and Fatihu, M.Y. 2014. Changes in body and haematological parameters following the use of bone plates in management of tibial fracture in kano brown goats. J. Anim. Vet. Adv. 13(9), 549–597. Gupta, S. 2015. Fracture healing using biphasic calcium phosphate with dynamic compression plating in goats. PhD Thesis, Nanaji Deshmukh Veterinary Science University, Jabalpur, India. Hart, N.H., Nimphius, S., Rantalainen, T., Ireland, A., Siafarikas, A. and Newton, R.U. 2017. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. JMNI 17(3), 114–139. Holstein, J.H., Menger, M.D., Scheuer, C., Meier, C., Culemann, U., Wirbel, R.J., Garcia, P. and Pohlemann, T. 2007. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 80, 893–900. Holstein, J.H., Orth, M., Scheuer, C., Tami, A., Becker, S.C., Garcia, P., Histing, T., Mörsdorf, P., Klein, M., Pohlemann, T. and Menger, M.D. 2011. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone 49, 1037–1045. Janes, G.C., Collopy, D.M., Price, R. and Sikorski, J.M. 1993. Bone density after rigid plate fixation of tibial fractures. A dual-energy X-ray absorptiometry study. J. Bone Joint Surg. 75(6), 914–917. Jelkmann, W. 2016. Erythropoietin. Front. Horm. Res. 47, 115–127. Jeon, S., Jang, J., Lee, G., Park, S., Lee, S.K., Kim, H. and Choi, J. 2020. Assessment of neovascularization during bone healing using contrast-enhanced ultrasonography in a canine tibial osteotomy model: a preliminary study. J. Vet. Sci. 21(1), e10. Joslin, C.C., Eastaugh-Waring, S.J., Hardy, J.R. and Cunningham, J.L. 2008. Weight bearing after tibial fracture as a guide to healing. Clin. Biomech. 23(3), 329–333. Kumar, D., Bhargava, M.K. and Singh, R. 2016. Haematological changes during fracture healing in goats. IOSR-JAVS 9(09), 1–3. Kumar, V., Varshney, A.C., Singh, M., Sharma, S.K. and Nigam, J.M. 1999. Haemato-biochemical changes during fracture repair with hydroxyapatite–fibrillar collagen implants in calves. Indian J. Vet. Surg. 20(2), 92–93. Langley-Hobbs, S. 2003. Biology and radiological assessment of fracture healing. In Practice 25(1), 26–35. McGee, S., Havens, A., Shiozawa, Y., Jung, Y. and Taichman, R. 2012. Effects of erythropoietin on the bone microenvironment. Growth Factors 30, 22−28. Menghini, T.L., Shriwise, G. and Muir, P. 2023. Fracture healing in 37 dogs and cats with implant failure after surgery (2013-2018). Animals 13(9), 1549. Nairz, M., Schroll, A., Moschen, A.R., Sonnweber, T., Theurl, M., Theurl, I., Taub, N., Jamnig, C., Neurauter, D., Huber, L.A., Tilg, H., Moser, P.L. and Weiss, G. 2011. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 34(1), 61–74. Piermattei, D.L., Flo, G. and DeCamp. 2006. Brinker, Piermattei and Flo’s handbook of small animal orthopedics and fracture repair, 4 ed. Philadelphia, PA: Saunders. Rajhans, M. 2013. Stabilization of splinters of long bone fracture in dogs. Jabalpur, India: Nanaji Deshmukh Veterinary Science University. Rölfing, J.H., Bendtsen, M., Jensen, J., Stiehler, M., Foldager, C.B., Hellfritzsch, M.B. and Bünger, C. 2012. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J. Orthop. Res. 30(7), 1083–1088. Schoener, B. and Borger, J. Erythropoietin stimulating agents. Treasure Island, FL: StatPearls Publishing; 2023. Scott, H.W. and McLaughlin, R. 2007. Introduction to feline orthopedic surgery, 9-16. In Feline orthopedics. Ed., Ibid. London, UK: Manson Publishing. Seo, S.H., Lee, J. and Park, I.H. 2014. Efficacy of dual energy X-ray absorptiometry for evaluation of biomechanical properties: bone mineral density and actual bone strength. J. Bone. Metab. 21(3), 205–212. Singbrant, S., Russell, M.R., Jovic, T., Liddicoat, B., Izon, D.J., Purton, L.E., Sims, N.A., Martin, T.J., Sankaran, V.G. and Walkley, C.R. 2011. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 117(21), 5631–5642. Tamir, L., Fradin, Z., Fridlander, M., Ashkenazi, U., Zeidman, A., Cohen, A.M., Hendel, D. and Mittelman, M. 2000. Recombinant human erythropoietin reduces allogeneic blood transfusion requirements in patients undergoing major orthopedic surgery. Haematologia 30(3), 193–201. Tembhurne, R.D., Gahlod, B.M., Dhakate, M.S., Akhre, M.S., Upadhye, S.V. and Bawaskar, S. 2010. Management of femoral fracture with the use of horn peg in canine. Vet. World 3(1), 37–41. Trassanidou-Chatzifotiadou, I. 2014. Mögliche Einsatzgebiete von Erythropoietin in der regenerativen Medizin. PhD Thesis, Medical University of Graz, Graz, Switzerland. Vasileva, R. and Chaprazov, T. 2022. Long bone fractures in cats: a five-year retrospective study (2016-2020). Trakia J. Sci. 1, 45–49. Vasileva, R. and Chaprazov, T. 2023. Bone healing of critical-sized femoral defects in rats treated with erythropoietin alone or in combination with xenograft. Vet. Sci. 10(3), 196. Wan, L., Zhang, F., He, Q., Tsang, W.P., Lu, L., Li, Q., Wu, Z., Qui, G., Zhou, G. and Wan, C. 2014. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS One 9, e102010. | ||
| How to Cite this Article |
| Pubmed Style Vasileva R, Chaprazov T. Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Vet. J.. 2024; 14(4): 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 Web Style Vasileva R, Chaprazov T. Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. https://www.openveterinaryjournal.com/?mno=181907 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i4.8 AMA (American Medical Association) Style Vasileva R, Chaprazov T. Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Vet. J.. 2024; 14(4): 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 Vancouver/ICMJE Style Vasileva R, Chaprazov T. Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Vet. J.. (2024), [cited January 12, 2026]; 14(4): 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 Harvard Style Vasileva, R. & Chaprazov, . T. (2024) Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Vet. J., 14 (4), 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 Turabian Style Vasileva, Radina, and Tsvetan Chaprazov. 2024. Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Veterinary Journal, 14 (4), 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 Chicago Style Vasileva, Radina, and Tsvetan Chaprazov. "Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures." Open Veterinary Journal 14 (2024), 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 MLA (The Modern Language Association) Style Vasileva, Radina, and Tsvetan Chaprazov. "Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures." Open Veterinary Journal 14.4 (2024), 1012-1018. Print. doi:10.5455/OVJ.2024.v14.i4.8 APA (American Psychological Association) Style Vasileva, R. & Chaprazov, . T. (2024) Evaluation of recombinant human erythropoietin as promoter of bone healing in cats with femoral fractures. Open Veterinary Journal, 14 (4), 1012-1018. doi:10.5455/OVJ.2024.v14.i4.8 |