| Research Article | ||
Open Vet. J.. 2024; 14(4): 962-972 Open Veterinary Journal, (2024), Vol. 14(4): 962–972 Original Research Embryonic development of the scorpion mud turtle (Kinosternon scorpioides) bred in captivityLianne Polliane Fernandes Araujo1, Diego Carvalho Viana2, Ligia Tchaika3, Juliana Maria Alves Caldas4,5, Antônio Chaves Assis Neto6, Maria Angélica Miglino6 and Alana Lislea de Sousa4,5*1Biotechnology (BIONORTE), São Luís, Brazil 2Center for Advanced Morphophiological Studies (NEMO), Centre of Agrarian Sciences, State University of the Tocantina Region of Maranhão (UEMASUL), Imperatriz, Brazil 3Programa de Pós-Graduação em Ecologia e conservação da biodiversidade, Centro de Educação, Ciências Exatas e Naturais, Universidade Estadual do Maranhão (UEMA), São Luís, Brazil 4Program in Animal Science, State University of Maranhão, University City Paulo VI, São Luís, Brazil 5Biotechnology, State University of Maranhão (UEMA), São Luís, Brazil 6Department of Surgery, School of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil *Corresponding Author: Alana Lislea de Sousa. Anatomy Group (Granato), Program in Animal Science, State University of Maranhão, University City Paulo VI, São Luís, Brazil. Email: alislea [at] hotmail.com Submitted: 26/09/2023 Accepted: 06/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
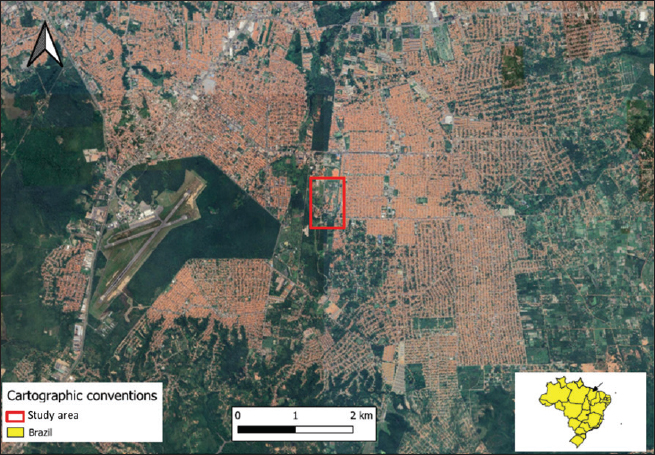
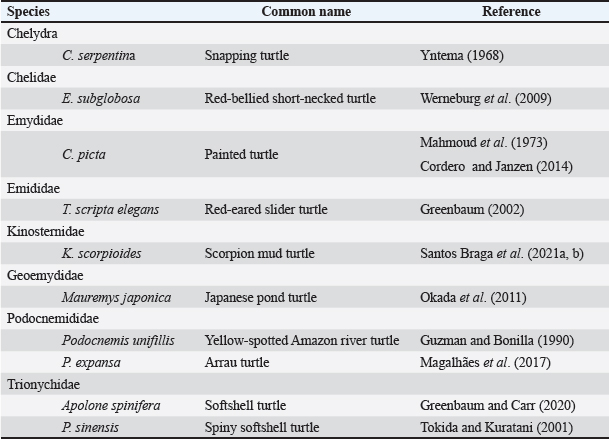
AbstractBackground: The developmental biology of Kinosternon scorpioides is described, based on the phenotype. This species is important for the flora because they are excellent seed disseminators. In addition, basic embryological information is not yet fully clarified, and this research provides unprecedented information on the chelonian embryology of the Amazonian fauna. Aim: The present study aims to identify the embryology of K. scorpioides in captivity during different periods. Methods: Females were monitored throughout the reproductive cycle, by video monitoring, to identify nests and the presence of newly laid eggs. At regular weekly intervals, embryo samples were collected fixed in a 4% paraformol solution and preserved in 70% alcohol. For the embryonic characterization, we used a stereomicroscope and the scanning electron microscopy method. Results: We describe 15 embryonic stages for a 15-week (105-day) incubation process. Only at 42 days (6th week) was the morphological characterization of a chelonian observed and at the 12th week (Stage XII), the phenotypic characterization of the species K. scorpioides. Conclusion: In view of the evidence, we found that these phases are similar to the other turtles, with structural variations in the appearance and disappearance of structures due to the specific characteristics of the species. Keywords: Biodiversity, Conservation, Turtles. IntroductionInvestigations into the reproduction of turtles are essential to ensure sustainability, conservation, and establishment of reproductive management plans (Ceriani et al., 2019). Despite the longevity of the scorpion mud turtle, freshwater and sea turtles, they still show slow growth may further complicate a populations recovery when it is under pressure from hunting and habitat loss (Roberts, 2021), compiled and analyzed data indicate distinct patterns of decline in various regions, highlighting the vulnerability of these animals in the face of environmental pressures and highlighting the significant contribution of ecotoxic factors in the reduction of amphibian and reptile populations (Todd et al., 2010). Unfavorable conditions make the attainment of birth challenging, resulting in elevated mortality rates. This, in turn, reduces the recruitment of the younger generation, posing a threat to the overall maintenance of the population (Ferreira Júnior, 2009). Some species of the Testudines order had their embryonic stages determined Chelydra serpentina (Yntema, 1968); Lepidochelys olivacea (Crastz, 1982); Pelodiscus sinensis (Tokita and Kuratani, 2001); Trachemys scripta (Greenbaum, 2002); Emydura subglobosa (Werneburg et al., 2009); Podocnemis expansa (Magalhães et al., 2017); and Kinosternon scorpioides (Santos Braga et al., 2021a). The species K. scorpioides benefits from an unparalleled biological description detailing the stages of embryonic development in captive animals. This captive setting offers a controlled environment, enabling meticulous observations. The controlled conditions and observational opportunities provided shed light on developmental processes that might be challenging to discern in the unpredictable setting of the wild. With a broad geographical distribution, K. scorpioides is a semi-aquatic turtle belonging to the Kinosternidae family and is a notable member of the Amazonian fauna. Its presence extends across various regions, particularly in the north and northeast of Brazil, including the states of Amazonas, Pará, Amapá, Rondônia, Mato Grosso, Maranhão, Tocantins, Goiás, and Minas Gerais (Vogt et al., 2015). In addition, this species has been documented in several South American countries, spanning Venezuela, Colombia, Ecuador, Peru, Bolivia, Paraguay, and Argentina, as well as some Central American countries. Kinosternon scorpioides is not globally assessed on the International Union for Conservation of Nature (IUCN) red list. This study is part of a dossier that we intend to cover to demonstrate technical and viable studies to implement a law to protect this animal, which is very important for the ecosystem in which it is found. The semi-aquatic nature of K. scorpioides implies an affinity for habitats associated with slow-moving or stagnant water bodies, aligning with its presence in diverse ecosystems across this extensive geographical range. Biological data on this species has been investigated for years by scholars from Maranhão, mainly on its reproductive biology (Sousa et al., 2014; Viana et al., 2015; Fernandes Araujo Chaves et al., 2020; Viana et al., 2023). In studies conducted on the reproduction of K. scorpioides, it has been observed that females typically lay clutches containing six eggs. The timing of egg-laying, often referred to as the nesting season, occurs from December to May. In terms of nesting behavior, K. scorpioides demonstrates a preference for mating in water, where they excavate nests to a depth of 4–6 cm (Chaves, 2010). Understanding these aspects of reproductive biology not only contributes to our knowledge of the species but also has implications for effective conservation management. Considering the species’ multifaceted importance to Amazonian culture, economy, and ecology. Culturally, these turtles may hold importance in indigenous traditions or local communities, playing a role in folklore, rituals, or traditional practices. Economically, they might contribute to local economies through subsistence or commercial uses, such as for food or traditional medicine. Ecologically, K. scorpioides is likely to play a crucial role in the ecosystem as a seed disseminator. Describing embryo stages in captivity rather than in the field provides researchers with controlled conditions and allows for detailed, systematic observation and documentation, enabling researchers to establish comprehensive timelines and milestones. The knowledge gained from studying embryonic stages in captivity has direct implications for conservation in the wild. It provides a baseline for understanding normal developmental processes, which is crucial for identifying abnormalities or disruptions that could occur in natural habitats. This understanding aids in the detection of potential threats to reproductive success, such as environmental changes, pollution, or habitat degradation. Moreover, it informs conservation strategies, allowing for targeted efforts to protect and manage critical nesting sites and habitats necessary for the species’ survival. Captive breeding initiatives can leverage specific knowledge about embryonic stages, ensuring that controlled environments replicate the conditions necessary for successful reproduction. This not only aids in bolstering population numbers but also provides a safety net against potential threats in the wild. This study gains significance considering the species’ importance in Amazonian fauna. The inclusion of embryological information within the framework of sustainable exploration further underscores the relevance of this research, which aims to describe the embryonic stages of the species, based on the phenotypic description. The present study aims to identify the embryology of K. scorpioides in captivity during different development stages. Materials and MethodsAnimalsThe animals belong to the experimental breeding ground for the species K. scorpioides (License IBAMA N° 1899339/2008), in São Luís-MA (02° 31’ 47” S 44° 18’ 10” W) (Fig. 1). A group of 15 females (Table 1) and seven males, sexually mature adults (age over 3 years and average carapace length of 14 cm) were monitored by video and descriptions were based on studies by Yntema (1968). Observations in the Experimental Breeding Ground were conducted on a daily basis to identify copulation, nesting, and laying behaviors. In addition, females underwent regular radiographic and ultrasound monitoring to detect the presence of eggs in the oviducts. The radiographic examination involved capturing internal images to assess the development and location of eggs, while ultrasonography provided real-time visualization of the reproductive structures. This comprehensive monitoring approach aimed to gather detailed data on reproductive processes, facilitating a thorough understanding of the species’ reproductive biology in captive conditions. Collection of eggs and embryosDuring the reproductive cycle of the year 2018 (May to September), the nesting area was monitored, and the eggs were collected immediately after laying, then placed in Jaeger Brutgerät fur reptilien D-65607 (Germany) incubators with vermiculite substrate and an average temperature of 28°C (the pivotal temperature for sex determination of K. scorpioides as per Ewert et al. (2004).
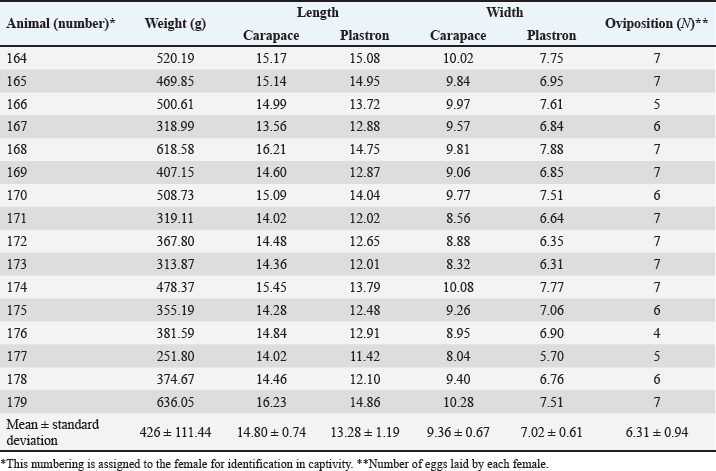
Fig. 1. Location of the experimental breeding ground for the species K. scorpioides, São Luís, Maranhão, Brazil. Table 1. Biometrics of female K. scorpioides obtained from captivity. São Luís, Maranhão, Brazil, 2018.
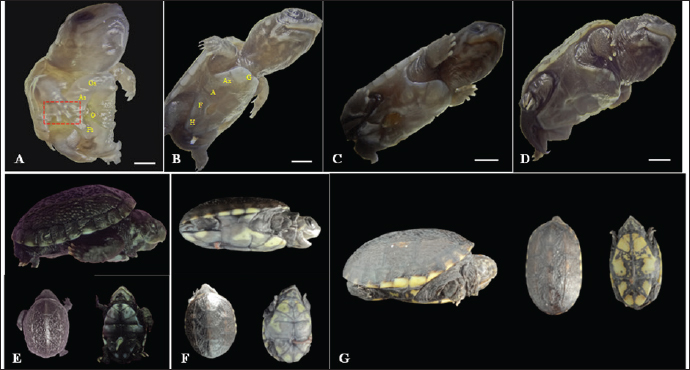
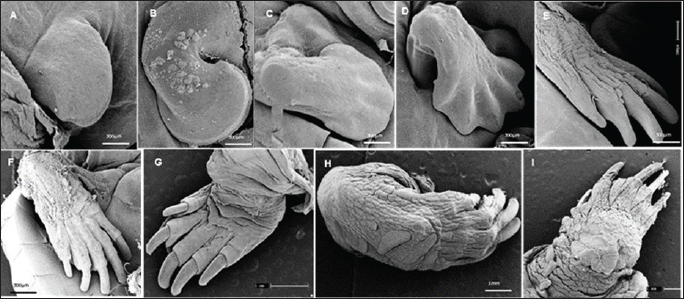
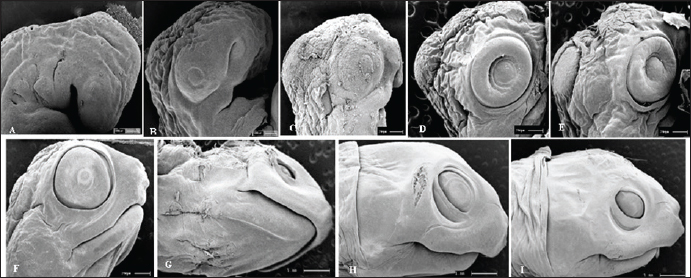
The eggs were collected after laying and immediately identified before being placed in incubators, representing the starting point for establishing the embryological sequence. The hatching time ranged from 75 to 164 days; however, the average hatching time was approximately 105 days. Based on the science of Animal Welfare, embryos were euthanized by infiltrating directly into the egg with sodium thiopental solution (20 mg/kg) (Carpenter, 2006). Description of the embryonic stagesThe description of embryonic development was based on the appearance of external morphological characteristics, observed through the stereomicroscope (LEICA EZ4) and the images recorded with a camera (Sony DSC Modelo, 16.2 Mega pixels). The individuals for macroscopic analysis were fixed and preserved in 4% formaldehyde. The embryos collected for scanning microscopy were fixed in 2.5% gluteraldehyde and then frozen for 72 hours. They were then preserved in 70% alcohol and dehydrated in an increasing series of alcohol (80%, 90%, and 100%), dried in a CO2 oven at 24°C, and glued onto a support (stub). Samples were dried with a critical-point apparatus Balzers CPD 020, Balzers Union Ltd., Liechtenstein). The samples were then sputter coated with gold (Emitech K550, Emitech Ltd. Ashford, Kent, UK) (Viana et al., 2014). Subsequently, a metal coating with gold was applied by sputtering and observed using the scanning microscopy (Zeiss LEO 435VP, Cambridge, UK) device for analysis and characterization of the structures. Ethical approvalAll handling was authorized by the Chico Mendes Institute for Biodiversity Conservation (CMIBio)/Brazilian Institute for the Environment and Renewable Resources (IBAMA-MA) with Biodiversity Authorization and Information System (SISBIO) licensing for in situ collection of animals under nº 47635-4/2016 and also by the Animal Ethics and Experimentation Committee (AEEC) of State University of Maranhão (UEMA) under nº 20/2016. ResultsThe average incubation period for the species K. scorpioides, submitted to a controlled temperature of 28°C was 105 days (15 weeks) with the identification of 15 stages of development, based on the studies of Yntema (1968) for the turtle C. serpentina. The descriptions of this study were based on the morphological characteristics of the formation of the eye, mandibular process, limbs, and dermal shield, which served as a basis for comparison with other species of aquatic chelonians described in specific literature (Table 2). Head and axial developmentWe characterized Stage I (7 days) by the formation of the embryonic disc, yolk sac, and albumen (Fig. 2A); at this stage, the embryo was not visible. From stage II (14 days), the organization of tissues in the formation of the systems was noticed, showing the primitive heart and the formation of blood vessels, and the appearance of cerebral and optical vesicles, pharyngeal arches, and curvature of the spine (Fig. 2B). In Stage IV (28 days), body flexion was evident and continuous, with the embryo looking like an arch, with the formation of the head and cephalic region with four cerebral vesicles, and the beginning of the organization of the nervous system. The optic vesicle is defined with a pigmented retina, choroidal fissure, and pupil (Fig. 2D). In stage V (35 days), the formation of the head with a well-defined optical placode with a completely pigmented retina and iris and a choroidal fissure like a line is notable, in addition to the maxillary process extending close to the eyes and fused to the nasal-front process. In K. scorpioides, the lower eyelid appeared in stage VI (42 days), while the upper eyelid appeared in stage VII (49 days), being formed and covering most of the eye in stage IX (63 days) (Figs. 2G and 3A), we emphasize that the vision organs gain notoriety. The formation of the caruncle, a structure that helps in the opening of the eggshell, is a determining process for its hatching. It is observed in stage VIII (56 days), when the jaw and mandible are formed and remain until Stage XV (105 days/birth) (Fig. 5A–I). The dermal shields of the K. scorpioides carapace and plastron are fully formed in the final third of development, after 70 days (stage X) and 98 days (stage XIV), they are formed with claws, cutaneous folds, and scales (Fig. 4C–I), completely showing the characteristic of the species. These structures will form the protective exoskeleton of the viscera (Fig. 3B). Initially, the carapace is very dark, with spots slightly yellowed at the edges and a black plastron in the center and slightly yellowed at the ends (Fig. 3E and F). Appendicular developmentThe appearance of the shoots to originate from the thoracic and pelvic members occurred simultaneously in stage III (21 days), and in stage IV (28 days), the shoots of the thoracic members were larger than the pelvic ones (Figs. 1C, D, 4A, and 5B). In stage V (35 days), a delimitation in the trunk, through a longitudinal groove shows the beginning of the carapace formation (Fig. 2E, 4B, and 5C), is well defined in stage VI (42 days), and still, in continuous growth and marked characteristic of the limbs and skin pigmentation, appearance of digital plates showing the peculiar shape of the Chelonia order (Fig. 2F, 4C, and 5D). Macroscopic sexual differentiationThe urogenital papilla, a structure that changes to sexual differentiation, was observed after 56 days (stage VIII), beginning to be surrounded by the cloacal structure at 63 days (stage IX), with the cloaca in formation at 84 days (stage XII) (Figs. 2H, 3A and D). The tail is short, and after 70 days (stage X), the formation of a corneal appendix at the extremity is observed, which gives the name to the species, due to the similarity to the scorpion’s tail. At 105 days of incubation (stage XV), hatching occurred with the birth of the cubs. At this stage, the phenotypic pattern of the species is very marked; however, there is still no differentiation from sexual dimorphism. All babies were similar, with a dark gray triangular head with yellow spots and a well-defined elongated snout, a neck with gray or black skinfolds, and the presence of three pairs of mentonian barbels. The carapace has thirty-six dermal plates (nuchal, vertebral, costal, and marginal) while the plastron is outerally covered by six pairs of corneal shields called gular, humeral, pectoral, abdominal, femoral, and anal. Thoracic and pelvic members with reduced interdigital membranes with five fingers with pointed nails. The vitellinic peduncle closed and marked by a central ring in the plastron and a small vitelline bag may be persistent (Fig. 3G). The tail is the same size for all newly hatched, not being a differentiation item, as occurs in adults, where the male has a long tail in relation to the female. Table 2. List of freshwater chelonian with determined embryonic stages.
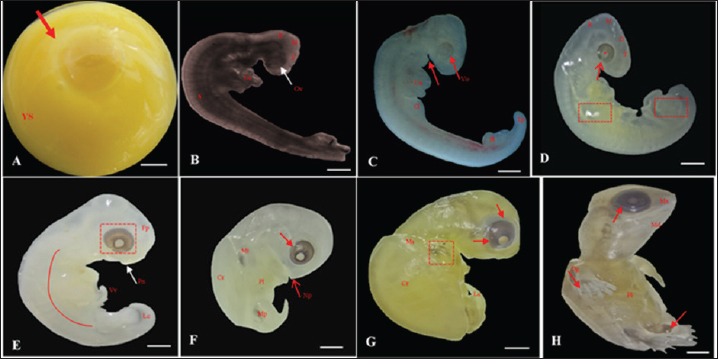
Fig. 2. Embryonic development of K. scorpioides. Side view: A—stage I: yolk sac (YS), blastodisc (arrow); B—stage II: cerebral vesicles—forebrain (F), midbrain (M), rhombencephalon (R), optic vesicle (Ov), somites (S), cardiac area (Ca). C—stage III: pharyngeal arch (arrow), cardiac area (Ca), chest limb bud (Cl), pelvic limb bud (Pl), tail process (Tp). D—stage IV: cerebral vesicles—telencephalon (T), diencephalon (D), midbrain (M), rombencephalon (R), pupil (asterisk), choroidal fissure (arrow). E—stage V: placode (dashed rectangle), fronto-nasal process (Fp), longitudinal groove (red line), vitelline vein (Vv). F—stage VI: nasal pit (Np), carapace (Cr), pastron in formation (Pl), upper eyelid (arrow); G—stage VII: marginal shields (Ms), pigmentation of the base of the digits (dashed rectangle), upper and lower eyelid (arrow); H—stage VIII: maxilla (Mx), mandible (Md), interdigital membrane (arrow), urogenital papilla (up) (bar scale: 0.1 cm).
Fig. 3. Embryonic development of K. scorpioides. Side view: A—stage IX: abdominal shield (As), gular shield (Gs) and femoral shield (Fs), opening of the plastron (Op); B—stage X: dermal shields: gular (G), humeral (H), abdominal (A), femoral (F), axillary (Ax), inguinal shield (I). C—stage XI: head is light gray with small white spots and claws are thick and curved; D—stage XII: neck and limbs take on a dark gray color; scales on the limbs and longer claws. Dorsal and ventral view: E–G—stage XIII to XV (day of birth): marked development of the phenotypic patterns of the species (barrel scale: 0.5 cm).
Fig. 4. Electromicrograph of the thoracic limbs of K. scorpioides embryos. A: stage III—beginning of bud formation. B: stage V—bulging of the shoots in the lateral regions. C: stage VI—emergence of digital cards. D: stage VII—member with a serrated edge. E: stage VIII—five digits separated by digital membranes. F: stage X—digits with folded skin and claws; G: stage XI—higher number of skin folds, scales, and thick and curved claws; H: stage XII—higher number of scales. I: stage XIV—Member fully formed.
Fig. 5. Electromicrograph of the head of K. scorpioides embryos. A: stage II—optic vesicle slightly recognized; pharyngeal arches. B: stage III—more evident optic vesicle; maxillary and mandibular process. C: stage V—head region presents placode formed with retina and iris; maxillary process fused with the fronto-nasal process. D: stage VI—growth of the lower eyelids. E: Stage VII—prominent placebo and formation of the upper eyelids; F: stage VIII—upper and lower eyelids beyond the edge of the eye; fully formed maxilla and mandible; presence of caruncle. G: stage IX—upper and lower eyelids; jaw, rhamphoteca, and dewlap. H: stage XI—Projections of the dermal papillae and a greater number of skin folds in the neck. I: stage XII to stage XIV—most prominent and numerous neck folds; fully formed eyelids, clear pupils, and presence of caruncle. DiscussionThe embryos of K. scorpioides can be distinguished from other species by their phenotypic pattern of pigmentation, characteristics of the longitudinal keel of the carapace, and dermal shields of the exoskeleton, formed by the junction of the carapace with the plastron. Comparisons of the development between chelonian species are still complex, due to differences in size and time involved in embryonic phases (Greenbaum and Carr, 2002). The use of measures of body size, chronological age, and morphological stage are tools used to standardize these interspecific comparisons (Hall and Miyake, 1995). Traditionally, two “test models” have been used for testudines, those of Yntema (1968) and Miller (1985). They serve as a basis for studies that have described the embryology of turtle development, and there are differences in development between species. The appearance of the chelonian carapace is a variable element between species, both in the stages of development, as well as in morphology and color. At 42 days (stage VI) the carapace of the K. scorpioides embryo is defined, with the beginning of the formation of the plastron, in a characteristic pattern of the turtles. These findings are consistent with other species of turtles, such as the Chrysemys picta and Chelonia mydas, in studies similar to this one, appearing between 22 and 28 days of incubation, which is shorter, but assigned to a shorter incubation time (Andrews, 2004). The initial formation of the eyes is initiated by the appearance of the optic vesicle, highlighting the visual apparatus in a marked way after the development of the retina, iris, pupil, and lower and upper eyelids. The optic vesicle is clearly visible in P. expansa in stage 6 (6 days), while the optic vesicle is visible in stage 7 (9 days) in Mauremys japonica (Okada et al., 2011) and C. serpentina (Yntema 1968) in stage 8 (4 days) in P. sinensis (Tokita and Kuratani, 2001). In the red turtle (E. subglobosa), the lower eyelid appears early, around the 13th day (stage 5) and already begins to overlap the eye, reaching the lens level (Werneburg et al., 2009). In this aspect, in the C. serpentina turtle, these characteristics were observed at 30 days of development and at 20 days in P. sinensis (Yntema 1968; Tokita and Kuratani, 2001). In M. japonica (stone turtle), the caruncle was initially evidenced as a small white process under the nostril (stage 17), showing a sharp point in stage 26, a peculiar characteristic of the species (Okada et al., 2011). At 63 days (stage IX), the ranfoteca is observed covering the branches of the mandible together with the formation of the barbel in the ventral region of the mandible (Fig. 2A). It helps animals to select, shatter and ingest their food to replace teeth. It has its own characteristics that vary according to the diet, and can therefore be used to identify different species of turtles (Wyneken, 2001). Differently from the description by Greenbaum (2002), for T. scripta, which was based only on the characteristics of the thoracic members as a criterion for delimiting the stages. In K. scorpioides, we have deepened the characterization of the various stages of development starting from the initial stage until the hatching. For Greenbaum (2002), the morphology of the thoracic limbs is the main criterion for delimiting the stages, as it is easily analyzed until the second half of development, and is highly conserved among most turtles. The tendency in the development of the limbs is that the thoracic ones precede the pelvic ones, an event described in the species (C. serpentina, P. sinensis, E. subglobosa, M. japonica and K. scorpioides), or both manifest simultaneously and synchronously (P. expansa and C. picta) (Tokita and Kuratani, 2001; Santos Braga et al., 2021b). The sexual dimorphism of the adults of K. scorpioides is striking, with males showing long tails and concave plastrons and females with short tails and straight plastrons (Rocha and Molina, 1990). In newly hatched turtles, the tail in males and females is the same size, being short and the plastron straight. Another important structure in sexual differentiation is the urogenital papilla, Magalhães et al. (2017) describes that it is the precursor of the cloaca in the Amazon turtle (P. expansa), appearing at 30–35 days (stage 19); in E. subglobosa, this structure becomes noticeable at 28 days (stage 9) (Werneburg et al., 2009). The growth rates of vertebrate embryos are highly variable within and between species and depend on the incubation temperature (Reiss, 1989). The incubation temperature influences its duration and the degree of development (Janzen and Morjan, 2002), at low temperatures they increase the time and decrease the rate of development, while at high temperatures, they decrease the duration and increase the rate of development (Bull and Vogt, 1981; Alho et al. 1984; Souza and Vogt, 1994; Valenzuela, 2001). In this way, these temperatures need to be taken into account when comparing, since eggs incubated at different temperatures can present divergent results. In K. scorpioides, the eggs were incubated with a controlled average temperature of 28°C. The observations, though similar to other reported species, had some important differences and they may be linked to the morphological characters of the interspecific variations, while the incubation temperature influences the differences related to the embryonic stage. The morphological characteristics of the embryonic development of the species K. scorpioides showed similar states, with few differences, to other turtles. Some characteristics were found delayed or accelerated in comparison to the findings of Yntema (1968), for the turtle C. serpentina. In addition, strong evidence from this study supports the hypothesis that the morphological characteristics of the embryonic development of this species, resemble the other chelonians (Santos Braga et al., 2021b), presenting some variations in the appearance and disappearance of structures. The primary differences among the families Emydidae, Geomydidae, and Testudinidae lie primarily in the number of phalanges in the digits and the number of proximal tarsals (Fritz et al. 2006; Ludwig et al., 2007; Sánchez-Villagra et al., 2008; Sheil and Portik, 2008). Testudinidae, in particular, exhibit a reduction in phalangeal elements, as each digit of members of this family possesses only two phalanges (Mautner et al., 2017). This characteristic is directly associated with their terrestrial lifestyle and the external morphology of their limbs, which take on a columnar shape, characterized by a few distinct digits and claws that facilitate locomotion. The evolution of the turtle shell has been a longstanding focal point in discussions within comparative anatomy. Comprising both dorsal and ventral components—the carapace and plastron, respectively—the turtle shell represents a distinctive feature. The rearrangement of skeletal elements, resulting in this unique topological shift, positions the carapace as an exemplar of evolutionary novelty, deviating from the ancestral body plan of tetrapods (Nagashima et al., 2012). According to the study of Santos Braga et al. (2021b) for K. scorpoioides, ossification began in the humerus and femur at stage 20, and continued into the radius, ulna, tibia, and fibula bones. By stage 23, it was already effectively directed toward the bone epiphyses in both limbs. At stage 26 and hatching, only articular cartilages remained, and in the majority of samples, the carpal region showed no affinity for alcian blue or alizarin red staining. The information generated by the analysis of the embryonic development of K. scorpioides provides a basis for research with species of the same genus and also with other species of freshwater turtles, the conditions of temperature and humidity during incubation play a crucial role. They influence the pace of metabolic reactions, consequently impacting the developmental processes of the embryo. In essence, the specific environmental conditions experienced by the eggs throughout incubation significantly contribute to the variations observed in the developmental timeline and overall embryonic development. Another aspect in the difference between the chronology of development in turtles can be attributed to the necessary morphological characteristics of each species according to their habitat such as streamlined shells for efficient swimming for K. scorpioides, as a result of the evolution process itself (MacCord et al., 2015). While the primary emphasis of the study centers on the broader theme of environmental sex determination, there is an indirect acknowledgment of the potential for intraspecific variability in the mechanisms governing sex determination. This nuanced discussion suggests a recognition of the possibility that intraspecific variations could contribute to the diverse outcomes observed in the context of sex determination processes (Janzen and Paukstis, 1991). This study significantly enhances our comprehension of the diverse stages of embryonic evolution and reproductive management in the controlled species K. scorpioides, considering factors such as incubation temperature. Noteworthy is the study’s emphasis on the importance of expanding research to comprehend intraspecific variations. By delving into the intricacies of embryonic development, the findings not only provide essential insights for targeted conservation actions and improved habitat management but also contribute to a more profound scientific understanding of turtle evolution. Moreover, the study holds the potential to engage the public in conservation efforts, fostering a broader awareness of the species’ importance and conservation needs. A significant contribution to understanding the maternal transfer of persistent organic pollutants to sea turtle eggs. Through a comprehensive meta-analysis, the authors address knowledge and data gaps, providing an enhanced synthesis of existing research findings. Results consistently highlight the presence of persistent organic pollutants in turtle eggs, indicating the extent of maternal exposure to these toxic substances. This vertical transfer of contaminants highlights the vulnerability of turtle embryos from the earliest stages of development, emphasizing the critical importance of conservation strategies that address not only threats in the environment but also maternal sources of exposure. Future studies are interesting in terms of using these techniques as monitoring tools (Muñoz and Vermeiren, 2020). The study by Muñoz and Vermeiren (2023) highlights the relevance of evaluating the load of organic pollutants in turtle eggs as biological indicators, using both the yolk and the white as biomonitoring matrices. This discovery not only contributes to the understanding of contamination dynamics in turtle populations, but also highlights the importance of conservation strategies that address reducing sources of pollution in marine habitats. An improved understanding of contamination patterns found in turtles can thus inform more effective conservation strategies aimed at mitigating threats arising from pollution in coastal and marine environments. In K. scorpioides, the stages of embryonic development occurred on average 105 days, from laying to hatching, in moments of structural formations similar to other turtles, even at different times. Temperature and humidity play pivotal roles in the incubation process, particularly for species like K. scorpioides. Maintaining a relatively high-humidity environment is crucial to prevent desiccation, ensuring the proper development of turtle eggs. The interplay between temperature and humidity in the incubation setting significantly influences the success of embryonic development, mirroring the conditions necessary for the species in its natural habitat. AcknowledgmentsThe authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for the research grant to author LPFAC and FAPEMA. FundingThis study was funded by Alana Lislea de Sousa (grant number Rebax Edital 030/2013 processo 03634/2013); Alana Lislea de Sousa (grant number Edital universal 40/2014 processo 00716/2015). The funding bodies are not involved in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsAll authors contributed to this study. All authors read and approved the final manuscript. Data availabilityAll data from the study are provided in the manuscript. ReferencesAlho, C.J.R., Danni, T.M.S. and Pádua, L.F.M. 1984. Influência da temperatura de incubação na determinação do sexo da tartaruga-da-amazônia (Podocnemis expansa, Testudinata: Pelomedusidae). Rev. Bras. Biol. 44(3), 1305–1311. Andrews, R.M. 2004. Patterns of embryonic development. Reptilian incubation: environment, evolution, and behaviour. Ed. D. C. Deeming. Nottingham University Press, Nottingham, pp: 75–102. Bull, J.J. and Vogt, R.C. 1981. Temperature-sensitive periods of sex determination in Emydid turtles. J. Exp. Zool. 218, 435–440. Carpenter, J. 2006. Formulario de animales exóticos. 3rd ed. Buenos Aires, Argentina: Inter-Medica, pp: 560. Ceriani, S.A., Casale, P., Brost, M., Leone, E.H. and Witherington, B.E. 2019. Conservation implications of sea turtle nesting trends: elusive recovery of a globally important loggerhead population. Ecosphere 10(11), 1–19. Chaves, E.P. 2010. Morfologia reprodutiva e dosagem hormonal em fêmea de jurará (Kinosternon scorpioides) criada em cativeiro, Doctoral dissertation, UEMA, São Luís, Brazil. Cordero, G.A. and Janzen, F.J. 2014. An enhanced developmental staging table for the painted turtle, Chrysemys picta (Testudines: Emydidae). J. Morphol. 275, 442–455. Crastz, F. 1982. Embryological stages of the marine turtle Lepidochelys olivacea (Eschscholtz). Rev. Biol. Trop. 30(2), 113–120. Ewert, M.A., Etchberger, C.R. and Nelson, C.E. 2004. Turtle sex-determining modes and TSD patterns, and some TSD pattern correlates. In Temperature-dependent sex determination in vertebrates. Eds., Valenzuela, N. and Vance, V.A. Washington, DC: Smithsonian Institution Press, pp: 21–32. Fernandes Araujo Chaves, L.P., Viana, D.C., Chaves, E.P., Miglino, M.A. and Sousa, A.L. 2020. Reproductive morphophysiology of the male scorpion mud turtle ( Kinosternon scorpioides Linnaeus, 1766) in captivity. Vet. Med. Sci. 1, 1–9. Ferreira Júnior, P.D. 2009. Aspectos ecológicos da determinação sexual em tartarugas. Acta Amazon. 39, 139–154. Fritz, U., Petzold, A. and Auer, M. 2006. Osteology in the Cuora gal-binifrons complex suggests conspecifity of C. bourreti and C. galbinifrons, with notes on shell osteology and phalangeal for-mulae within the Geoemydidae. Amphib-Reptil. 27, 195–205. Greenbaum, E. and Carr, J.L. 2020. Staging criteria for embryos of the spiny softshell turtle, Apalone spinifera (Testudines: Trionychidae). J. Morphol. 254, 272–291. Greenbaum, E. 2002. A standardized series of embryonic stages for the emydid turtle Trachemys scripta. Can. J. Zool. 80, 1350–1370. Guzmán, N. and Bonilla, H.O. 1990. Serie normal del desarrollo morfológico embrionario de Podocnemis unifilis (Troschel, 1848) (Testudinata: Pelomedusidae). Acta Biol. Colombiana, 2, 129–150. Hall, B.K. and Miyake, T. 1995. How do embryos measure time? In Evolutionary change and heterochrony. Ed., McNamara K.J. New York, NY: John Wiley and Sons, pp: 3–20. Janzen, F.J. and Morjan, C.L. 2002. Egg size, incubation temperature, and posthatching growth in painted turtles (Chrysemys picta). J. Herpetol. 36, 308–311. Janzen, F.J. and Paukstis, G.L. 1991. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Quart. Rev. Biol. 66(2), 149–179. Ludwig, M., Auer, M. and Fritz, U. 2007. Phalangeal formulae ofgeoemydid terrapins (Batagut, Callagur, Hardella, Heosemys, Kachuga, Orlitia, Pangshura, Rhinoclemmys) reflect distinctmodes of life. Amphib-Reptil. 28, 574–576. MacCord, K., Caniglia, G., Moustakas-Verho, J.E. and Burke, A.C. 2015. The dawn of chelonian research: turtles between comparative anatomy and embryology in the 19th century. J. Exper. Zool. Part B: Mol. Develop. Evol. 324(3), 169–180. Magalhães, M.S., Vogt, R.C., Sebben, A., Dias, L.C., Oliveira, M.F. and Moura, C.E.B. 2017. Embryonic development of the Giant South American River Turtle, Podocnemis expansa (Testudines: Podocnemididae). Zoomorphology 136(4), 523–537. Mahmoud, I., Hess, G.L. and Klicka, J. 1973. Normal embryonic stages of the western painted turtle, Chrysemys picta bellii. J. Morphol. 141, 269–280. Mautner, A.K., Latimer, A.E., Fritz, U. and Scheyer, T.M. 2017. Anupdated description of the osteology of the pancake tortoise Malacochersus tornieri (Testudines: Testudinidae) with special focuson intraspecific variation. J. Morphol. 278, 321–333. Miller, J.D. 1985. Criteria for staging reptilian embryos. In Biology of Australasian frogs and reptiles. Eds., Grigg, G., Shine, R. and Ehmann, H. New South Wales, Australia: Royal Zoological Society of New South Wales, pp: 305–310. Muñoz, C.C. and Vermeiren, P. 2020. Maternal transfer of persistent organic pollutants to sea turtle eggs: a meta-analysis addressing knowledge and data gaps toward an improved synthesis of research outputs. Environmen. Toxicol. Chem. 39(1), 9–29. Muñoz, C.C. and Vermeiren, P. 2023. Sea turtle egg yolk and albumen as biomonitoring matrices for maternal burdens of organic pollutants. Marine Pollut. Bull. 194, 1–10. Nagashima, H., Kuraku, S., Uchida, K., Kawashima-Ohya, Y., Narita, Y. and Kuratani, S. 2012. Body plan of turtles: an anatomical, developmental and evolutionary perspective. Anat. Sci. Int. 87, 1–13. Okada, Y., Yabe, T. and Sen-Ichi, O. 2011. Embryonic development of the Japanese pond turtle, Mauremys japonica (Testudines: Geoemydidae). Herpetologica 30, 89–102. Reiss, J.O. 1989. The meaning of developmental time: a metric for comparative embryology. Am. Nat. 134(2), 170–189. Roberts, P. 2021. Jungle: How tropical forests shaped world history–and us. London, UK: Penguin. Rocha, M.B. and Molina, F.B. 1990. Reproductive Biology of Kinosternon scorpioides (Testudines: Kinosternidae) in Capitivity. Tortoises and Turtles, 5, 8. Sánchez-Villagra, M.R., Ziermann, J.M. and Olsson, L. 2008. Limb chondrogenesis in Graptemys nigrinoda (Emydidae), with com-ments on the primary axis and the digital arch in turtles. Amphib.-Reptil. 29, 85–92. Santos Braga, B.S., Fernandes-Neto, D.L., Leal, R.P., Silva, S.R., Ferreira, M.A.P., Oliveira-Bahia, V.R. and Araujo Guimaraes, D.A. 2021a. Embryonic development of Kinosternon scorpioides (testudines: kinosternidae). Zoomorphology 140, 279–290. Santos Braga, B.S., Fernandes-Neto, D.L., Teixeira, L.C., Silva Costa, J., Ferreira, M.A.P., Oliveira-Bahia, V.R. and Araújo Guimarães, D.A. 2021b. Skeletal development of Kinosternon scorpioides limbs (Chelonia: Kinosternidae). Anat. Rec. 304(6), 1280–1293. Sheil, C.A. and Portik, D. 2008. Formation and ossification of limbelements in Trachemys scripta and a discussion of autopodialelements in turtles. Zool. Sci. 25, 622–641. Souza, R.R. and Vogt, R.C. 1994. Incubation temperature influences sex and hatchling size in the neotropical turtle Podocnemis unifilis. J. Herpetol. 28(4), 453–464. Sousa, A.L., Campos-Junior, P.H., Costa, G.M. and de França, L.R. 2014. Spermatogenic cycle length and sperm production in the freshwater turtle Kinosternon scorpioides. Biol. Reprod. 90(2), 35. Todd, B.D., Willson, J.D. and Gibbons, J.W. 2010. The global status of reptiles and causes of their decline. Ecotoxicol Amphibians Reptiles 2010, 47–67. Tokita, M. and Kuratani, S. 2001. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae). Zool. Sci. 18, 705–715. Valenzuela, N. 2001. Maternal effects on life-history traits in the Amazonian giant river turtle Podocnemis expansa. J. Herpetol. 2001, 368–378. Viana, D.C., Anunciação, A.R.D.A.D., Santos, A.C.D., Assis Neto, A.C., Miglino, M.A., Oliveira, C.A. and Sousa, A.L. 2014. Plasma testosterone and seasonal reproductive changes in the scorpion mud turtle. Pakistan J. Zool. 46(6), 1641–1650. Viana, D.C., Santos, A.C. and Assis Neto, A.C. 2015. Spermiogenesis in scorpion mud turtle, Kinosternon scorpioides. Rev. Bras. Med. Vet. 37(4), 389–396. Viana, D.C., Santos, A.C., Sousa, A.L. and Assis Neto, A.C. 2023. Steroidogenesis during dry and wet season of free-living male scorpion mud turtle (Kinosternon scorpioides). In Theriogenology—recent advances in the field. Eds., Silva, A.R and Pereira, A.F. London, UK: IntechOpen Limited, pp: 1–17. Vogt, R.C., Fagundes, C.K., Bataus, Y.S.L., Balestra, R.A.M., Batista, F.R.W., Uhlig, V.M., Silveira, A.L., Bager, A., Batistella, A.M., Souza, F.L., Drummond, G.M., Reis, I.J., Bernhard, R., Mendonça, S.H.S.T. and Luz, V.L.F. 2015. Avaliação do Risco de Extinção de Kinosternon scorpioides (Linnaeus, 1766) no Brasil. Processo de avaliação do risco de extinção da fauna brasileira. ICMBio. 2015. Available via http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/estado-de-conservacao/7407-repteis-kinosternon-scorpioides-mucua.html Werneburg, I., Hugi, J., Muller, J. and Sánchez-Villagra, M.R. 2009. Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev. Dynam. 238, 2770–2786. Wyneken, J. 2001. The anatomy of sea turtles. Miami: National Oceanic and Atmospheric Administration, U.S. Department of Commerce. NOAA Technical Memorandum NMFS-SEFSC. 2001, 470. Yntema, C. 1968. A series of stages in the embryonic development of Chelydra serpentina. J. Morphol. 125(2), 219–251. | ||
| How to Cite this Article |
| Pubmed Style Chaves LPFA, Viana DC, Tchaika L, Caldas JMA, Neto ACA, Miglino MA, Sousa ALD. Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Vet. J.. 2024; 14(4): 962-972. doi:10.5455/OVJ.2024.v14.i4.3 Web Style Chaves LPFA, Viana DC, Tchaika L, Caldas JMA, Neto ACA, Miglino MA, Sousa ALD. Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. https://www.openveterinaryjournal.com/?mno=169384 [Access: January 11, 2026]. doi:10.5455/OVJ.2024.v14.i4.3 AMA (American Medical Association) Style Chaves LPFA, Viana DC, Tchaika L, Caldas JMA, Neto ACA, Miglino MA, Sousa ALD. Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Vet. J.. 2024; 14(4): 962-972. doi:10.5455/OVJ.2024.v14.i4.3 Vancouver/ICMJE Style Chaves LPFA, Viana DC, Tchaika L, Caldas JMA, Neto ACA, Miglino MA, Sousa ALD. Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Vet. J.. (2024), [cited January 11, 2026]; 14(4): 962-972. doi:10.5455/OVJ.2024.v14.i4.3 Harvard Style Chaves, L. P. F. A., Viana, . D. C., Tchaika, . L., Caldas, . J. M. A., Neto, . A. C. A., Miglino, . M. A. & Sousa, . A. L. D. (2024) Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Vet. J., 14 (4), 962-972. doi:10.5455/OVJ.2024.v14.i4.3 Turabian Style Chaves, Lianne Polliane Fernandes Araujo, Diego Carvalho Viana, Ligia Tchaika, Juliana Maria Alves Caldas, Antônio Chaves Assis Neto, Maria Angélica Miglino, and Alana Lislea De Sousa. 2024. Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Veterinary Journal, 14 (4), 962-972. doi:10.5455/OVJ.2024.v14.i4.3 Chicago Style Chaves, Lianne Polliane Fernandes Araujo, Diego Carvalho Viana, Ligia Tchaika, Juliana Maria Alves Caldas, Antônio Chaves Assis Neto, Maria Angélica Miglino, and Alana Lislea De Sousa. "Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive." Open Veterinary Journal 14 (2024), 962-972. doi:10.5455/OVJ.2024.v14.i4.3 MLA (The Modern Language Association) Style Chaves, Lianne Polliane Fernandes Araujo, Diego Carvalho Viana, Ligia Tchaika, Juliana Maria Alves Caldas, Antônio Chaves Assis Neto, Maria Angélica Miglino, and Alana Lislea De Sousa. "Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive." Open Veterinary Journal 14.4 (2024), 962-972. Print. doi:10.5455/OVJ.2024.v14.i4.3 APA (American Psychological Association) Style Chaves, L. P. F. A., Viana, . D. C., Tchaika, . L., Caldas, . J. M. A., Neto, . A. C. A., Miglino, . M. A. & Sousa, . A. L. D. (2024) Embryonic development of scorpion mud turtle (Kinosternon scorpioides) from captive. Open Veterinary Journal, 14 (4), 962-972. doi:10.5455/OVJ.2024.v14.i4.3 |