| Research Article | ||
Open Vet J. 2023; 13(5): 576-587 Open Veterinary Journal, (2023), Vol. 13(5): 576–587 Original Research Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitroAisyah Retno Wulandari1, Andini Nurlaelasari1, Herjuno Ari Nugroho2, Muhamad Cahyadi1, Wahyu Kurniawan3 and Penny Humaidah Hamid1*1Department of Animal Science, Faculty of Agriculture, Universitas Sebelas Maret, Surakarta, Indonesia 2Reseach Center for Applied Zoology, National Research and Innovation Agency, Jakarta Pusat, Indonesia 3Department of Animal Production, Agency of Livestock and Fishery Services, Boyolali District, Central Java, Indonesia *Corresponding Author: Penny Humaidah Hamid. Department of Animal Science, Faculty of Agriculture, Universitas Sebelas Maret, Surakarta, Indonesia. Email: pennyhumaidahhamid [at] staff.uns.ac.id. Submitted: 13/12/2022 Accepted: 11/04/2023 Published: 11/05/2023 © 2023 Open Veterinary Journal
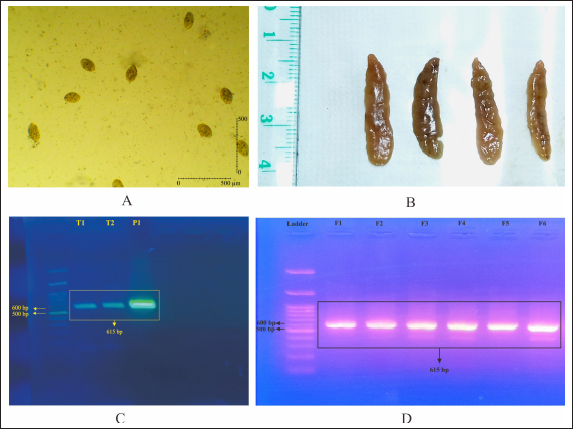
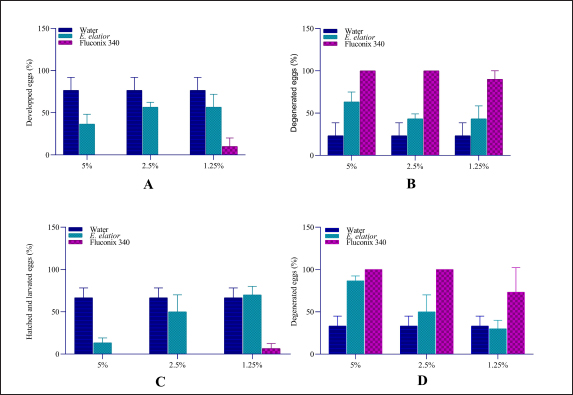
AbstractBackground: Fasciolosis is a parasitic disease affecting the hepatobiliary system of livestock worldwide. The control of the fluke is important to be performed in endemic regions. Aim: This study aims to evaluate the effect of Etlingera elatior ethanolic extract on egg and adult stadia of Fasciola gigantica. Methods: Fasciola gigantica in different stages were incubated with E. elatior ethanolic extract in different concentrations and time points. Results: The number of developed eggs with different concentrations of 1.25%, 2.5%, and 5% was significantly decreased by 36.67%, 56.67%, and 56.67% on day 11 post-incubation, which showed an ovicidal effect of the herb. The developed eggs on day 14, which were represented by hatched larvae, were also decreased by 70%, 50%, and 13.33%, respectively. Significant flukicidal effects were observed in the incubation time of 80 minutes for the concentration of 20% (p =0.007) and 640 minutes for 10% concentration (p =0.003). Surface microscopy of adult F. gigantica showed damaged skin and spina with the erosion of the inner membrane and detached syncytium from the tegument. Conclusion: Overall, the results indicate that E. elatior has a promising anthelmintic property against F. gigantica in both ova and adult stages. Keywords: Fasciola gigantica, Etlangiera elatior, Ovicidal, Flukicidal, Bioresources. IntroductionFasciolosis is a parasitic disease that infects the hepatobiliary system of livestock, with Fasciola gigantica being the causative agent in tropical countries (Good and Scherbak, 2022), and Fasciola hepatica in temperate regions (Arjona et al., 1995). The infection is transmitted through food or drinking water contaminated by metacercaria of Fasciola sp. (Najib et al., 2020). Furthermore, infection causes serious liver damage, with constraints to nutrient metabolism in livestock husbandry (Luo et al., 2021). The infected liver is characterized by the presence of various lesions, including fibrosis in the parenchyma area (Mendes et al., 2012). Fasciolosis also adversely affects the livestock carcass production (Rashid et al., 2019) and causes economic losses of up to US$3.2 billion/year worldwide (Mehmood et al., 2017). The high prevalence in tropical countries is supported by warm climatic conditions and the ability of the intermediate hosts to live throughout the year. In Indonesia, F. gigantica is commonly found in bovines, small ruminants, and buffaloes (Pleasance et al., 2011). Fasciola sp. is also well-known as a zoonotic parasite and human fascioliasis is currently identified transmitted through raw foods. Metacercaria cysts on the leaves are accidentally eaten together with vegetables or salad (Mas-Coma et al., 2014). The global prevalence of Fasciola sp. infection is estimated at 17 million people from 61 countries. Another 180 million people are at risk of infection because they are in endemic areas (McManus, 2020). Moreover, human fasciolosis has been identified on various continents and countries. In America, infections were reported in Peru (Esteban et al., 2002), Bolivia (Valero et al., 2009), Argentina (Mera y Sierra et al., 2011), Mexico (Zumaquero-Ríos et al., 2013), and Brazil (Pritsch and Molento, 2018). While in Asia, several cases have been found in China (Chen et al., 2013), the Philippines (Kumari et al., 2013), Vietnam (Carrique-Mas and Bryant, 2013), and Singapore (Ahamed et al., 2013) Various countries in the African continent have reported cases of human fasciolosis such as Senegal (Ka et al., 2002), Zimbabwe (Esteban et al., 2003), Cameroon (Mbuh and Mbwaye, 2005), and South Africa (Black et al., 2013). Europe also has a history of this disease such as in France (Rondelaud et al., 2000), Turkey (Yılmaz and Gödekmerdan, 2004), Greece (Assimakopoulos et al., 2010), England (Fox et al., 2011), Spain (Mas-Coma et al., 2014), and Italy (Mas-Coma et al., 2018). The most commonly used method of controlling F. gigantica is the treatment using commercial drugs from the classes of triclabendazole, albendazole, and benzimidazole (Merachew and Alemneh, 2020). The disadvantage of using certain types of anthelmintic for a long time is the emergence of resistance. Anthelmintic resistance can also occur because of a poor understanding of farmers in inappropriate dosing and lack of drug efficacy testing (Fairweather, 2011; Kelley et al., 2016). Although there are several reports of anthelmintic resistance in the world, it was first discovered in Australia in 1995 (Overend and Bowen, 1995), 2014 (Brockwell et al., 2014), and 2015. In Europe, there have been cases of anthelmintic resistance in Spain (Alvarez-Sanchez et al., 2006), Scotland (Sargison and Scott, 2011), and the Republic of Ireland (Kelley et al., 2016). Resistance also occurs in America, Argentina (Olaechea et al., 2011), Peru (Ortiz et al., 2013), and Chile (Gil et al., 2014). Using natural ingredients can be an alternative to anthelmintic because they are environmentally friendly and based on biological resources available. Indonesia is a country rich in biodiversity, including medicinal plants that have been used for decades (Sofowora et al., 2013). The Kecombrang plant (Etlingera elatior) is native to Indonesia and belongs to the Zingiberaceae group that has been used in the manufacture of traditional medicine and vegetables (Naufalin, 2019). Phytochemical screening of the Kecombrang-flower methanol extract showed the presence of flavonoids, terpenoids, saponins, and tannins (Lachumy et al., 2010), which have anthelmintic activities to different fluke (Wang et al., 2010). In this study, we aim to determine the effect of E. elatior extract on the ova and adult stages of F. gigantica in vitro. Materials and MethodsEggs and adult F. gigantica collectionFasciola gigantica was collected from naturally infected bovines in Ampel Abbatoir, Boyolali, Central Java. The eggs were isolated from the gallbladder, while the living flukes were manually collected from the liver bile ducts of infected bovines. The infected gallbladder was removed and transported on ice. The liver observed with living flukes were transported all together at room temperature to the Integrated Laboratory Unit, Sebelas Maret University, Indonesia. The gallbladders were dissected, and fluid was removed. Bile fluid was filtered using a 100-mesh filter to separate bile and egg debris, then the eggs were deposited using tap water in a 1,000 ml glass beaker for 30 minutes. The liquid was slowly drained leaving the egg deposits at the bottom of the beaker glass. Sedimentation was repeated until the eggs were clean of tissue debris (Hegazi et al., 2018). Afterward, the eggs were washed by using dH2O, centrifuged at 5,500-rpm for 10 minutes, and stored in the fridge until use. Parasite identificationFasciola gigantica eggs were identified morphologically under a light microscope with a magnification of 40 times. About 50 µl volume containing 100–200 eggs was placed on an object glass and observed in three categories, namely normal, degenerated, and developed. The developing eggs were described as being morulated on day 5 and early larval development on day 9. The normal development was followed by hatching after 12 days of incubation. The eggs were categorized as degenerated when there were undeveloped morula, destruction of the morula inside of eggs, and or broken egg wall before normal hatching time (Arafa et al., 2015). Biomolecular identification was carried out to determine whether the eggs and flukes were a homogenous F. gigantica population or hybrid. DNA isolation was carried out with pooled eggs containing at least 90–100. The pooled sample contained 900–1,000 eggs in a 1.5 ml micro tube. DNA from collected eggs was isolated using the DNeasy isolation kit (Qiagen, Germany) according to the manufacturer’s protocol. The results of DNA isolation with 100 µl volume were stored at −20°C until use. Identification of F. gigantica was performed by a duplex PCR (Le et al., 2012) which contains: 1 µl of 10 pmol FHF primer (5′GTTTTTTAGTTGTTTTGGGGTTTG-3′), 1 µl of 10 pmol FGF primer (5′TGTTATGATTCATTGTTTGTAC-3′), and 1 µl of 10 pmol primer FHGR (5′-ATAAGAACCGACCTGGCTCCAC-3′), 2.5 µl BioLine HS (BioLine, USA), 8.5 µl nuclease-free water, and 1 µl DNA sample. The PCR reaction was carried out as follows; pre-denaturation at 95°C for 3 minutes, 30 cycles of denaturation at 95°C for 30 minutes, annealing at 52°C for 30 seconds, extension at 72°C for 30 seconds, and final elongation at 72°C for 2 minutes (T100 Thermal cycler, Singapore). Afterward, the PCR results were loaded onto 1.5% agarose gel and visualized in UV-light gel documentation. Etlingera elatior preparationThe E. elatior flower used in the study was collected from Cilacap, Central Java, Indonesia. The sample was identified at the Department of Plant Systematics, Faculty of Biology, Universitas Gadjah Mada, and approved by letter no. 072/S.T.b./IV/2022. About 10 kg of E. elatior flowers were cleaned, cut into small pieces, dried under direct sunlight for 7 days, and then ground into powder using a grinding machine. The powder was macerated at the LPPT, Gadjah Mada University, Indonesia by adding 96% ethanol. The mixture of powder and ethanol was stirred using an ultraturaq for 30 minutes, soaked for 48 hours, and then filtered. The maceration process was repeated 2 to 3 times. The extract was evaporated using a vacuum rotary evaporator with a water bath heater at a temperature of 50°C until thickened (Lachumy et al., 2010). The thick extract was heated using a water bath at 70°C with occasional stirring and stored at 4°C until use. UV-vis spectrophotometric test of extract on E. elatiorEtlingera elatior extract samples were prepared and sent to LPPT UGM, for UV-vis spectrophotometry measurements. The compounds tested were flavonoids, alkaloids, tannins, and saponins. Ovicidal assay of E. elatior effects on F. gigantica eggThe isolated eggs were incubated in E. elatior extract with different concentrations at room temperature. Aquadest is used as a basic medium for ovicidal assay. Etlingera elatior ethanolic extract was diluted and added to the aquadest to a total of three concentrations, i.e., 5%, 2.5%, and 1.25%. Fluconix 340 containing Nitroxynil was used as a positive control and diluted in the same concentration as E. elatior, while the negative control was aquadest. The total volume was adjusted to 2 ml on a 48-well plate. All treatments were performed in triplicates, while the observations of egg development were carried out on days 5, 9, 11, and 14. Flukicidal assay of E. elatior effect on adult F. giganticaThe flukes were immersed in a solution of E. elatior extract with concentrations of 40%, 20%, 10%, 5%, 2.5% and 1.25% in physiologic NaCl with five living-flukes each. The positive control used was also set up with the appropriate concentrations, respectively. Physiologic NaCl was used as negative control and each treatment was repeated three times. Fluke mortalities were observed at 5, 10, 20, 40, 80, 160, 320, and 640 minutes post-incubation. The absence of movement was characterized as fluke mortalities. Fasciola gigantica tissue sectionsFasciola gigantica in different concentrations and the time of incubations were fixed in formaldehyde 10%. Transversal-section of the samples were processed for hematoxylin-eosin staining at the Anatomy Pathology Laboratory, Faculty of Medicine, Gadjah Mada University, Indonesia. Scanning electron microscopy (SEM)The effect of E. elatior extract on F. gigantica surface membranes was assessed by using the SEM. Observations were made at a magnification of 40,000×. SEM at the Integrated Laboratory (iLab), National Research and Innovation Agency, Cibinong, Indonesia. The A. vulgaris-treated samples, Fluconix-340 and control F. gigantica flukes were cleaned by soaking in caccodylate buffer for 2 hours, followed by the agitation process in an ultrasonic cleaner for 5 minutes. Afterward, the samples were placed into 2.5% glutaraldehyde for hours. Fixation was performed by immersion in 2% tannic acid for 6 hours, followed by washing with caccodylate buffer for 5 minutes with 4 times repetitions. The samples were then immersed at room temperature in 50% alcohol for 5 minutes 4 times, 70% for 20 minutes, 85% for 20 minutes, 95% for 20 minutes, and finally in absolute alcohol for 10 minutes. The dehydrated samples were immersed in butanol for 10 minutes twice, frozen, and then freeze-dried. The specimens were glued to the specimen stub as needed and coated with gold using an ion coater, while observations were carried out under SEM (JEOL JSM-6510LA, Belgium). Statistical analysisThe data visualization and statistical analysis were performed using the GraphPad Prism 4.0 (GraphPad Inc., USA). Ethical approvalThe experiments related to adult F. gigantica collection from bovines were approved by the ethics committee of Ahmad Dahlan University with approval no. 022206036. ResultsIsolation of eggs and adult stages of F. giganticaThe eggs were collected from 15 gall-bladders of naturally infected bovines to get sufficient samples for all experiments and mixed. They were successfully isolated from bovine bile, while adult F. gigantica was obtained from bile ducts. Each egg contains a one-cell stage embryo surrounded by a group of oval body yolk cells. It has a distinct, inner concaved operculum and an umbilicus-like invagination at the posterior end of the shell. Almost all the eggs collected, possessed intact walls and were full of body yolk cells (Fig. 1A). The adult stages were collected from the bile ducts and directly used for experiments (Fig. 1B). Only fresh, live, and active flukes were used for adult Fasciola since the living abilities were shorter after they were separated from the host tissue. The isolated eggs are homogeneously recognized as F. gigantica eggs as shown in the PCRs of pooled eggs (Fig. 1C), compared to tissue section PCR from the adult stage (Fig. 1D). All the eggs and adult F. gigantica in these experiments showed a single band of 615 bp regarding a homogenous population. Spectrophotometric test of E. elatior ethanol extractFreshly extracted E. elatior was found to contain flavonoids 1.29% b/b, alkaloid 1.49% b/b, tannin 3.09% b/b, and saponin 1.23% b/b. Ovicidal efficacy of E. elatior on F. gigantica eggsThe maturation of eggs was completed within a period of 13–16 days under a normal laboratory condition of temperature 25°C–27°C. The developed miracidia exhibited some movements inside the eggshells before escaping through repeated and strong pushing to the operculum, which became partially opened. The emergence of miracidia occurred within 4 days of their maturation. The miracidia of F. gigantica had a size ranging from 98–119 (110 ± 0.1) and 63–77 (70 ± 0.04) µm. Etlingera elatior ethanolic extract showed a markedly ovicidal effect on F. gigantica ova compared to the untreated groups (Figs. 2 and 3). The total developed eggs from the 1.25%, 2.5%, and 5% concentration samples on day 11 were 56.67%, 56.67%, and 36.67%, respectively (Fig. 2A and B). However, this result was not significantly different compared to untreated samples, with the concentration of 1.25% (p =0.18), 2.5% (p =0.1), and 5% (p =0.02). The developed eggs on day 14, which were represented by hatched larvae for the three concentrations, were 70%, 50%, and 13.33%, respectively (Fig. 2C and D). The total developed eggs were not significantly reduced compared to untreated samples on day 14 with the concentration of 1.25% (p =0.72), and 2.5% (p =0.27), while 5% with (p =0.002) differed significantly. Furthermore, for the Fluconix-340 treated samples, the total developed eggs for 1.25%, 2.5%, and 5% were 6.67%, 0%, and 0%, respectively. They were significantly reduced compared to the untreated samples on day 11 with the concentration of 1.25% (p =0.003), 2.5% ( p =0.00009), and 5% (p =0.0009) (Fig. 2A and B). The developed eggs on day 14, mostly represented by hatched larvae for the 1.25%, 2.5%, and 5% concentrations were 6.67%, 0%, and 0%, respectively. The values were significantly reduced compared to untreated samples with the concentration of 1.25% (p =0.001), 2.5% ( p =0.0005), and 5% p =(0.0005) (Fig. 2C and D), respectively.
Fig. 1. Screening of F. gigantica population on ova and adult stages by duplex PCR. (A) Egg from freshly dissected gall bladder, (B) Adult F. gigantica isolated from bile ducts, (C) PCR from pooled eggs, and (D) PCR from adult F. gigantica, 8–10 adult worms per lane.
Fig. 2. Ovicidal efficacy of E. elatior in different concentration and incubation compared to negative control, aquadest and synthetic anthelmintic, Fluconix 340. (A) Hatched and developed egg after incubation on day 11; (B) Degenerated eggs after incubation on day 11; (C) Hatched and developed eggs after incubation on day 14; and (D) Degenerated eggs after incubation on day 14.
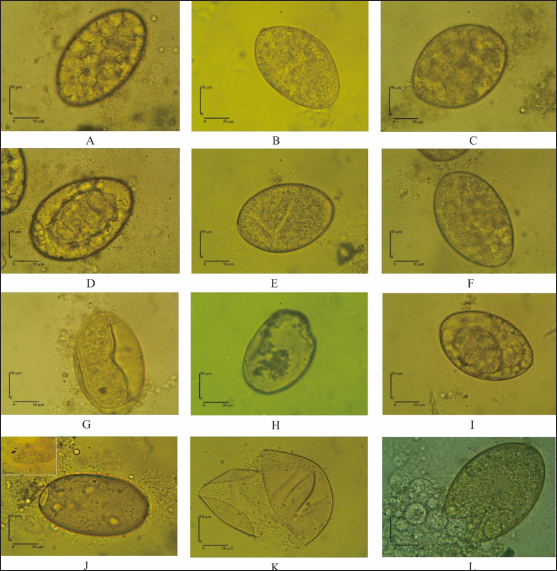
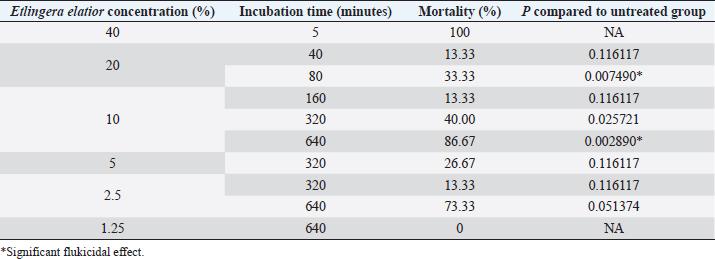
Fig. 3. Eggs development of F. gigantica showed different morphology during treatment in 5 days A–C; 9 days D–F; 11 days G–I; and 14 days J–L. F. gigantica wall destruction obviously observed during the treatment, which is showing inhibition of morulation since day 6 post treatment, followed by damage of the wall by E. elattior ethanolic extract in the highest concentration (K). Morula inside the eggs also out followed by the egg damage (L) which implied undeveloped both of undeveloped morula and egg destruction. In the untreated samples, almost all the eggs started embryo development on day 11, as observed under a light microscope (Fig. 3G). The miracidium was hatched as indicated by the larvae outside or an opened operculum (Fig. 3J). Meanwhile, Figure 3I shows the treated samples exhibited incomplete embryo development, and Figure 3H, K, and L demonstrate the destruction of egg walls. The initiation of larva development was observed in the Fluconix-340 and E. elatior-treated samples. Additionally, the destruction of egg walls was documented clearly on Fluconix-340 treated samples (Fig. 3K). Flukicidal effect of E. elatior on adult F. giganticaNo flukes were observed dead during incubation with NaCl 0.9% until 640 minutes (Table 1). In the 20% E. elatior concentration, mortalities reached 0% in 5 minutes, 0% in 10 minutes, 0% in 20 minutes, 13.33% in 40 minutes, 33.33% in 80 minutes, 100% in 160 minutes, 100% in 320 minutes, and 100% in 640 minutes as shown in Table 1. Furthermore, mortalities reached 0% in 5 minutes, 0% in 10 minutes, 0% in 20 minutes, 0% in 40 minutes, 0% in 80 minutes, 13.33% in 160 minutes, 40% in 320 minutes, and 86.67% in 640 minutes for the 10% concentration (Table 1). In the 5% concentration, mortalities reached 0% in 5 minutes, 0% in 10 minutes, 0% in 20 minutes, 0% in 40 minutes, 0% in 80 minutes, 0% in 160 minutes, 26.67% in 320 minutes, and 100% in 640 minutes as described in Table 1. For the 2.5% concentration, mortalities reached 0% in 5 minutes, 0% in 10 minutes, 0% in 20 minutes, 0% in 40 minutes, 0% in 80 minutes, 0% in 160 minutes,13.33% in 320 minutes, and 73.33% in 640 minutes as shown in Table 1. In the 1.25% concentration, no flukes were dead, as stated in Table 1. A significant flukicidal effect was observed in the incubation time of 80 minutes for the concentration of 20% (p =0.007) and 640 minutes for 10% (p =0.003). Surface and internal organs change of F. giganticaSEM of treated flukes with the concentration of 20% E. elatior showed a significantly folded ventral-sucker compared to the control as stated in Figure 4B and A, respectively. Damage to the tegument was quite prominent from the ventral and dorsal parts of the fluke (Fig. 4C and D), while the acetabulum was wrinkled and folded. Furthermore, complete erosion and loss of the spina occurred in the ventral and dorsal parts of the flukes’ body, leaving holes in the former spina position (Fig. 4C and D). In samples treated with Fluconix-340, the fluke was also wrinkled (Fig. 4E) and the spines rounded (Fig. 4F). The tegument destruction was shown by the cross-section and stained with hematoxylin-eosin illustrated in Figure 5A and D, which differ completely from negative control in Figure 5B and Fluconix-340 treated flukes in Figure 5C. The tegument of the E. elatior-treated samples was separated from the cuticle in Figure 5A, while the intestines were found to have lost their epithelium compared to the control and Fluconix-340 treated samples as presented in Figure 6C, A, and B, respectively. DiscussionThe worldwide losses in animal production due to liver fluke infection are estimated to be over 3.2 US$ billion/year. In addition, the World Health Organization declared that 180 million people are at risk of infection and 2.4 million people are infected with fasciolosis (Cwiklinski et al., 2016) with several cases occurring in all continents including temperate and tropical climates (Mehmood et al., 2017). The economic impact of the liver fluke is mainly because of antihelmintic control (Odeniran et al., 2020), condemned livers (Opio et al., 2021), and failure to achieve efficient production (Rashid et al., 2019). A previous study reported that resistance in antihelmintic, commonly used to control fasciolosis, is frequent and has continued to increase (Brennan et al., 2007; Olaechea et al., 2011; Brockwell et al., 2014). Meanwhile, the use of natural herbs to control flukes has promising potential considering that no resistance and side effects have been reported (Bauri et al., 2015). Etlingera elatior is an herb used traditionally for anthelmintic control against humans in villages of Kulawi, Central Sulawesi, Indonesia (Samarang et al., 2018). It has various active compounds which have great potential against diseases. These include tannins, flavonoids, alcohols, aldehydes, and terpenes (Chan et al., 2011). Because of its rich content, E. elatior is reportedly effective as an insecticide (Tarigan et al., 2014), anticancer (Ghasemzadeh et al., 2015), and antibacterial (Ernilasari et al., 2021). However, its anti-trematode effect against F. gigantica has not been studied. Table 1. Flukicidal efficacy of E. elatior extract to adult F. gigantica in different concentration and incubation time compared to untreated control.
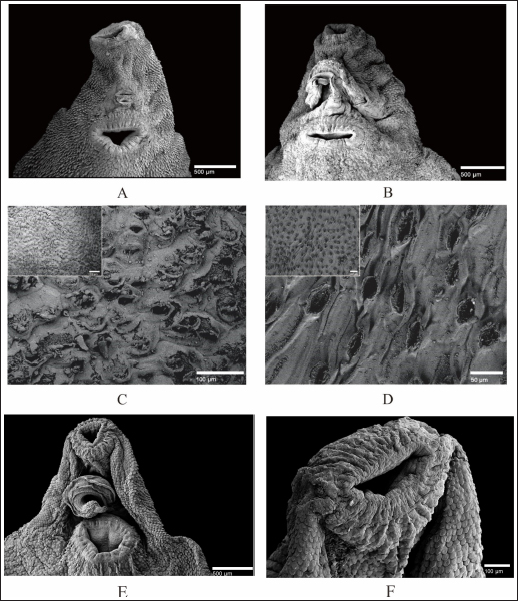
Fig. 4. SEM of F. gigantica, (A) Ventral sucker of control fluke, (B) Etlingera elatior treated samples of ventral sucker; (C) Ventral tegument; and (D) Dorsal tegument. Spines and tegument of treated samples were almost all eroded. The remaining spines were rounded (B) compared to the untreated sample with sharp-end (A). Besides, damaged skin is also erosion of the inner membrane until the tegument. € Fluconix-340 treated F. gigantica showed a wrinkled fluke and spines became rounded (F). In this study, E. elatior showed ovicidal effects against F. gigantica eggs, this is demonstrated by lethal activities on blastomeres development as shown by the incomplete formation of miracidium. On day 11, the development was significantly less with the 1.25% concentration. The results were then followed by low hatched larva on day 14, as shown by the empty-intact eggs with opened operculum. Compared to the Fluconix-340-treated sample, E. elatior showed a lower impact on the destruction of egg walls but successfully inhibited the development of larva. During the adulticidal assay, erosion of the spines was indicated by holes in the tegument surface of the flukes. Teguments showed blebs and were separated from the cuticle as demonstrated by the cross-section in the E. elatior-treated sample. The effect was completely different with Fluconix-340-treated samples, which showed shrinkage of almost all fluke tissue. The intestine of the treated sample was completely eroded leaving villi without epithelial lining in the lumen. Furthermore, Fluconix-340 showed a strong effect in vitro, as indicated by 100% mortality from the lowest concentration used, for 5 minutes of incubation time. Although both substances showed powerful effects, the E. elatior extract had a significant effect on F. gigantica compared to Fluconix-340 with different mechanisms of action which are still unknown.
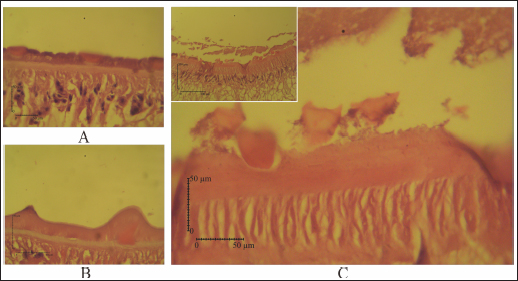
Fig. 5. (A) Tegument of untreated-control, (B) Fluconix-340, and (C) Etlingera elatior. The tegumental syncytium of E. elatior treated fluke, 40% 5 minutes, was detached from the basal lamina of tegument (A, C).
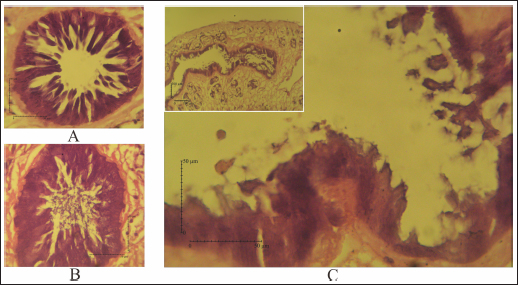
Fig. 6. (A) Hematoxylin eosin staining for the intestine of untreated-control; (B) Fluconix-340; and (C) Etlingera elatior. Intestine of F. gigantica treated with E. elatior 40% for 5 minutes showed disruption of the intestine lumen. Compared to Fluconix-340, E. elatior was less destructive in the equal concentration used but showed significant ovicidal and flukicidal effects compared to the untreated samples. Fluconix-340 contains Nitroxynil 340 mg/100 ml and its application for treating flukes has been widely reported with various concentrations in in-vitro and in-vivo studies. Furthermore, the ultrastructural changes in the tegument of flukes have long been studied as an important parameter for the determination of anthelmintic drugs and natural products’ efficacy (Abdel-Fatah et al., 2022). The tegument damage makes the parasite more vulnerable to the drug, which can percolate within the flukes, affecting several internal tissues and processes. Osmotic stress is believed to alter β tubulin molecules; hence, it may debilitate the tegument structure (Stitt and Fairweather, 1993). The weakened tegument structure also severely alters the energy-dependent NA+-K+ transport. Keiser and Morson (2008) observed internal Na+ and water content will then increase, causing swelling of the syncytium in nitroxinil treatment (Keiser and Morson, 2008). The tegumental damage may alter tegumental function in excretory and secretory via trans-tegumental uptake, disturbing signaling pathway and metabolic processes (Halton, 2004). Several herb extracts also have been studied for their anthelmintic properties. Alcoholic extract of M. oleifera green leaves showed an ovicidal effect on Fasciola non-embryonated and developed eggs with a concentration of 24.39 mg/ml (Hegazi et al., 2018). Nigella sativa and Curcuma longa also exhibited flukicidal effects by pronounced tegmental disruptions and erosion of spines in the posterior region and around the acetabulum (Ullah et al., 2017). Plant extracts of Lantana camara, Bocconia frutescens, Piper auritum, Artemisia Mexicana, Cajanus cajan (Alvarez-Mercado et al., 2015), Ocimum sanctum L. (Mahardika et al., 2017), Peganum harmala (Moazeni et al., 2017), Acacia senegal (Alsadeg et al., 2015), Zingiber officinale (Moazeni and Khademolhoseini, 2016), Cleome rutidosperma (Luis et al., 2021), Moringa oleifera (Kandil et al., 2018), Terminalia catappa L. (Anuracpreeda et al., 2017), ( Curcuma aeruginosa Roxb (Vanda et al., 2020), and Areca catechu (Yamson et al., 2019) demonstrated an in vitro anthelmintic effect against F. hepatica and F. gigantica. All the aforementioned herbs evidently have alkaloids, saponins, and tannins which handle the anthelmintic properties. Products derived from natural resources can cause lesser side effects for the host and less environmental damage. The results obtained in this in vitro assay can be considered for further in vivo study. Etlingera elatior is also suitable for feed enrichment with the expected anthelmintic effect and is highly abundant in tropical regions. This ethnopharmacological product is expected to reduce the use of chemical-based substances with side effects that might affect animals during pregnancy (El-Makawy et al., 2006). Further studies are needed on the active compounds involved, and the docking modeling for anthelmintic activities. ConclusionEtlingera elatior in this study showed a promising effect both in egg and adult stages of F. gigantica. It exhibited detrimental effects on F. gigantica tegument, reduced egg hatchability, and increased mortality in the adult stage. This is because of the extract’s high level of tannic acid, alkaloid, saponin, and flavonoid, which possess anthelmintic properties. AcknowledgmentsThe authors are grateful to Ampel abattoir staff who help during sample collection, as well as the Planning, Research, and Development Agency of Boyolali District, Indonesia, who gives permission to perform this study in the region. Conflicts of interestThe authors declare that there is no conflict of interest Author contributionsConceptualization: Penny H. Hamid. Funding acquisition: Penny H. Hamid, Muhammad Cahyadi. Investigation: Aisyah Wulandari, Andini Nurlaelasari, Wahyu Kurniawan, Herjuno Ari Nugroho. Writing: Aisyah Retno Wulandari, Penny H. Hamid, Herjuno Ari Nugroho, Muhammad Cahyadi. ReferenceAbdel-Fatah, O.R., Arafa, W.M., Wahba, A.A. and El-Dakhly, K.M. 2022. Tegumental alterations and resistance of Fasciola gigantica adult worms exposed to flukicides in Egypt. Beni-Suef. Univ. J. Basic. Appl. Sci. 11, 1–11. Ahamed, S.H., Ho, J. and Venkatesh, S.K. 2013. A fluke diagnosis. Ann. Acad. Med. Singap. 42, 368–370. Alsadeg, A.M., Koko, W.S., Osman, E., Kabbashi, A., Dahab, M. and Garbi, M. 2015. In vitro anthelminthic activity of the methanol stem bark extract of Acacia senegal against Fasciola gigantica. Inter. Inven. J. Biochem. Bioinform. 3, 18–22. Alvarez-Mercado, J.M., Ibarra-Velarde, F., Alonso-Díaz, M.Á., Vera-Montenegro, Y., Avila-Acevedo, J.G. and García-Bores, A.M. 2015. In vitro antihelmintic effect of fifteen tropical plant extracts on excysted flukes of Fasciola hepatica. BMC. Vet. Res. 11, 1–6. Alvarez-Sanchez, M.A., Mainar-Jaime, R.C., Perez-Garcia, J. and Rojo-Vazquez, F.A. 2006. Resistance of Fasciola hepatica to triclabendazole and albendazole in sheep in Spain. Vet. Rec. 159, 424–425. Anuracpreeda, P., Chawengkirttikul, R., Ngamniyom, A., Panyarachun, B., Puttarak, P., Koedrith, P. and Intaratat, N. 2017. The in vitro anthelmintic activity of the ethanol leaf extracts of Terminalia catappa L. on Fasciola gigantica. Parasitology 144, 1931–1942. Arafa, W.M., Shokeir, K.M. and Khateib, A.M. 2015. Comparing an in vivo egg reduction test and in vitro egg hatching assay for different anthelmintics against Fasciola species, in cattle. Vet. Parasitol. 214, 152–158. Arjona, R.R., Aguado, J.M., Salesa, R. and González-Macías, J. 1995. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine 74, 13–23. Assimakopoulos, S.F., Psilopanagioti, A., Michail, A., Papakonstantinou, C., Gogos, C. and Labropoulou-Karatza, C. 2010. Severe eosinophilia and hepatic lesion: a rare case of fascioliasis from Greece. J. Gastrointestin. Liver. Dis. 19, 125. Bauri, R.K., Tigga, M. and Nisha Kullu, S.S. 2015. A review on use of medicinal plants to control parasites. Indian. J. Nat. Prod. Resour. 6, 268–277. Black, J., Ntusi, N., Stead, P., Mayosi, B. and Mendelson, M. 2013. Human fascioliasis in South Africa. S. Afr. Med. J. 103, 658–659. Brennan, G.P., Fairweather, I., Trudgett, A., Hoey, E.M., McConville, M., Meaney, M., Robinson, M., McFerran, N., Ryan, L., Lanusse, C., Mottier, L., Alvarez, L., Solana, H., Virkel, G. and Brophy, P.M. 2007. Understanding triclabendazole resistance. Exp. Mol. Pathol. 82(2), 104–109. Brockwell, Y.M., Elliott, T.P., Anderson, G.R., Stanton, R., Spithill, T.W. and Sangster, N.C. 2014. Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int. J. Parasitol. Drugs. Drug. Resist. 4, 48–54. Carrique-Mas, J.J. and Bryant, J.E. 2013. A review of foodborne bacterial and parasitic zoonoses in Vietnam. EcoHealth 10, 465–489. Chan, E.W.C., Lim, Y. and Wong, S.K. 2011. Phytochemistry and pharmacological properties of Etlingera elatior: a review. Pharmacogn. J. 3, 6–10. Chen, J.X., Chen, M.X., Ai, L., Xu, X.N., Jiao, J.M., Zhu, T.J.,Guo, Y.H. and Zhou, X.N. 2013. An outbreak of human Fascioliasis gigantica in southwest China. PloS One 8(8), e71520. Cwiklinski, K., O’Neill, S.M., Donnelly, S. and Dalton, J.P. 2016. A prospective view of animal and human fasciolosis. Parasite. Immunol. 38, 558–568. El-Makawy, A., Radwan, H.A., Ghaly, I.S. and Abd El-Raouf, A. 2006. Genotoxical, teratological and biochemical effects of anthelmintic drug oxfendazole maximum residue limit (MRL) in male and female mice. Reprod. Nutr. Dev. 46, 139–156. Ernilasari, E., Walil, K., Fitmawati, F., Roslim, D.I., Zumaidar, Z., Saudah, S. and Rayhannisa, R. 2021. Antibacterial activity of leaves, flowers, and fruits extract of Etlingera elatior from Nagan Raya district, Indonesia against Escherichia coli and Staphylococcus aureus. Biodiversitas 22, 658–659. Esteban, J.G., González, C., Bargues, M.D., Angles, R., Sánchez, C., Náquira, C. and Mas-Coma, S. 2002. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health. 7, 339–348. Esteban, J.G., Gonzalez, C., Curtale, F., Muñoz-Antoli, C., Valero, M.A., Bargues, M.D. and Mas-Coma, S. 2003. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am. J. Trop. Med. Hyg. 69, 429–437. Fairweather, I. 2011. Raising the bar on reporting ‘triclabendazole resistance’. Vet. Rec. 168, 514–515. Fox, N.J., White, P.C., McClean, C.J., Marion, G., Evans, A. and Hutchings, M.R. 2011. Predicting impacts of climate change on Fasciola hepatica risk. PLoS One 6, e16126. Ghasemzadeh, A., Jaafar, H.Z., Rahmat, A. and Ashkani, S. 2015. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) RM Sm grown in different locations of Malaysia. BMC. Complement. Altern. Med. 15, 1–10. Gil, L.C., Diaz, A., Rueda, C., Martinez, C., Castillo, D. and Apt, W. 2014. Resistant human fasciolasis: report of four patients. Rev. Med. Chil. 142, 1330–1333. Good, R. and Scherbak, D. 2022. Fascioliasis. Treasure Island, FL: StatPearls. Halton, D.W. 2004. Microscopy and the helminth parasite. Micron 35, 361–390. Hegazi, A.G., Megeed, K.N.A., Hassan, S.E., Abdelaziz, M., Toaleb, N.I., El Shanawany, E.E. and Aboelsoued, D. 2018. Comparative ovicidal activity of Moringa oleifera leaf extracts on Fasciola gigantica eggs. Vet. World. 11, 215–220. Ka, M.M., Mbengue, M., Diop, B.M., Pouye, A., Da Veiga, J.A., Dia, D. and Diop, T.M. 2002. Two unexpected cases of hepatobiliary fascioliasis in Dakar (Senegal). Dakar. Med. 47, 202–205. Kandil, O.M., Hassan, N.M., Sedky, D., Ata, E.B., Nassar, S.A., Shalaby, H.A. and Gabrashanska, M. 2018. Anthelmintic efficacy of Moringa oleifera seed methanolic extract against Fasciola hepatica. J. Parasit. Dis. 42, 391–401. Keiser, J. and Morson, G. 2008. Fasciola hepatica: surface tegumental responses to in vitro and in vivo treatment with the experimental fasciolicide OZ78. Exp. Parasitol. 119(1), 87–93. Kelley, J.M., Elliott, T.P., Beddoe, T., Anderson, G., Skuce, P. and Spithill, T.W. 2016. Current threat of triclabendazole resistance in Fasciola hepatica. Trends. Parasitol. 32, 458–469. Kumari, V., Banerjee, T., Negi, N., Gupta, M.I., Tiwari, K. and Gupta, M. 2013. Human fascioliasis with biliary complications. J. Commun. Dis. 45, 91–93. Lachumy, S.T.J., Sasidharan, S., Sumathy, V. and Zuraini, Z. 2010. Pharmacological activity, phytochemical analysis and toxicity of methanol extract of Etlingera elatior (torch ginger) flowers. Asian. Pac. J. Trop. Med. 3, 769–774. Le, T.H., Nguyen, K.T., Nguyen, N.T.B., Doan, H.T.T., Le, X.T.K., Hoang, C.T.M. and De, N.V. 2012. Development and evaluation of a single-step duplex PCR for simultaneous detection of Fasciola hepatica and Fasciola gigantica (family Fasciolidae, class Trematoda, phylum Platyhelminthes). J. Clin. Microbiol. 50, 2720–2726. Luis, H.C., Matias, F.B.R., Tubalinal, G.A.S. and Mingala, C.N. 2021. In vitro anthelminthic activity of fringed spiderflower (Cleome rutidosperma) ethanolic leaf extract against Fasciola spp. Ann. Parasitol. 67, 243–248. Luo, X.C.K., Wang, Z., Li, Z. and Wu, Z. 2021. High-quality reference genome of Fasciola gigantica: insights into the genomic signatures of transposon-mediated evolution and specific parasitic adaption in tropical regions. PLoS. Negl. Trop. Dis. 15, e0009750. Mahardika, M.M., Sudjarwo, S.A. and Koesdarto, S. 2017. Anthelmintic activity of Ocimum sanctum L. leaves ethanol extract against Fasciola gigantica in vitro. KnE. Life. Sci. 3, 266–277. Mas-Coma, S., Bargues, M. and Valero, M.A. 2018. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 145, 1665–1699. Mas-Coma, S.V., Valero, M.A. and Bargues, M.D. 2014. Fascioliasis. Adv. Exp. Med. Biol. 766, 77–114. Mbuh, J.V. and Mbwaye, J. 2005. Serological changes in goats experimentally infected with Fasciola gigantica in Buea sub-division of S.W.P. Cameroon. Vet. Parasitol. 131, 255–259. McManus, D.P. 2020. Recent progress in the development of liver fluke and blood fluke vaccines. Vaccines 8, 553. Mehmood, K., Zhang, H., Sabir, A.J., Abbas, R.Z., Ijaz, M., Durrani, A.Z. and Li, J. 2017. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 109, 253–262. Mendes, E.A., Vasconcelos, A.C. and Lima, W.D.S. 2012. Histopathology of Fasciola hepatica infection in Merionesunguiculatus. Rev. Patrol. Trop. 41, 55–62. Mera y Sierra, R.A.V., Cuervo, P. and Mas-Coma, S. 2011. Human fascioliasis in Argentina: retrospective overview, critical analysis and baseline for future research. Parasit. Vectors. 4, 104. Merachew, W. and Alemneh, T. 2020. Review on triclabendazole resistance in Fasciola. J. Vet. Sci. Med. 8, 1–8. Moazeni, M. and Khademolhoseini, A.A. 2016. Ovicidal effect of the methanolic extract of ginger (Zingiber officinale) on Fasciola hepatica eggs: an in vitro study. J. Parasit. Dis. 40, 662–666. Moazeni, M., Saadaty Ardakani, Z.S., Saharkhiz, M.J., Jalaei, J., Khademolhoseini, A.A., Shams Esfand Abad, S. and Mootabi Alavi, A. 2017. In vitro ovicidal activity of Peganum harmala seeds extract on the eggs of Fasciola hepatica. J. Parasit. Dis. 41, 467–472. Najib, M.A.I.N., Amilah, W., Faez, A.M. and Shafizol, Z. 2020. A scoping review of the prevalence of fascioliasis in Malaysia and risk factors for infection. Malays. J. Med. Sci. 27, 22–36. Naufalin, R. Natural preservation opportunities and challenges in improving food safety. In AIP Conference Peoceedings, 2019, p 020032. Odeniran, P.O., Omolabi, K.F. and Ademola, I.O. 2020. Economic model of bovine fasciolosis in Nigeria: an update. Trop. Anim. Health. Prod. 52, 3359–3363. Olaechea, F., Lovera, V., Larroza, M., Raffo, F. and Cabrera, R.J.V. 2011. Resistance of Fasciola hepatica against triclabendazole in cattle in Patagonia (Argentina). Vet. Parasitol. 178, 364–366. Opio, L.G., Abdelfattah, E.M., Terry, J., Odongo, S. and Okello, E. 2021. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at Lira Municipality Abattoir in Northern Uganda. Animals 11, 681. Ortiz, P., Scarcella, S., Cerna, C., Rosales, C., Cabrera, M., Guzmán, M. and Solana, H.J. 2013. Resistance of Fasciola hepatica against triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Vet. Parasitol. 195, 118–121. Overend, D. and Bowen, F.L. 1995. Resistance of Fasciola hepatica to triclabendazole. Aust. Vet. J. 72, 275–276. Pleasance, J., Raadsma, H.W., Estuningsih, S.E., Widjajanti, S., Meeusen, E. and Piedrafita, D. 2011. Innate and adaptive resistance of Indonesian thin tail sheep to liver fluke: a comparative analysis of Fasciola gigantica and Fasciola hepatica infection. Vet. Parasitol. 178, 264–272. Pritsch, I.C. and Molento, M.B. 2018. Recount of reported cases of human fascioliasis in Brazil over the last 60 years. J. Trop. Pathol. 47, 75–86. Rashid, M., Rashid, M., Akbar, H., Ahmad, L., Hassan, M., Ashraf, K., Saeed K. and Gharbi, M. 2019. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitology 146, 129–141. Rondelaud, D., Dreyfuss, G., Bouteille, B. and Dardé, M.L. 2000. Changes in human fasciolosis in a temperate area: about some observations over a 28-year period in central France. Parasitol. Res. 86, 753–757. Samarang, S., Isnawati, R. and Murni, M. 2018. Potensi kandungan Karondo (Etlingera elatior) sebagai obat cacing tradisional masyarakat Kulawi di Sulawesi Tengah. J. Penyakit. Bersumber. Binatang. 2, 1–8. Sargison, N. and Scott, P.R. 2011. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet. Rec. 168, 159. Sofowora, A., Ogunbodede, E. and Onayade, A. 2013. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 10, 210–229. Stitt, A. and Fairweather, I. 1993. Fasciola hepatica: tegumental surface changes in adult and juvenile flukes following treatment in vitro with the sulphoxide metabolite of triclabendazole (Fasinex). Parasitol. Res. 79, 529–536. Tarigan, L.A., Batubara, R. and Sumardi, S. 2014. The giving variation of concentration Kecombrang flowers extract (Etlingera elatior Jack RM Sm) as natural insectiside against Aedes spp. Peronema. Forest. Sci. J. 3, 56–61. Ullah, R., Rehman, A., Zafeer, M.F., Rehman, L., Khan, Y.A., Khan, M.A. and Abidi, S.M. 2017. Anthelmintic potential of Thymoquinone and Curcumin on Fasciola gigantica. PLoS One 12, e0171267. Valero, M.A.P.C.I., Periago, M.V., Khoubbane, M. and Mas-Coma, S. 2009. Fluke egg characteristics for the diagnosis of human and animal fascioliasis by Fasciola hepatica and F. gigantica. Acta. Trop. 111(2), 150–159. Vanda, H., Parindra, R., Hambal, M. and Athaillah, F. 2020. Anthelmintic activity of Curcuma Aeruginosa Roxb extract on Fasciola gigantica in vitro. In E3S Web of Conferences, 2020, Vol. 151, p 01046. Wang, G.X., Han, J., Zhao, L.W., Jiang, D.X., Liu, Y.T. and Liu, X.L. 2010. Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine 17, 1102–1105. Yamson, E.C., Tubalinal, G.A.S., Viloria, V.V. and Mingala, C.N. 2019. Anthelmintic effect of betel nut (Areca catechu) and neem (Azadirachta indica) extract against liver fluke (Fasciola spp.). J. Adv. Vet. Anim. Res. 6, 44. Yılmaz, H. and Gödekmerdan, A.J. 2004. Human fasciolosis in Van province, Turkey. Acta. Trop. 92, 161–162. Zumaquero-Ríos, J.L., Sarracent-Perez, J., Rojas-Garcia, R., Rojas-Rivero, L., Martinez-Tovilla, Y., Valero, M.A. and Mas-Coma, S. 2013. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla state, Mexico: epidemiology and treatment with nitazoxanide. PLoS. Negl. Trop. Dis. 7, e2553. | ||
| How to Cite this Article |
| Pubmed Style Wulandari AR, Nurlaelasari A, Nugroho HA, Cahyadi M, Kurniawan W, Hamid PH. Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Vet J. 2023; 13(5): 576-587. doi:10.5455/OVJ.2023.v13.i5.10 Web Style Wulandari AR, Nurlaelasari A, Nugroho HA, Cahyadi M, Kurniawan W, Hamid PH. Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. https://www.openveterinaryjournal.com/?mno=92860 [Access: July 14, 2025]. doi:10.5455/OVJ.2023.v13.i5.10 AMA (American Medical Association) Style Wulandari AR, Nurlaelasari A, Nugroho HA, Cahyadi M, Kurniawan W, Hamid PH. Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Vet J. 2023; 13(5): 576-587. doi:10.5455/OVJ.2023.v13.i5.10 Vancouver/ICMJE Style Wulandari AR, Nurlaelasari A, Nugroho HA, Cahyadi M, Kurniawan W, Hamid PH. Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Vet J. (2023), [cited July 14, 2025]; 13(5): 576-587. doi:10.5455/OVJ.2023.v13.i5.10 Harvard Style Wulandari, A. R., Nurlaelasari, . A., Nugroho, . H. A., Cahyadi, . M., Kurniawan, . W. & Hamid, . P. H. (2023) Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Vet J, 13 (5), 576-587. doi:10.5455/OVJ.2023.v13.i5.10 Turabian Style Wulandari, Aisyah Retno, Andini Nurlaelasari, Herjuno Ari Nugroho, Muhammad Cahyadi, Wahyu Kurniawan, and Penny Humaidah Hamid. 2023. Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Veterinary Journal, 13 (5), 576-587. doi:10.5455/OVJ.2023.v13.i5.10 Chicago Style Wulandari, Aisyah Retno, Andini Nurlaelasari, Herjuno Ari Nugroho, Muhammad Cahyadi, Wahyu Kurniawan, and Penny Humaidah Hamid. "Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro." Open Veterinary Journal 13 (2023), 576-587. doi:10.5455/OVJ.2023.v13.i5.10 MLA (The Modern Language Association) Style Wulandari, Aisyah Retno, Andini Nurlaelasari, Herjuno Ari Nugroho, Muhammad Cahyadi, Wahyu Kurniawan, and Penny Humaidah Hamid. "Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro." Open Veterinary Journal 13.5 (2023), 576-587. Print. doi:10.5455/OVJ.2023.v13.i5.10 APA (American Psychological Association) Style Wulandari, A. R., Nurlaelasari, . A., Nugroho, . H. A., Cahyadi, . M., Kurniawan, . W. & Hamid, . P. H. (2023) Ethanolic extract of Etlingera elatior flower exhibits anthelmintic properties to Fasciola gigantica in vitro. Open Veterinary Journal, 13 (5), 576-587. doi:10.5455/OVJ.2023.v13.i5.10 |