| Original Article | ||
Open Vet J. 2020; 10(3): 267-271 doi: 10.4314/ovj.v10i3.4 Open Veterinary Journal, (2020), Vol. 10(3): 267–271 Original Research http://dx.doi.org/10.4314/ovj.v10i3.4 Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection-site sarcomas: A retrospective studyEnrico P. Spugnini1*, Bruno Vincenzi2, Francesca Carocci3, Chiara Bonichi3, Francesco Menicagli3 and Alfonso Baldi4,51Biopulse srl, Naples, Italy 2University Campus Biomedico, Rome, Italy 3Ambulatorio Veterinario Le Accademie, Rome, Italy 4Department of Environmental, Biological and Pharmaceutical Sciences and Technologies Campania University “Luigi Vanvitelli”, Caserta, Italy 5Institute of Biosciences and BioResources, CNR, Naples, Italy *Corresponding Author: Enrico P. Spugnini. Biopulse srl, Naples, Italy. Email: info [at] enricospugnini.net Submitted: 04/02/2020 Accepted: 02/07/2020 Published: 31/07/2020 © 2020 Open Veterinary Journal
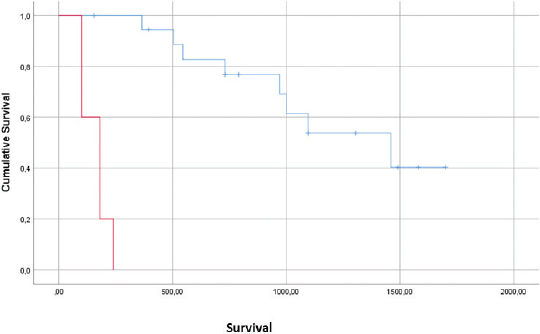
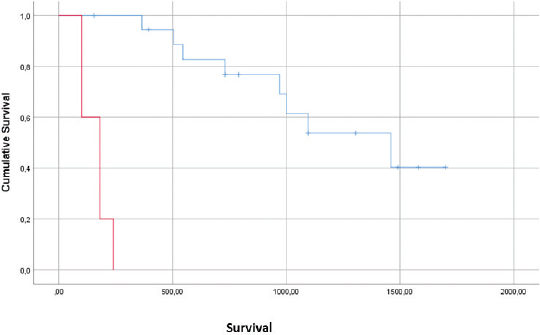
AbstractBackground: Feline injection-site sarcomas (FISSs) are mesenchymal tumors that can occur in cats after injections of different medical agents and are easily prone to recurrence. Aim: The aims of this study were to report treatment outcomes for cats with feline injection-site sarcomas (FISSs) treated with both bleomycin and cisplatin, per adjuvant electrochemotherapy (ECT) protocol. Methods: The medical records of cats with a diagnosis of FISS that were treated with ECT using both bleomycin and cisplatin were retrospectively evaluated. A total of 27 cats were available for statistical evaluation of their response. The cats received intravenous 20 mg/m2 bleomycin, and the tumor bed and margins were infiltrated with cisplatin at the dose of 0.5 mg/cm2. Then, the trains of permeabilizing biphasic electric pulses lasting 50 + 50 µseconds each were delivered in bursts of 1,300 V/cm using caliper electrodes under sedation. A second session was performed 2 weeks later. Results: Side effects were limited to local inflammation in three cats. Three cats developed local tumor recurrence at days 180, 180, and 545 after surgery, two cats developed recurrence and metastases at 100 and 505 days after surgery, and two cats experienced distant metastases. A median time to recurrence could not be calculated as over 80% of the study population remained disease free or were censored due to death from other causes. Mean survival time was 985 days, and median cumulative survival for all cases was 1,000 days. Conclusion: When compared to historical controls, the results of this study demonstrate the superior rates of tumor-free survival and disease-free interval. This adjuvant therapy could be a useful addition to the current options for FISS in consideration of its efficacy, limited toxicity, and ease of administration. Keywords: Bleomycin, Cat, Cisplatin, Electrochemotherapy, Sarcoma. IntroductionFeline injection-site sarcomas (FISSs) are mesenchymal tumors that can occur in cats after injections of different medical agents, including vaccines, antibiotics, and corticosteroids (Martano et al., 2011; Hartmann et al., 2015; Zabielska-Koczywąs et al., 2017). Pathogenic mechanisms seem to be centered on an excessive and nonspecific inflammatory reaction to the substances introduced within the tissues (Martano et al., 2011; Wilcock et al., 2012; Graf et al., 2018). Besides multiple drugs, this cancer has also been associated with the presence of the tissues of other materials that have been perceived as “non-self” by the feline immune system such as non-absorbable sutures, surgical sponges, glass fragments, vegetable thorns, and microchips (Gagnon, 2000; Buracco et al., 2002; de Man and Ducatelle, 2007; Carminato et al.,. 2011; Munday et al., 2011; Martano et al., 2012; Srivastav et al., 2012). Since its identification, this neoplasm showed an aggressive behavior characterized by rapid recurrence after surgery. These tumors also have a metastatic potential. Early therapeutic attempts at curing this neoplastic entity with marginal excision resulted in short-lived control with a median time to recurrence of 79 days (Hershey et al., 2000). More aggressive surgeries improved the outcome to 325 days of control (Hershey et al., 2000). Local control and overall survival greatly benefited from surgeries performed by oncology surgeons (Bray and Polton, 2016) and from the inclusion in the treatment protocols of radiation therapy (neoadjuvant and adjuvant) and/or chemotherapy (Bregazzi et al., 2001; Martano et al., 2005; Eckstein et al., 2009). The current guidelines strongly advocate the adoption of a multimodality approach to this disease, with the rates of local recurrence for FISS varying from 14% to 59% (Hershey et al., 2000; Bregazzi et al., 2001; Martano et al., 2005; Eckstein et al., 2009; Bray and Polton, 2016). The challenge posed by these tumors is reinforced by the reports that even wider en bloc resection with 5-cm margins was not a guarantee of local control (Phelps et al., 2011). Novel approaches have been pursued by veterinary oncologists including hyperthermia (Siddiqui et al., 2007; Zimmermann et al., 2016) and electrochemotherapy (ECT) (Spugnini et al., 2016; 2017). Currently, there are only three previous studies and a case report describing the use of ECT for the control of feline sarcomas (including FISS) (Mir et al., 1997; Spugnini et al., 2007, 2010; 2011). The first study was a preliminary investigation to treat macroscopic feline sarcoma with ECT reporting only one partial response and a mean survival time of 6.1 months (Mir et al., 1997). The other two studies reported the adjuvant use of ECT after an incomplete surgical excision of feline sarcomas using local bleomycin (Spugnini et al., 2007) or cisplatin (Spugnini et al., 2011) with control times of 12–19 months and 666 days, respectively. In this study, we evaluated the tolerability and effectiveness of a combined ECT protocol involving local and systemic administration of chemotherapy agents (cisplatin and bleomycin, respectively) in cats with incompletely excised FISS. The hypothesis of this study was that adjuvant ECT combining two chemotherapy agents might result in extended control and overall survival for cats with FISS when compared to other protocols. Materials and MethodsThe clinical records of cats referred from 2013 to 2018 for adjuvant ECT for FISS were reviewed, searching for feline patients treated with the combination of bleomycin and cisplatin. Cats treated with single-agent ECT were not included. The data were collected by the retrospective review of clinical records and included breed, age, sex, location of tumor, prior surgical treatment, dose and number of treatments of ECT, toxicities attributed to the chemotherapy agents or electroporation procedure, overall survival time, cause, and date of death. The patients were staged before ECT, with complete blood cell count, chemistry profile, urinalysis, chest radiographs, and abdominal ultrasonography. Cats received two sessions of ECT 2 weeks apart, starting at the time of suture removal (10–14 days after surgery). Cats were pre-medicated with butorphanol, ketamine, and medetomidine, followed by the propofol to effect. Bleomycin was intravenously injected as a bolus at the dose of 20 mg/m2. Moreover, aiming to improve the local control, the tumor bed (a surface of approximately 2 cm deep under the surgical scar, or in case of leg location, as deep as feasible and two additional centimeters of margins at each side) was infiltrated with cisplatin at the dose of 0.5 mg/cm2. Five minutes after the chemotherapy injections, the sequences of 8 biphasic pulses lasting 50+50 µs each were delivered in bursts of 1,300 V/cm using caliper electrodes until treatment coverage was achieved as elsewhere described (Spugnini et al., 2017). Pulses were generated using an electroporator certified for veterinary use (Onkodisruptor®). Contact between the patients and the electrodes was optimized using an electroconductive gel. The treatment was repeated after 2 weeks. During the ECT sessions, the patients were monitored using a cardiac monitor and pulse oximeter. Survival time (ST) was calculated from the date of the first ECT session to the date of death. Time to disease recurrence was calculated from the date of first ECT treatment until progressive disease was documented (local or metastatic) and toxicity was graded using VCOG criteria (Veterinary cooperative oncology group, 2016). The survival time for cats alive at the time of submission or died of other diseases was censored at the last date, and they were reported to be disease free. Kaplan–Meier product limit was used to estimate the median disease-free interval (DFI) and median ST (Kaplan and Meier, 1958). A statistical analysis was performed using the SPSS version 23. Ethical approvalAll procedures were in accordance with institutional guidelines under the control of the Italian Ministry of Public Health (Italian Law D.lgs 26/2014). ResultsAbout 31 feline patients were referred for the adjuvant treatment of FISS for 6 years and received ECT with the combination of systemic bleomycin and local cisplatin. Four cats were lost to follow up, leaving 27 patients for statistical analysis. The patients’ breeds included one Siamese, one Persian, two Norwegian forest cats, and 23 domestic shorthair cats. There were 13 male castrated cats and 14 female spayed cats; age varied from 6 to 16 years. Tumors were localized in the dorsum (17 cats), lateral thorax (4 cats), flank (4 cats), and thigh (2 cats). Three cats had multiple surgeries (3-5) before ECT, three cats were referred at the time of their second surgery, and 21 cats were referred after the first tumor removal. The treatment was well tolerated, and side effects were confined to local inflammation in three cats (Grade I VCOG toxicity) which were managed through the administration of an anti-inflammatory drug (meloxicam as per the manufacturer’s instructions). Systemic side effects were not identified. In terms of response, 20 (74%) had no evidence of recurrence at different times, three cats had a recurrence, two experienced a local recurrence and metastasis, and the remaining two had distant metastases alone. Seven patients died of unrelated causes at different times and were censored in the survival analysis (four had renal failure, one cardiac disease, one developed oral squamous cell carcinoma, and one died of lymphoma). The mean survival time was 985 days with a median survival time of 1,000 days. A median disease-free interval could not be calculated as too many patients remained alive at the time of writing (13) or died of unrelated causes (7). Figure 1 shows the Kaplan–Meier survival curve for the whole study population. The only identified prognostic factor was the number of surgeries before referral (Fig. 2). In fact, the median survival time for cats which underwent multiple surgeries before ECT was 180 days versus 1,460 days for patients which underwent a single surgery before ECT (p > 0.0001). DiscussionThe formerly published results for FISS highlight the challenges posed by this neoplastic entity. FISS needs to be approached with aggressive surgeries performed by oncology surgeons to achieve local control (Phelps et al., 2011; Cantatore et al., 2014). This approach results in a longer survival time but could also lead to wound complications. Survival time with aggressive surgery for FISS has been reported to be 901 days with recurrence and distant metastases as factors negatively influencing survival (499 and 388 days, respectively) (Phelps et al., 2011). The same research reports a survival time of 1,461 days in cats, which not experience recurrence, and 1,528 days in cats, which not experience metastases (Phelps et al., 2011). A similar approach combining aggressive surgery with chemotherapy reports a survival in excess of 2 years and a DFI not reached (Bray and Polton, 2016). The older studies describing the outcome of surgery alone (Hershey et al., 2000) reported a significant (p=0.005) difference in RFI, with a median of 274 days for excision performed at a referral institution, compared with 66 days for surgery performed by a referring veterinarian (Hershey et al., 2000), or more recently the median survival times of >395 days (Davidson et al., 1997), 576 days (Phelps et al., 2011), and 804 days (Romanelli et al., 2008).
Fig. 1. Kaplan–Meier curve showing the survival time for cats that underwent multiple surgery before ECT versus cats that underwent single surgery. The adoption of adjuvant therapies (mostly radiation therapy) reported survival times, ranging from 600 to 842 days (Bregazzi et al., 2001; Hahn et al., 2007) with a recent study reporting 1,300 days (Eckstein et al., 2009). ECT is a treatment modality that combines the administration of chemotherapy drugs with the delivery of permeabilizing electrical pulses to improve the drug uptake by cancer cells (Spugnini et al., 2016; 2017). There are currently three original studies and one case report describing its outcome in cats with FISS as previously reported (Mir et al., 1997; Spugnini et al., 2007; 2011). These works showed adjuvant effectiveness as indicated by the local control and survival times. The current study is the first describing the outcome of a combination of ECT with both systemic bleomycin and local cisplatin in cats with FISS. In the literature, there is only another study describing a similar approach in dogs with soft tissue sarcoma (Spugnini et al., 2019). This retrospective study, with all the limitations related to this type of analysis, shows that the bleomycin/cisplatin combination is well tolerated by cats, with limited dermatological toxicities. The incidence of local recurrence, 5 cases out of 27 (18%), is slightly higher than the best outcomes reported in the literature but still within the upper control range. The metastatic rate of 4 out of 27 (15%) is consistent with the other reports. The mean survival time of 985 days and median survival time of 1000 days favorably compare with other reports of adjuvant ECT in FISS (Mir et al., 1997; Spugnini et al., 2007; 2011). These data are of particular interest when one considers that all the surgeries were performed by referring veterinarians; excisions would be more likely to be associated with early tumor recurrence as reported (Hershey et al., 2000). ECT with drugs in combination can be a novel field of investigation to further enhance the options available to oncologists adopting this technique.
Fig. 2. Kaplan–Meier curve showing the survival time for cats that underwent multiple surgery before ECT versus cats that underwent single surgery. Conflicts of interestEnrico P. Spugnini and Alfonso Baldi are the stockholders of Biopulse S.r.l. Authors contributions:Enrico P. Spugnini and Alfonso Baldi conceived the study and wrote the paper. Bruno Vincenzi performed the statistical analysis. Francesca Carocci, Chiara Bonichi, and Francesco Menicagli were involved in the handling of the patients and clinical outcome follow-up. ReferencesBray, J. and Polton, G. 2016. Neoadjuvant and adjuvant chemotherapy combined with anatomical resection of feline injection-site sarcoma: results in 21 cats. Vet. Comp. Oncol. 14, 147–160. Bregazzi, V.S., LaRue, S.M., McNiel, E., Dernell, W.S., Powers, B.E. and Withrow, S.J. 2001. Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with vaccine-associated sarcomas: 25 cases (1995–2000). J. Am. Vet. Med. Assoc. 18, 547–550. Buracco, P., Martano, M., Morello, E. and Ratto, A. 2002. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet. J. 163, 105–107. Cantatore, M., Ferrari, R., Boracchi, P., Gobbetti, M., Travetti, O., Ravasio, G., Giudice, C., Di Giancamillo, M., Grieco, V. And Stefanello, D. 2014. Factors influencing wound healing complications after wide excision of injection site sarcomas of the trunk of cats. Vet. Surg. 43, 783–790 Carminato, A., Vascellari, M., Marchioro, W., Melchiotti, E. And Mutinelli, F. 2011. Microchip-associated fibrosarcoma in a cat. Vet. Dermatol. 22, 565–569. Davidson, E.B., Gregory, C.R. and Kass, P.H. 1997. Surgical excision of soft tissue fibrosarcomas in cats. Vet. Surg. 26, 265–269. de Man, M.M. and Ducatelle, R.V. 2007. Bilateral subcutaneous fibrosarcomas in a cat following feline parvo-, herpes- and calicivirus vaccination. J. Feline Med. Surg. 9, 432–434. Eckstein, C., Guscetti, F., Roos, M., Martin de las Mulas, J., Kaser-Hotz, B. and Rohrer Bley, C. 2009. A retrospective analysis of radiation therapy for the treatment of feline vaccine-associated sarcoma. Vet. Comp. Oncol. 7, 54–68. Gagnon, A. 2000. Drug injection-associated fibrosarcoma in a cat. Feline Pract. 28, 18–21. Graf, R., Guscetti, F., Welle, M., Meier, D. and Pospischil, A. 2018. Feline Injection Site Sarcomas: Data from Switzerland 2009-2014. J. Comp. Pathol. 163, 1–5. Hahn, K.A., Endicott, M.M., King, G.K. and Harris-King, F.D. 2007. Evaluation of radiotherapy alone or in combination with doxorubicin chemotherapy for the treatment of cats with incompletely excised soft tissue sarcomas: 71 cases (1989–1999). J. Am. Vet. Med. Assoc. 231, 742–745. Hartmann, K., Day, M.J., Thiry, E., Lloret, A., Frymus, T., Addie, D., Boucraut-Baralon, C., Egberink, H., Gruffydd-Jones, T., Horzinek, M.C., Hosie, M.J., Lutz, H., Marsilio, F., Pennisi, M.G., Radford, A.D., Truyen, U., Möstl, K. and European Advisory Board on Cat Diseases. 2015. Feline injection-site sarcoma: ABCD guidelines on prevention and management. J. Feline Med. Surg.17, 606–613. Hershey, A., Sorenmo, K. and Hendrick, M.J. 2000. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J. Am. Vet. Med. Assoc. 216, 58–61. Kaplan, E.L. and Meier, P. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481. Martano, M., Morello, E. And Buracco, P. 2011. Feline injection-site sarcoma: past, present and future perspectives. Vet. J. 188, 136–141. Martano, M., Morello, E., Iussich, S. and Buracco, P. 2012. A case of feline injection-site sarcoma at the site of cisplatin injections. J. Feline Med. Surg. 14, 751–754. Martano, M., Morello, E., Ughetto, M., Iussich, S., Petterino, C., Cascio, P. and Buracco, P. 2005. Surgery alone versus surgery and doxorubicin for the treatment of feline injection-site sarcomas: a report on 69 cases. Vet. J. 170, 84–90. Mir, L.M., Devauchelle, P., Quintin-Colonna, F., Delisle, F., Doliger, S., Fradelizi, D., Belehradek, J. Jr. and Orlowski, S. 1997. First clinical trial of cat soft-tissue sarcomas treatment by electrochemotherapy. Br. J. Cancer 76, 1617–1622. Munday, J.S., Banyay, K., Aberdein, D. and French, A.F. 2011. Development of an injection site sarcoma shortly after meloxicam injection in an unvaccinated cat. J. Feline Med. Surg. 13, 988–991. Phelps, H., Kuntz, C., Milner, R., Powers, B. and Bacon, N. 2011. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002). Vet. Surg. 239, 97–106. Romanelli, G., Marconato, L., Olivero, D., Massari, F. and Zini, E. 2008. Analysis of prognostic factors associated with injection-site sarcomas in cats: 57 cases (2001–2007). J. Am. Vet. Med. Assoc. 232, 1193–1199. Siddiqui, F., Li, C.Y., Larue, S.M., Poulson, J.M., Avery, P.R., Pruitt, A.F., Zhang, X., Ullrich, R.L., Thrall, D.E., Dewhirst, M.W. and Hauck, M.L. 2007. A phase I trial of hyperthermia-induced interleukin-12 gene therapy in spontaneously arising feline soft tissue sarcomas. Mol. Cancer Ther. 6, 380–389. Spugnini, E.P., Azzarito, T., Fais, S., Fanciulli, M. and Baldi, A. 2016. Electrochemotherapy as First Line Cancer Treatment: Experiences from Veterinary Medicine in Developing Novel Protocols. Curr. Cancer Drug Targets 16, 43–52. Spugnini, E.P., Fais, S., Azzarito, T. and Baldi, A. 2017. Novel Instruments for the Implementation of Electrochemotherapy Protocols: From Bench Side to Veterinary Clinic. J. Cell Physiol. 232, 490–495. Spugnini, E.P., Baldi, A., Vincenzi, B., Bongiorni, F., Bellelli, C., Citro, G. and Porrello, A. 2007. Intraoperative versus postoperative electrochemotherapy in high grade soft tissue sarcomas: a preliminary study in a spontaneous feline model. Cancer Chemother. Pharmacol. 59, 375–381. Spugnini, E.P., Renaud, S.M., Buglioni, S., Carocci, F., Dragonetti, E., Murace, R., Cardelli, P., Vincenzi, B., Baldi, A. and Citro, G. 2011. Electrochemotherapy with cisplatin enhances local control after surgical ablation of fibrosarcoma in cats: an approach to improve the therapeutic index of highly toxic chemotherapy drugs. J. Transl. Med. 9, 152. Spugnini, E.P., Filipponi, M., Romani, L., Dotsinsky, I., Mudrov, N., Citro, G. and Baldi, A. 2010. Electrochemotherapy treatment for bilateral pleomorphic rhabdomyosarcoma in a cat. J. Small Anim. Pract. 51, 330–332. Spugnini, E.P., Vincenzi, B., Amadio, B. and Baldi, A. 2019. Adjuvant electrochemotherapy with bleomycin and cisplatin combination for canine soft tissue sarcomas: a study of 30 cases. Open Vet. J. 9, 88–93. Srivastav, A., Kass, P.H., McGill, L.D., Farver, T.B. and Kent, M.S. 2012. Comparative vaccine-specific and other injectable-specific risks of injection-site sarcomas in cats. J. Am. Vet. Med. Assoc. 241, 595-602. Veterinary cooperative oncology group. 2016. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 14, 417–446. Wilcock, B., Wilcock, A. and Bottoms, K. 2012. Feline postvaccinal sarcoma: 20 years later. Can. Vet. J. 53, 430–434. Zabielska-Koczywąs, K., Wojtalewicz, A. and Lechowski, R. 2017. Current knowledge on feline injection-site sarcoma treatment. Acta Vet. Scand. 13, 47. Zimmermann, K., Hossann, M., Hirschberger, J., Troedson, K., Peller, M., Schneider, M., Brühschwein, A., Meyer-Lindenberg, A., Wess, G., Wergin, M., Dörfelt, R., Knösel, T., Schwaiger, M., Baumgartner, C., Brandl, J., Schwamberger, S. and Lindner, L.H. 2016. A pilot trial of doxorubicin containing phosphatidyldiglycerol based thermosensitive liposomes in spontaneous feline soft tissue sarcoma. Int. J. Hyperthermia 25, 1–13. | ||
| How to Cite this Article |
| Pubmed Style Spugnini EP, Vincenzi B, Carocci F, Bonichi C, Menicagli F, Baldi A. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Vet J. 2020; 10(3): 267-271. doi:10.4314/ovj.v10i3.4 Web Style Spugnini EP, Vincenzi B, Carocci F, Bonichi C, Menicagli F, Baldi A. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. https://www.openveterinaryjournal.com/?mno=85377 [Access: July 13, 2025]. doi:10.4314/ovj.v10i3.4 AMA (American Medical Association) Style Spugnini EP, Vincenzi B, Carocci F, Bonichi C, Menicagli F, Baldi A. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Vet J. 2020; 10(3): 267-271. doi:10.4314/ovj.v10i3.4 Vancouver/ICMJE Style Spugnini EP, Vincenzi B, Carocci F, Bonichi C, Menicagli F, Baldi A. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Vet J. (2020), [cited July 13, 2025]; 10(3): 267-271. doi:10.4314/ovj.v10i3.4 Harvard Style Spugnini, E. P., Vincenzi, . B., Carocci, . F., Bonichi, . C., Menicagli, . F. & Baldi, . A. (2020) Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Vet J, 10 (3), 267-271. doi:10.4314/ovj.v10i3.4 Turabian Style Spugnini, Enrico Pierluigi, Bruno Vincenzi, Francesca Carocci, Chiara Bonichi, Francesco Menicagli, and Alfonso Baldi. 2020. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Veterinary Journal, 10 (3), 267-271. doi:10.4314/ovj.v10i3.4 Chicago Style Spugnini, Enrico Pierluigi, Bruno Vincenzi, Francesca Carocci, Chiara Bonichi, Francesco Menicagli, and Alfonso Baldi. "Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study." Open Veterinary Journal 10 (2020), 267-271. doi:10.4314/ovj.v10i3.4 MLA (The Modern Language Association) Style Spugnini, Enrico Pierluigi, Bruno Vincenzi, Francesca Carocci, Chiara Bonichi, Francesco Menicagli, and Alfonso Baldi. "Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study." Open Veterinary Journal 10.3 (2020), 267-271. Print. doi:10.4314/ovj.v10i3.4 APA (American Psychological Association) Style Spugnini, E. P., Vincenzi, . B., Carocci, . F., Bonichi, . C., Menicagli, . F. & Baldi, . A. (2020) Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection site sarcomas: a retrospective study. Open Veterinary Journal, 10 (3), 267-271. doi:10.4314/ovj.v10i3.4 |