| Original Article | ||
Open Vet J. 2020; 10(1): 74-79 doi: 10.4314/ovj.v10i1.12 Open Veterinary Journal, (2020), Vol. 10(1): 74–79 Original Research DOI: http://dx.doi.org/10.4314/ovj.v10i1.12 Giardia duodenalis infection in dogs affected by primary chronic enteropathyStefania Perrucci1, Federica Berrilli2, Cristina Procopio1, Margherita Montalbano Di Filippo2, Alessio Pierini1 and Veronica Marchetti1*1Department of Veterinary Sciences, University of Pisa, Pisa, Italy 2Department of Clinical Science and Translational Medicine, University of Rome “Tor Vergata”, Rome, Italy *Corresponding Author: Veronica Marchetti. Department of Veterinary Sciences, University of Pisa, Pisa, Italy. Email: veronica.marchetti [at] unipi.it Submitted: 27/08/2019 Accepted: 05/03/2020 Published: 18/03/2020 © 2020 Open Veterinary Journal
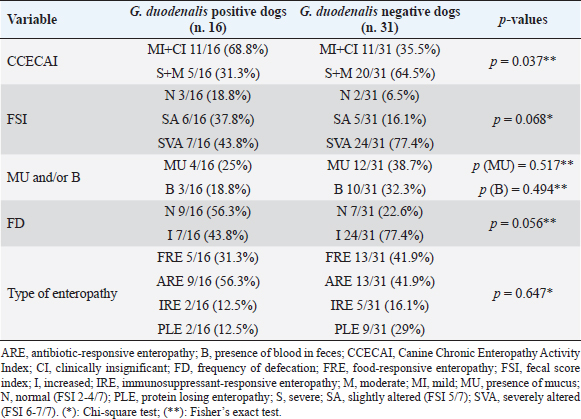
AbstractBackground: Canine primary chronic enteropathy (CE) includes a heterogeneous group of diseases characterized by chronic gastrointestinal signs. Aim: This study evaluated the occurrence of Giardia duodenalis infection in primary CE-affected dogs. Methods: Forty-seven CE-affected dogs of different age and sex were enrolled in the study. For each dog, frequency of defecation, fecal consistency, and eventual fecal abnormalities were evaluated. A clinical scoring index of CE severity (clinical chronic enteropathy activity index) was also assessed, and the type of enteropathy was retrospectively classified. For parasitological analysis, fresh fecal samples collected from each dog were examined by fresh and Lugol stained smears, flotation test, and a rapid immunoassay. Giardia duodenalis genotypes were identified by molecular analysis. Differences of clinical parameters between G. duodenalis positive and G. duodenalis negative dogs were statistically evaluated. Results: Among the CE canine patients, 16 out of 47 (34%) dogs were found positive for G. duodenalis and assemblages C and D were identified. No statistical differences emerged according to the types of CE between G. duodenalis-positive and G. duodenalis-negative dog groups. The clinical index of CE severity was indicative of significant less severe clinical forms in G. duodenalis-positive dogs (p=0.037). Conclusion: Results here obtained shows how G. duodenalis may be present in primary CE-affected dogs and further investigations are needed to clarify the real significance of mild clinical presentation in G. duodenalis-positive dogs affected by CE. Keywords: Canine, Clinical forms, Genotypes, Giardiasis, Primary chronic enteropathy. IntroductionGiardia duodenalis is a worldwide intestinal protozoan parasite that infects a wide range of hosts, including humans, domestic, and wild mammals (Monis et al., 2009). The localization site of G. duodenalis is the small intestine, mainly duodenum and jejunum, and in the infected hosts, it may be responsible for gastrointestinal signs (Hawrelak, 2003; Tangtrongsup and Scorza, 2010). Based on genetic analysis, G. duodenalis is considered as a species complex, which includes at least eight distinct genetic groups or assemblages, from A to H (Ryan and Cacciò, 2013). Zoonotic assemblages A and B and canine-specific assemblages C and D have been reported in dogs (Ballweber et al., 2010; Ryan and Cacciò, 2013; Sommer et al., 2018). The prevalence of G. duodenalis infection in dogs may vary depending on the population examined and the diagnostic method used (Ballweber et al., 2010; Epe et al., 2010). Younger animals and some dog communities, as stray and kennels dogs, have a higher risk of G. duodenalis infection than other dog populations (Huber et al., 2005; Tysnes et al., 2014), while dogs kept as pets are less likely to be positive (Bouzid et al., 2014). The prevalence of G. duodenalis in pet dogs in Italy is about 4%–29% (Riggio et al. 2013; Pipia et al., 2014; Zanzani et al., 2014), while it is about 25% in symptomatic dogs from Europe (Epe et al., 2010). Giardia duodenalis-infected dogs may show a spectrum of clinical signs ranging from subclinical forms to acute and intermittent forms or chronic diarrhea (Epe et al., 2010; Tangtrongsup and Scorza, 2010). Factors associated with the onset of the disease include the age and the clinical, nutritional, and immune status of the infected dog (Roxstrom-Lindquist et al., 2006). Although some studies suggested a possible relation between G. duodenalis assemblage and the severity of clinical disease, other studies suggested the opposite (Tysnes et al., 2014). The pathogenic mechanisms proposed for G. duodenalis infections include production of toxins, disruption of normal intestinal microbiota, inhibition of normal enterocyte enzymatic function, blunting of microvilli, intestinal motility disorders, intestinal epithelial cell apoptosis, and intestinal inflammation (Tangtrongsup and Scorza, 2010). To explain variations in signs both among dogs and over time within the same dog, it has been suggested that microbiota modifications may influence the pathogenicity of G. duodenalis (Tysnes et al., 2014). Associations with bacterial pathogens have been also hypothesized (Tysnes et al., 2014) although results from a recent study suggest that G. duodenalis may also protect against gastrointestinal disease induced by a co-infecting bacterial enteropathogen (Manko et al., 2017). In dogs, chronic enteropathy (CE) includes a heterogeneous group of diseases characterized by chronic gastrointestinal signs lasting from longer than 3 weeks, as diarrhea, vomiting, hyporexia, abdominal pain, weight loss, with the exclusion of extra-intestinal or intestinal diseases of other etiology (Dandrieux, 2016). At present, chronic enteropathies are classified by treatment response in food-responsive enteropathy (FRE), antibiotic-responsive enteropathy (ARE), immunosuppressant-responsive enteropathy (IRE), and non-responsive enteropathy (NRE). Moreover, to avoid misclassification, an early treatment with fenbendazole for Giardia is performed even if G. duodenalis is not detected by routinely laboratory tests (Hall and Day, 2017). However, because the complexity of interactions between dietary components, microbiota, intestinal epithelial integrity, and immune system is supposed to play the main role in maintaining chronic inflammation, the role of G. duodenalis in dogs with chronic enteropathy needs to be clarified. The aim of this prospective study was to evaluate the occurrence of G. duodenalis infection in dogs affected by primary CE. Materials and MethodsAnimalsForty-seven dogs presented at the Veterinary Teaching Hospital (VTH) “M. Modenato” of the University of Pisa from January 2016 to March 2017, were enrolled in the study. At presentation to our VTH, parasitological examination for intestinal parasites was performed by using the same methods reported below. All dogs were treated then with fenbendazole (50 mg/kg once daily, for five consecutive days) before to be enrolled in the study even if there were negative for parasites. All 47 dogs had a history of recurrent gastrointestinal signs and were diagnosed with a primary CE before the enrollment in the study, since extra-intestinal or intestinal diseases of other etiology had been excluded by previous diagnostic and therapeutic work-up. More specifically, all dogs underwent a complete blood cell count, a complete biochemical profile, including serum trypsin-like immunoreactivity and an abdominal ultrasound. At the time of inclusion in the study, when the diagnosis of CE was made, a fresh fecal sample was newly obtained for the parasitological and molecular analysis. Written informed consent was obtained from all the owners of dogs enrolled in this study. Clinical analysisFor each dog enrolled in the study, the frequency of defecation (normal ≤ 3 times/day) and fecal abnormalities, including the presence of blood and/or mucus, were evaluated. In addition, the use of a fecal consistency scoring system (Purina Prolan Veterinary Diets. Fecal Scoring Chart. https://www.proplanveterinarydiets.ca/wp-content/uploads/2018/05/180107_PPPVD-Fecal-Scoring-Chart-UPDATE-EN-FINAL.pdf) allowed us to assign to each dog fecal sample a different score on a scale from 1 to 7. A clinical index of CE severity was also assessed based on the clinical chronic enteropathy activity index (CCECAI) (Allenspach et al., 2007; 2016). Moreover, the type of enteropathy was retrospectively classified as FRE, ARE, or IRE. More specifically, FRE diagnosis was based on a positive response to a change of diet using an elimination diet. ARE was diagnosed when a positive clinical response to antibiotic treatment (tylosin 10 mg/kg/bid) was observed, intestinal signs reoccurred after the discontinuation of the treatment, and no other underlying etiology was identified. Finally, IRE was diagnosed in dogs with persistent or recurrent gastrointestinal signs who had a histopathological evidence of intestinal inflammation and all the possible causes of this condition were excluded (Hall and Day, 2017). Based on serum albumin (Alb) concentrations, CE was further classified as protein-losing enteropathy (PLE; Alb < 2.0 g/dl) or non-protein-losing enteropathy. Parasitological and molecular analysisFor parasitological analysis, individual fresh fecal samples collected from each dog were examined within 24 hours by fresh and Lugol stained fecal smears and by flotation test with a low-density solution (33% ZnSO4 solution, specific gravity 1.18). A commercial rapid immunoassay for the search of G. duodenalis and Cryptosporidium spp. fecal antigens (RIDA QUICK® Cryptosporidium/Giardia Combi, R-Biopharm, Darmstadt, Germany) was also used. Positivity for G. duodenalis at least to one of these methods was assumed to indicate the positivity of examined samples. For molecular analysis, G. duodenalis-positive samples were processed by a commercial kit (QIAamp DNA Stool Mini Kit, QIAGEN, Valencia, CA) for DNA extraction. A nested PCR protocol was applied to amplify a fragment of the small subunit ribosomal RNA gene (Read et al., 2002). Amplification products were run on 2% ethidium bromide agarose gel and visualized under ultraviolet light. Positive amplicons were purified using mi-PCR Purification Kit, Metabion International AG. Amplification products were sent to an external laboratory for sequencing (Bio-Fab Research, Rome, Italy); sequence multiple alignment was carried out by ClustalW to identify G. duodenalis assemblages. After performing all clinical and parasitological evaluations, all dogs that in the present study scored positive for endoparasites were treated with previously recommended antiparasitic protocols. Statistical analysisFor all parameters considered in this study, i.e. age and sex, CCECAI, fecal score index (FSI), frequency of defecation (FD), and type of enteropathy, including PLE, ARE, FRE, and IRE, possible statistical differences between G. duodenalis positive and G. duodenalis negative dogs were evaluated. Data analysis was performed using the statistical software GraphPad Prism 7 and data were analyzed by the Chi square test and the Fischer’s exact test. The significance level was set at p < 0.05. Ethical approvalIn this study, only fecal samples were collected from dogs with primary chronic entheropathy, thus a formal ethics approval was not applicable. ResultsAnimals included in this study were 20 females and 27 male dogs, ranging from 5 months to 14 years of age. Among the 47 dogs included in the study, 22/47 dogs (46.8%) were found affected by ARE, 18/47 dogs (38.3%) by FRE and 7/47 dogs (14.9%) by IRE. Moreover, PLE was diagnosed in 11/47 (23.4%) examined dogs. At parasitological analysis, 16 out of the 47 examined dogs (34 %) were found positive for G. duodenalis. More specifically, all 16 dogs were positive at the immunoassay, while 12 and 14 dog fecal samples scored positive for G. duodenalis at the fresh fecal smear and at the remaining tests performed in the study, respectively. The mean age of the G. duodenalis positive group was 5.3 years, with a median of 3 years (6 months–14 years), and only three subjects were younger than one year. In the G. duodenalis negative group, the mean age was 4.8 years with a median of 4 years (5 months–13 years). Table 1. Comparison of clinical aspects in Giardia duodenalis positive and negative dogs affected by different type of chronic enteropathy.
Due to the small amount of fecal material available, PCR was performed only on 9/16 dog samples found positive for G. duodenalis at parasitological examination, while only for 6/9 positive dogs it was possible to identify also G. duodenalis assemblage. G. duodenalis assemblage D was identified in five of them and assemblage C in only one. From the evaluation of possible differences between G. duodenalis positive and G. duodenalis negative dogs according to the FSI, a higher frequency of fecal consistency alteration was observed in the G. duodenalis negative dog group, although no significant differences emerged at statistical analysis (Table 1). Indeed, fecal consistency was greatly reduced (FSI 6 or 7) in the 77.4% (24/31) of G. duodenalis negative dogs and in the 44% (7/16) of the G. duodenalis positive dogs. However, significant differences (p=0.037) emerged at statistical analysis between G. duodenalis positive and G. duodenalis negative dogs according to the CCECAI index, since most of Giardia-negative dogs had a moderate/severe CCECAI score (20/31, 64.5%), while among Giardia-positive dogs the same index was mainly low/negligible (11/16, 68.8%) (Table 1). No statistical difference was instead found between the two groups of dogs according to the types of enteropathies (PLE, FRE, ARE, and IRE) (Table 1) although a higher frequency of PLE was observed among G. duodenalis negative dogs (9/31, 29%) in respect to that observed among G. duodenalis positive dogs (2/16, 12.5%). DiscussionTo date, this is the first study evaluating G. duodenalis in a selected population of dogs with a diagnosis of primary CE. Our findings are taken from a population which underwent on a fenbenzadole treatment prior the inclusion. The prevalence of G. duodenalis in primary CE found in the present study (34%) is much higher than data reported in recent studies in symptomatic dogs in Europe (Epe et al., 2010; Volkmann et al., 2017). Furthermore, it is higher than prevalence of G. duodenalis found in different dog populations in Italy (Pipia et al., 2014; Zanzani et al., 2014; Paoletti et al., 2015; De Liberato et al., 2018), including privately owned (about 4%) and kennel (about 5%) dogs of the same area (Riggio et al., 2013; Sauda et al., 2018). In our opinion, this high prevalence which we found it is unlikely to be associated to a primary G. duodenalis infection. Although the reported efficacy of fenbenzadole treatment is very high (Barr et al., 1994; 0 et al., 1998), a fenbendazole resistance may occur in dogs. Another possible explanation is that the risk of G. duodenalis infection seems to increase with high frequency of anthelmintic treatment. This may be due to the change of the intestinal niche caused by the anthelmintic therapy on major parasites (Bugg et al., 1999). However, it is not possible to exclude that the positive dogs may be newly infected due to incomplete animal and/or environmental disinfection (Raza et al., 2018). Finally, in our opinion, this result may be related to the dog population here considered because the intestinal alterations caused by chronic inflammation may predispose CE- affected dogs to the acquisition of G. duodenalis infections. However, it is unlikely that G. duodenalis may have a significant role in the gastrointestinal signs in our dogs. Indeed, from the evaluation and scoring of clinical parameters, the presence of G. duodenalis was not associated with more severe clinical forms. On the contrary, the CCECAI score was indicative of significantly more severe clinical forms in G. duodenalis negative group. In addition, FSI and the presence of mucus or blood in stools were not statistically associated with G. duodenalis infection. No significant differences emerged also between G. duodenalis-positive and G. duodenalis-negative dogs according to the type of enteropathies (e.g., ARE, FRE, IRE, and PLE). Giardia duodenalis infections can be asymptomatic, subclinical, and clinically evident forms may occur especially when other factors are also present, such as concurrent entero-pathogenic bacteria or parasites, food intolerance or decreased host defense ability and stress conditions (Ballweber et al., 2010; Tysnes et al., 2014). Interestingly, all clinical parameters used in this study to score the severity of signs were indicative of less severe clinical forms in the group of G. duodenalis-infected dogs, especially the CCECAI score that gave statistically significant results. To the best of our knowledge, no previous studies specifically investigated the prevalence of G. duodenalis in canine primary CE; its possible associations with the type of enteropathy and the severity of clinical presentation. In fact, only the stool consistence and the presence of diarrhea were previously evaluated in dogs infected by G. duodenalis (Epe et al., 2010; Upjohn et al., 2010; Pipia et al., 2014; Volkmann et al., 2017). However, it is also possible that some dogs considered negative in this study were instead positive, since only a single fecal sample per dog was examined. In fact, due to the inconstant shedding of the cysts into the feces, for the detection of G. duodenalis it is advisable to examine three samples collected in three non-consecutive days, while the examination of a single fecal sample has a lower sensitivity (Tangtrongsup and Scorza, 2010). In this study, only the canine assemblages C and D were identified at genotyping of some dog fecal samples found positive for G. duodenalis at molecular analysis. These data seem to confirm the higher frequency of these canine-specific assemblages in privately owned dogs in Italy (Pipia et al., 2014; Paoletti et al., 2015; Simonato et al., 2017; Sauda et al., 2018). Nevertheless, it is not possible to exclude the presence of the zoonotic assemblages A and B in the remaining positive dog samples for which molecular analysis and genotyping were not possible. Indeed, the assemblages A and B have been also identified in owned dogs from Italy (Riggio et al., 2013; Zanzani et al., 2014; Simonato et al., 2017) and a high possibility of transmission of these zoonotic assemblages between dogs and humans has been supposed (Marangi et al., 2010; Feng and Xiao, 2011). We acknowledge that this study has several limitations. First, the number of dogs examined in this study was low and follow-up for clinical conditions was not performed. Secondly, at inclusion in the study only a single fecal sample per dog to assess positivity or negativity for G. duodenalis was possible to analyze. In conclusion, G. duodenalis may be present in primary CE-affected dogs and further investigations are needed to clarify the real significance of less severe clinical presentation observed in G. duodenalis-positive and CE-affected dogs and a possible higher diffusion of G. duodenalis in CE dog patients. Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionStefania Perrucci and Veronica Marchetti conceived and designed the study. All authors contributed to perform research, interpreting the results, writing and critically revising the manuscript. All authors approved the final version of the manuscript. ReferencesAllenspach, K., Culverwell, C. and Chan, D. 2016. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet. Rec. 178, 368. Allenspach, K., Wieland, B., Gröne, A. and Gaschen, F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Int. Med. 21, 700–708. Ballweber, L.R., Xiao, L., Bowman, D.D., Kahn, G. and Cama, V.A. 2010. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 26, 180–189. Barr, S.C., Bowman, D.D. and Heller, R.L. 1994. Efficacy of fenbendazole against giardiasis in dogs. Am. J. Vet. Res. 55(7), 988–990. Bouzid, M., Halai, K., Jeffreys, D. and Hunter, P.R. 2014. The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Vet. Parasitol. 207, 181–202. Bugg, R.J., Robertson, I.D., Elliot, A.D. and Thompson, R.C. 1999. Gastrointestinal parasites of urban dogs in Perth, Western Australia. Vet. J. 157, 295–301. Dandrieux, J.R.S. 2016. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J. Small. Anim. Pract. 57, 589–599. De Liberato, C., Berrilli, F., Odorizi, L., Scarcella, R., Barni, M., Amoruso, C., Scarito, A., Filippo, M.M.D., Carvelli, A., Iacoponi, F. and Scaramozzino, P. 2018. Parasites in stray dogs from Italy: prevalence, risk factors and management concerns. Acta. Parasitol. 63, 27–32. Epe, C., Rehkter, G., Schnieder, T., Lorentzen, L. and Kreienbrock, L. 2010. Giardia in symptomatic dogs and cats in Europe — Results of a European study. Vet. Parasitol. 173, 32–38. Feng, Y. and Xiao, L. 2011. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24, 110–140. Hall, E.J. and Day, M.J. 2017. Diseases of the small intestine. In Textbook of veterinary internal medicine: diseases of the dog and the cat, Eds., Ettinger, S., Feldman, E., Coté E. St. Louis, MO:Elsevier Saunders, pp: 3643–3820. Hawrelak, J. 2003. Giardiasis: pathophysiology and management. Alternat. Med. Rev. 8, 129–142. Huber, F., Bomfim, T.C.B. and Gomes, R.S. 2005. Comparison between natural infection by Cryptosporidium sp., Giardia sp. in dogs in two living situations in the West Zone of the municipality of Rio de Janeiro. Vet. Parasitol. 130, 69–72. Manko, A., Motta, J.P., Cotton, J.A., Feener, T., Oyeyemi, A., Vallance, B.A., Wallace, J.L. and Buret, A.G. 2017. Giardia co-infection promotes the secretion of antimicrobial peptides beta-defensin 2 and trefoil factor 3 and attenuates attaching and effacing bacteria-induced intestinal disease. PLoS One. 12, (6):e0178647. Marangi, M., Berrilli, F., Otranto, D. and Giangaspero, A. 2010. Genotyping of Giardia duodenalis among children and dogs in a closed socially deprived community from Italy. Zoonoses Public Health 57(7-8), e54–58. Monis, P.T., Cacciò, S.M. and Thompson, R.C.A. 2009. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol. 26, 93–100. Paoletti, B., Traversa, D., Iorio, R., De Berardinis, A., Bartolini, R., Salini, R. and Di Cesare, A. 2015. Zoonotic parasites in feces and fur of stray and private dogs from Italy. Parasitol. Res. 114, 2135–2141. Pipia, A.P., Varcasia, A., Tamponi, C., Sanna, G., Soda, M., Paoletti, B., Traversa, D. and Scala, A. 2014. Canine giardiosis in Sardinia Island, Italy: Prevalence, molecular characterization, and risk factors. J. Infect. Dev. Ctries. 8, 655–660. Raza, A., Rand, J., Qamar, A. G., Jabbar, A. and Kopp, S. 2018. Gastrointestinal Parasites in Shelter Dogs: Occurrence, Pathology, Treatment and Risk to Shelter Workers. Animals (Basel). 8(7), E108. Read, C., Walters, J., Robertson, I.D. and Thompson, R.C.A. 2002. Correlation between genotype of Giardia duodenalis and diarrhea. Int. J. Parasitol. 32, 229–231. Riggio, F., Mannella, R., Ariti, G. and Perrucci, S. 2013. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 193, 78–84. Roxstrom-Lindquist, K., Palm, D., Reiner, D., Ringqvist, E. and Svard, S.G. 2006. Giardia immunity—an update. Trends Parasitol. 22, 26–31. Ryan, U. and Cacciò, S.M. 2013. Zoonotic potential of Giardia. Int. J. Parasitol. 43, 943–956. Sauda, F., Malandrucco, L., Macrì, G., Scarpulla, M., De Liberato, C., Terracciano, G., Fichi, G., Berrilli, F. and Perrucci, S. 2018. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite 25(2). doi:10.1051/parasite/2018001. Simonato, G., Frangipane di Regalbono, A., Cassini, R., Traversa, D., Tessarin, C., Di Cesare, A. and Pietrobelli, M. 2017. Molecular detection of Giardia duodenalis and Cryptosporidium spp. in canine faecal samples contaminating public areas in Northern Italy. Parasitol. Res. 116, 3411–3418. Sommer, M.F., Rupp, P., Pietsch, M., Kaspar, A. and Beelitz, P. 2018. Giardia in a selected population of dogs and cats in Germany – diagnostics, coinfections and assemblages. Vet. Parasitol. 249, 49–56. Tangtrongsup, S. and Scorza, V. 2010. Update on the diagnosis and management of Giardia spp. infections in dogs and cats. Top. Comp. Anim. Med. 25, 155–162. Tysnes, K.R., Skancke, E. and Robertson, L.J. 2014. Subclinical Giardia in dogs: a veterinary conundrum relevant to human infection. Trends Parasitol. 30, 520–527. Upjohn, M., Cobb, C., Monger, J., Geurden, T., Claerebout, E. and Fox, M. 2010. Prevalence, molecular typing and risk factor analysis for Giardia duodenalis infections in dogs in a central London rescue shelter. Vet. Parasitol. 172, 341–346. Volkmann, M., Steiner, J.M., Fosgate, G.T., Zentek, J., Hartmann, S. and Kohn, B. 2017. Chronic diarrhea in dogs – retrospective study in 136 cases. J. Vet. Int. Med. 31, 1043–1055. Zajac, A.M., LaBranche, T.P., Donoghue, A.R. and Chu, T.C. 1998. Efficacy of fenbendazole in the treatment of experimental Giardia infection in dogs. Am. J. Vet. Res. 59(1), 61–63. Zanzani, S.A., Gazzonis, A.L., Scarpa, P., Berrilli, F. and Manfredi, M.T. 2014. Intestinal parasites of owned dogs and cats from metropolitan and micropolitan areas: prevalence, zoonotic risks, and pet owner awareness in Northern Italy. BioMed. Res. Int. 2014, ID 696508. | ||
| How to Cite this Article |
| Pubmed Style Perrucci S, Berrilli F, Procopio C, Filippo MMD, Pierini A, Marchetti V. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet J. 2020; 10(1): 74-79. doi:10.4314/ovj.v10i1.12 Web Style Perrucci S, Berrilli F, Procopio C, Filippo MMD, Pierini A, Marchetti V. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. https://www.openveterinaryjournal.com/?mno=62961 [Access: July 07, 2025]. doi:10.4314/ovj.v10i1.12 AMA (American Medical Association) Style Perrucci S, Berrilli F, Procopio C, Filippo MMD, Pierini A, Marchetti V. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet J. 2020; 10(1): 74-79. doi:10.4314/ovj.v10i1.12 Vancouver/ICMJE Style Perrucci S, Berrilli F, Procopio C, Filippo MMD, Pierini A, Marchetti V. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet J. (2020), [cited July 07, 2025]; 10(1): 74-79. doi:10.4314/ovj.v10i1.12 Harvard Style Perrucci, S., Berrilli, . F., Procopio, . C., Filippo, . M. M. D., Pierini, . A. & Marchetti, . V. (2020) Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet J, 10 (1), 74-79. doi:10.4314/ovj.v10i1.12 Turabian Style Perrucci, Stefani, Federica Berrilli, Cristina Procopio, Margherita Montalbano Di Filippo, Alessio Pierini, and Veronica Marchetti. 2020. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Veterinary Journal, 10 (1), 74-79. doi:10.4314/ovj.v10i1.12 Chicago Style Perrucci, Stefani, Federica Berrilli, Cristina Procopio, Margherita Montalbano Di Filippo, Alessio Pierini, and Veronica Marchetti. "Giardia duodenalis infection in dogs affected by primary chronic enteropathy." Open Veterinary Journal 10 (2020), 74-79. doi:10.4314/ovj.v10i1.12 MLA (The Modern Language Association) Style Perrucci, Stefani, Federica Berrilli, Cristina Procopio, Margherita Montalbano Di Filippo, Alessio Pierini, and Veronica Marchetti. "Giardia duodenalis infection in dogs affected by primary chronic enteropathy." Open Veterinary Journal 10.1 (2020), 74-79. Print. doi:10.4314/ovj.v10i1.12 APA (American Psychological Association) Style Perrucci, S., Berrilli, . F., Procopio, . C., Filippo, . M. M. D., Pierini, . A. & Marchetti, . V. (2020) Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Veterinary Journal, 10 (1), 74-79. doi:10.4314/ovj.v10i1.12 |