| Review Article | ||
Open Vet J. 2021; 11(2): 203-209 Open Veterinary Journal, (2021), Vol. 11(2): 203-209 Review Article The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decadesCurtis Wells Dewey1,2* and Huisheng Xie21Elemental Pet Vets, PLLC, Freeville NY, USA 2Chi University, 9650 West Highway 318, Reddick, FL 32686, USA *Corresponding Author: Curtis Wells Dewey. Elemental Pet Vets, PLLC, 1610 Dryden Road, Freeville, NY 13068, USA. Email: elementalpetvets [at] outlook.com Submitted: 27/01/2021 Accepted: 03/04/2021 Published: 15/04/2021 © 2021 Open Veterinary Journal
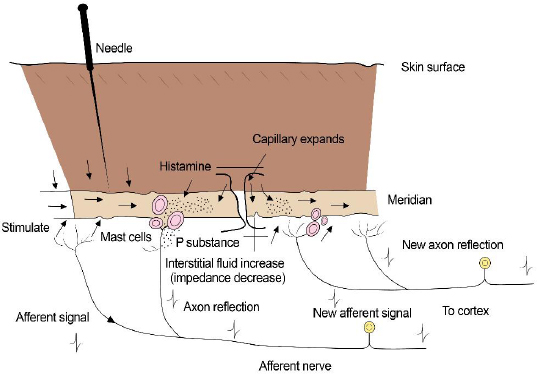
AbstractThe practice of acupuncture is becoming increasingly popular in veterinary medicine, especially as a method of providing pain relief. Originally based on principles derived from centuries of observation, conventional scientific mechanisms of action for acupuncture as a pain-relieving modality have recently been elucidated. Acupuncture points allow access to multiple regions of the body via the peripheral nervous system and its connection with the central nervous system. Local, segmental (spinal), and suprasegmental (brain) effects of acupuncture involve enhanced release of pain-relieving endogenous substances (e.g., opioids) and mitigated release of pain-inducing substances (e.g., inflammatory cytokines). In addition, there is evidence that acupuncture can induce positive neurochemical and cytoarchitectural change in the central nervous system via the phenomenon of neuroplasticity. Electroacupuncture is considered the most effective type of acupuncture delivery, allowing for more potent and long-lasting pain relief than is achieved via other methods (e.g., dry needling). The purpose of this review article is to summarize the relevant scientific literature from the last two decades relating to the physiological mechanisms of action of acupuncture as a pain-relieving modality. Keywords: Animal, Acupuncture, Electroacupuncture, Pain, Veterinary medicine. IntroductionThe appeal of acupuncture as a pain-relieving therapy is expanding in veterinary medicine. The practice of acupuncture in veterinary medicine is thousands of years old (Xie and Chrisman, 2009); however, recent experimental and clinical studies have shown both specific biological effects and measurable clinical benefits of acupuncture. Many of these studies have been carried out in laboratory animals and humans with painful conditions, but much of the knowledge from this literature can be applied to veterinary practice (Soligo et al., 2013; Cheng, 2014; Chen et al., 2014a,b; Fry et al., 2014; Zhou and Benharash, 2014; He et al., 2015; Kim et al., 2020). In addition to these investigations, some of the clinical studies demonstrating acupuncture efficacy for pain relief have been conducted in companion animal species (Xie et al., 2001, 2005, 2009; Skarda and Muir, 2003; Rungsri et al., 2009; de Sousa et al., 2012; Chomsiriwat and Ma, 2019). One of the barriers to integrating acupuncture into modern practice is a commonly held perception that it is based on archaic principles, rather than accepted (Western or contemporary) medical science. The individuals who developed acupuncture thousands of years ago did not have the tools at the disposal of contemporary clinicians to ascertain the true structural and functional properties of their patients. The medical system that they envisioned – the system upon which acupuncture was originally implemented – was based upon centuries of observations. It was also based upon logical conclusions that were derived from those observations. In many cases, the metaphorical terminology of traditional Chinese veterinary medicine can be effectively “translated” into conventional medical terms (Soligo et al., 2013; Xie and Preast, 2013; Cheng, 2014; Zhou and Benharash, 2014). Acupuncture includes manual acupuncture or “dry needling” (DN), aquapuncture, moxibustion, and electroacupuncture (EA). Aquapuncture and moxibustion may have a greater therapeutic effect than DN. Presumptive mechanisms for aquapuncture’s efficacy include enhanced stimulation of the injected acupuncture point via spatial distortion of the injected substance and pharmacologic effects of the injected substance. Moxibustion entails heating the acupuncture needles with a burning stick of dried mugwort plant (Artemisia vulgaris); heating the acupuncture needles is thought to induce a greater stimulation to the acupuncture point than would be achieved with DN alone. It is generally accepted that the clinical effects of EA are more potent and longer lasting than DN, aquapuncture, or moxibustion (Xie et al., 2009; Soligo et al., 2013; Xing et al., 2013; Chen et al., 2014a; Fry et al., 2014; Zhou and Benharash, 2014; Lu et al., 2015). Acupuncture points and meridiansRecent expansion of knowledge pertaining to the scientific basis of acupuncture is due mainly to advances in neuroimaging and molecular biology techniques. An acupuncture point can be envisioned as an anatomic unit that comprises free nerve endings, small arterioles, venules, lymphatics, and mast cells (Fig. 1). Acupuncture points may be larger than “points” and more accurately described as receptive regions or fields in the vicinity of the “point”. Acupuncture points are usually found physically in areas of surface depressions and physiologically in cutaneous regions typified by low electrical impedance and high electrical conductivity (Fry et al., 2014; Zhou and Benharash, 2014; Zhang et al., 2014; Pei et al., 2016; Lim et al., 2018; Kim et al., 2020). A high concentration of gap junctions has been demonstrated in cellular components of acupuncture points, which may partially explain the enhanced conductivity of bioelectrical signals in these regions. Nitric oxide (NO), a prevalent biological signaling molecule, has been shown to enhance conduction of electrical signals across gap junctions (Sheng-Xing, 2017). Subepidermal nerve fibers and dermal connective tissue cells associated with acupuncture points versus non-acupuncture points have been shown to have increased levels of neuronal NO synthase (nNOS) levels and higher concentrations of transient receptor potential vanilloid type-1 (TRPV-1) receptors (Sheng-Xing, 2017). TRPV-1 receptors are cation channels known to be integral to pain transmission. These receptors are particularly prevalent in small diameter C fibers, which are important for pain conduction from the periphery to the central nervous system (CNS). Experimentally, the expression of both nNOS and TRPV-1 at acupuncture sites is enhanced by electroacupuncture, suggesting that NO and TRPV-1 receptors play an important role in neurotransmission during acupuncture (Chen et al., 2014b; Theysohn et al., 2014; Sheng-Xing, 2017; Yen et al., 2019). Subcutaneous mast cells in acupuncture points contain adenosine triphosphate (ATP) which is released into local tissues upon acupuncture stimulation. Both ATP and its metabolite, adenosine, serve as important neurotransmitters in pain modulation peripherally and centrally (Takano et al., 2012; He et al., 2020). Most acupuncture points and their respective meridians are aligned with the peripheral nervous system (PNS) (Fig. 2). Others correspond to known fascial planes of the body. Some acupuncturists believe that acupuncture signals may also follow vascular routes. It is possible that signals are transmitted through a variety of anatomic scaffolds, but it is likely that the nerves play a prominent role in all the scenarios described (Zhao, 2008; Xing et al., 2013; Cheng, 2014; Chen et al., 2014a, 2015; Ding et al., 2014; Zhang et al, 2014; Lim et al., 2018). In general, the entire body – including the CNS and the viscera – is accessible via the PNS. Classification of acupuncture points is also based upon the location of nerves associated with those points. Type I or motor points are located where major nerves penetrate muscles. These are most common and comprise nearly 70% of all acupuncture points. These points are considered among the most effective points for achieving a positive clinical effect. Type II points are located where nerves intersect on the dorsal and ventral midlines. Type III acupuncture points are located where superficial nerves branch. Type IV acupuncture points are located where nerves penetrate tendons (e.g., Golgi tendon organs) (Gunn et al., 1976; Hwang, 1992). Experimental studies in rats have demonstrated that noxious sensory stimulation from visceral organs produce foci of cutaneous neurogenic inflammation in the dermatomes that overlap the areas of visceral stimulation; these neurogenic inflammatory spots correspond to traditional acupuncture points (Kim et al., 2017). In addition, stimulation of these spots produces visceral effects, lending further credence to that they represent specific acupuncture points (Kim et al., 2017).
Fig. 1. Schematic illustration depicting the anatomic components of an acupuncture point.
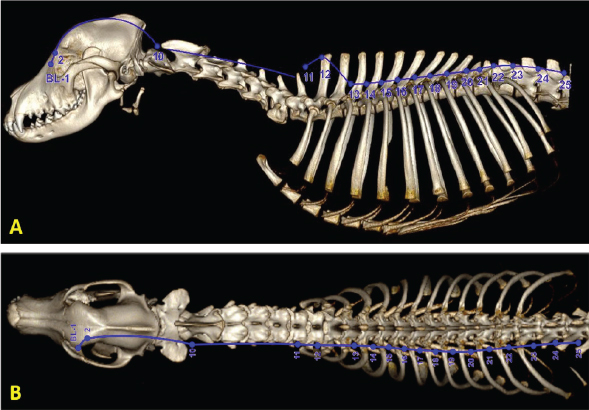
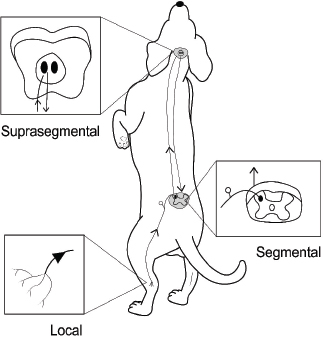
Fig. 2. Three-dimensional computed tomographic (CT) reconstruction showing the individual points and trajectory of the bladder meridian from lateral (A) and dorsal (B) views. Note how this meridian is aligned with the sympathetic chain. (CT image courtesy of Fred Wininger VMD, DACVIM-Neurology.) The analgesic effects of acupuncture can be conceptually divided into local, segmental (spinal), and suprasegmental (brain) effects (Fig. 3). Each of these divisions reflects a component of the nociceptive pathway. Local effects involve sensory afferents of tissues being stimulated (with neuronal cell bodies located in dorsal root ganglia). Segmental effects are associated with neurons located in the dorsal gray column of the spinal cord segments. These neurons provide ascending nociceptive tracts to the brain after being stimulated by sensory afferents. Suprasegmental (brain) effects correspond to a descending pain-mitigating system arising from several areas in the brain. These brain regions – when stimulated – send signals to the dorsal gray column neurons of the spinal cord, inhibiting their ability to provide ascending pain signals to the brain (Quandt and Dewey, 2019). Local effects of acupunctureAcupuncture is considered a method of counterirritation that provides sensory stimulation to local tissues. After providing a stimulus to the tissue with a needle, a local reaction is instigated that elicits several anti-inflammatory and immune responses. Acupuncture induces several physiological effects peripherally which contribute to pain relief. Many of these processes involve endogenous opioids (Zhao, 2008; Soligo et al., 2013; Xing et al., 2013; Cheng, 2014; Chen et al., 2014b; Fry et al., 2014; Zhang et al., 2014; Lim et al., 2018; Fan et al., 2019). Acupuncture induces local release of endogenous opioids from lymphocytes, macrophages, and granulocytes into tissue. These opioids then suppress the propagation of nociceptive signals by acting at receptors of the peripheral nerves in the tissue (Zhang et al., 2014; McDonald et al., 2015). Peripheral sympathetic nerve fibers are also activated, leading to increased levels of endogenous opioids in the region. Some of this effect is via adrenergic receptor activation of inflammatory cells, which causes them to release β endorphin into the tissue. Sympathetic nerve fiber activation also leads to increased expression of specific intracellular adhesion molecules in blood vessels of inflamed tissue. These adhesion molecules promote migration of neutrophils and mononuclear cells that contain β endorphin and met-enkephalin (Zhang et al., 2014). Acupuncture stimulation causes increased levels of cannabinoid CB2 receptors in tissue, which leads to upregulation of endogenous local opioids (Zhang et al., 2014). Acupuncture has the effect of decreasing levels of local inflammatory cytokines, including tumor necrosis factor-α (TNFα), interleukin 1β (IL-1β), and interleukin 6 (IL-6). This is also believed to involve endogenous opioids (Chen et al., 2014). Another local effect of acupuncture is the inhibition of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) production in the region being stimulated (Zhang et al., 2014; Yen et al., 2019). Elevated tissue levels of nerve growth factor (NGF-a neurotrophin) have been associated with local inflammation; electroacupuncture has been shown to decrease NGF levels in inflamed tissue (Soligo et al., 2013). Acupuncture stimulation leads to upregulation of TRPV-1 receptors in peripheral nerve endings, which has been associated with an analgesic effect (Sheng-Xing, 2017; Yen et al., 2019). Local tissue release of purine neurotransmitters ATP and adenosine via acupuncture stimulation has both direct and indirect analgesic effects (McDonald et al., 2015; Fan et al., 2017; Lim et al., 2018; Yen et al., 2019; He et al., 2020).
Fig. 3. Schematic illustration showing the three locations of acupuncture’s physiologic effects: local, segmental, and suprasegmental. Segmental (spinal) effects of acupunctureSegmental (spinal) and suprasegmental (brain) effects of acupuncture cannot be completely isolated into separate categories, as they are anatomically and physiologically interconnected. However, there are many anti-nociceptive effects induced by acupuncture (again, particularly EA) at the spinal level (Zhao, 2008; Chen et al., 2014b; Zhang et al, 2014; He et al., 2015; Fan et al, 2019). Acupuncture causes dampening of n methyl d aspartate (NMDA) receptor activity in spinal cord dorsal horn neurons via increasing endogenous opioids, noradrenaline, and serotonin levels (Fry et al., 2014; Zhang et al, 2014). Acupuncture stimulation leads to increased serotonin levels in the spinal cord which accentuates the ability of gamma aminobutyric acid (GABA) to inhibit pain signal transmission (Chen et al., 2014b; Zhang et al, 2014). Spinal glial cell activation is reduced by acupuncture stimulation, leading to decreased release of astrocyte and microglial-derived pain-promoting cytokines (IL-1β, IL-6, TNFα, COX-2, and PGE2) (Liang et al., 2016; Lin et al., 2016). Acupuncture also inhibits substance P release in spinal cord gray matter (substance P promotes nociceptive signals and activates glial cells) (McDonald et al., 2015). In addition to decreasing substance P and calcitonin gene-related peptide (CGRP, another major pain-provoking neuropeptide) release at dorsal root ganglion neuron synapses with spinal dorsal horn neurons, electroacupuncture facilitates GABA release at these synaptic connections (Lundeberg and Lund, 2016; Qiao et al., 2017). Acupuncture has been shown to cause upregulation of Nociceptin/Orphanin FQ (N/OFQ) receptors in the spinal cord gray matter. N/OFQ is a powerful opioid-related peptide that has potent analgesic properties and is widely distributed throughout the spinal cord. It inhibits C fiber-evoked responses and wind-up (Fu et al., 2006; Zhang et al., 2014). Increased spinal cord dorsal horn levels of acetylcholine and dopamine are achieved with acupuncture stimulation, both of which are believed to inhibit nociceptive signaling (Zhao, 2008). Acupuncture leads to activation of spinal cord δ opioid receptors, leading to reduced cellular GABA reuptake. Increased levels of GABA in the extracellular space enhance its anti-nociceptive effects (Zhao, 2008). Like its action in the periphery, acupuncture inhibits COX-2 expression at the spinal cord level (Chen et al., 2014b). In contrast to peripheral nerve endings, acupuncture has been shown to downregulate TRPV-1 expression in spinal cord dorsal horn neurons (McDonald et al., 2015). Suprasegmental (brain) effects of acupunctureThe mechanisms for suprasegmental effects of acupuncture are not as well understood as segmental effects, but they are primarily based on stimulation of a descending anti-nociceptive network that projects from several brain regions to the dorsal horn of spinal cord segments (Chen et al., 2014; Fry et al., 2014; He et al., 2015; Pei et al., 2016). These brain regions include the dorsolateral prefrontal cerebral cortex and cingulate gyrus, the hypothalamus, the periaqueductal gray (PAG) matter and ventral tegmental area of the midbrain, the locus ceruleus of the pons, and the raphe magnus nucleus of the medulla. These various brain regions directly or indirectly suppress nociceptive spinal cord dorsal horn neurons via several chemical messengers (β-endorphins, enkephalins, noradrenaline, dopamine, and serotonin). The pituitary gland is also part of this network and releases adrenocorticotrophic hormone and oxytocin into the bloodstream upon stimulation, both of which have analgesic effects (Quandt and Dewey, 2019). Evidence from functional magnetic resonance imaging and positron emission tomography studies in people, as well as experimental animal models, shows that specific acupuncture points – when stimulated – activate specific regions of the brain. This point specificity for stimulation of anti-nociceptive regions of the brainstem descending pain modulating pathway is most convincing for limb (vs. trunk) acupuncture points (Xing et al., 2013; Cheng, 2014; Theysohn et al., 2014; He et al., 2015; Lu et al., 2015). In addition, activation of such points with a sufficient stimulus will activate the intended brainstem center, without necessarily producing an appreciable segmental effect. On the contrary, stimulating an acupuncture point in the dermatomal segment of interest (e.g., a back Shu point for thoracolumbar pain) may produce a mainly segmental analgesic response, or a segmental and descending brain-stem response, depending on the stimulus intensity (if intense enough, the ascending pathways to brainstem nociceptive centers may also be recruited). In this way, local, segmental, and suprasegmental effects can be combined for maximum results. Some specific examples of suprasegmental effects of acupuncture (primarily EA) include activation of serotonergic nucleus raphe magnus (NRM) neurons in the medulla, activation of noradrenergic neurons in the locus ceruleus of the pons, stimulation of the hypothalamus to release β-endorphin, and activation of descending neurons of the PAG matter of the mesencephalon (Fry et al., 2014; Fan et al., 2017; Quandt and Dewey, 2019). The effects of acupuncture stimulation on TRPV-1 expression in the brain appear to be variable and location-dependent. In one experimental rodent study in which inflammatory pain was alleviated by electroacupuncture, TRPV-1 activity was reduced after electroacupuncture treatment in the prefrontal cortex and hypothalamus but potentiated in the PAG matter of the midbrain (Yen et al., 2019). Acupuncture and central nervous system neuroplasticityNeuroplasticity refers to the ability of the central nervous system to alter both its chemical and structural organization in response to chronic stimulation. Mechanisms involved in neuroplasticity include neurogenesis, axonal sprouting, and alterations in synaptic transmission efficiency (synaptic plasticity). These changes are mediated via neurotransmitters and neurotrophins (NTs), the latter of which comprises a family of proteins crucial to the phenomenon of CNS neuroplasticity. In chronic pain conditions, neuroplasticity is considered maladaptive, as the induced changes in the CNS facilitate pain transmission. There is considerable evidence that electroacupuncture can induce clinically beneficial CNS neuroplasticity, preventing and reversing the maladaptive neurochemical and structural changes caused by chronic pain (Soligo et al., 2013; Fan et al., 2017; Maeda et al., 2017; Wang et al., 2017; Xiao et al., 2018; Kim et al., 2020). In addition to the positive effects mediated by several neurotransmitters and NTs, there is experimental evidence from a rat spinal cord injury model that acupuncture provides neuroprotection via mitigating inflammation and microglial activation (Choi et al., 2010). Some specifics regarding electroacupuncture and DNCurrent scientific literature supports the concept that EA produces more potent and long-lasting analgesic effects compared to DN. It has also been shown that low frequency EA (2–10 Hz) is more effective for pain control than high frequency EA (100 Hz) (Zhang et al., 2014). However, there is some rationale for combining modes of acupuncture (DN and EA) and different frequencies of EA to produce a greater analgesic effect than would be possible with only one modality or one frequency. There is some evidence that DN may be more effective at mediating analgesic effects through C fibers than EA, with EA mediating analgesic effects mainly through Aβ and Aδ fibers. Electroacupuncture has been shown to cause the release of different mediators of analgesia, dependent upon the frequency applied. At low frequencies (2–10 Hz), endorphins and enkephalins are mainly released. At higher frequencies (50–100 Hz), the primary opioid released is dynorphin. At even higher frequencies (>200 Hz), the most prominent neurochemical mediator released is serotonin. In addition, there are other anti-nociceptive agents released, according to certain applied frequencies. For example, at low frequencies, there are also noradrenergic and muscarinic cholinergic mechanisms that are recruited. At high frequencies, there are muscarinic cholinergic and GABA-ergic mechanisms that are recruited to help mitigate nociceptive signaling. Endorphins and enkephalins may be the most effective EA-induced mediators of pain (at low frequency stimulation); however, it is likely that providing other pain-relieving agents at higher frequencies may have an additive effect (Zhao, 2008; Chen et al., 2014b; Liang et al., 2016). ConclusionNumerous molecular mechanisms of action have been discovered in recent years to explain the pain-mitigating effects of acupuncture. Through a variety of cellular messaging systems at the local, segmental (spinal), and suprasegmental (brain) levels, acupuncture exhibits pain-relieving effects, the most potent and long-lasting of which are achieved via electroacupuncture. In addition to this three-tiered system of pain alleviation, acupuncture may also help reverse maladaptive neuroplasticity changes induced by chronic pain states. Through improved understanding of how acupuncture works in a conventional physiological framework, it will continue to grow in popularity as a valuable tool in veterinary pain management. ReferencesChen, C.Y., Lin, C.N., Chern, R.S., Tsai, Y.C., Chang, Y.H. and Chien, C.H. 2014a. Neuronal activity stimulated by liquid substrates injection at zusanli (ST36) acupoint: the possible mechanism of aquapuncture. Evid. Based Complement. Alternat. Med. 2014, 627342; doi:10.1155/2014/627342. Chen, S., Wang, S., Rong P., Wang, S., Qiao L., Feng, X., Liu, J. and Zhang, J. 2014b. Acupuncture for visceral pain: neural substrates and potential mechanisms. Evid. Based Complement. Alternat. Med. 2014, 609594; doi:10.1155/2014/609594. Chen, X.M., Xu, J., Song, J.G., Zheng, B.J. and Wang, X.R. 2015. Electroacupuncture inhibits excessive interferon-γ evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br. J. Anaesth. 114, 150–157. Cheng, K.J. 2014. Neurobiological mechanisms of acupuncture for some common illnesses: a clinician’s perspective. J. Acupunct. Meridian Stud. 7, 105–114. Choi, D.C., Lee, J.Y., Moon, Y.J., Kim, S.W., Oh, T.H. and Yune, T.Y. 2010. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 39, 272–282. Chomsiriwat, P. and Ma, A. 2019. Comparison of the effects of electroacupuncture and laser acupuncture on pain relief and joint range of motion in dogs with coxofemoral degenerative joint disease. Am. J. Trad. Chin. Vet. Med. 14, 11–20. de Sousa, N.R., Luna, S.P.L., de Cápua, M.L.B., da Maia Lima, A., de Oliveira, F.A., de Viveiros, B.M. and Barbosa, L. 2012. Analgesia of preemptive pharmacopuncture with meloxicam or acupuncture in cats undergoing ovariohysterectomy. Ciênc. Rural 42, 1231–1236. Ding, S.S., Hong, S.H., Wang, C., Guo, Y., Wang, Z.K. and Xu, Y. 2014. Acupuncture modulates the neuro-endocrine-immune network. QJM. 107, 341–345. Fan, A.Y., Miller, D.W., Bolash, B., Bauer, M., McDonald, J., Faggert, S., He, H., Li, M.L., Matecki, A., Camardella, L., Koppelman, M.H., Stone, A.M., Meade, L. and Pang, J. 2017. Acupuncture’s role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management-white paper 2017. J. Integr. Med. 15, 411–425. Fan, A.Y, Ouyang, H., Qian, X., Wei, H., Wang, D.D., He, D., Tian, H., Gong, C., Matecki, A. and Alemi, S.F. 2019. Discussions on real-world acupuncture treatments for chronic low-back pain in older adults. J. Integr. Med. 17, 71–76. Fry, L.M., Neary, S.M., Sharrock, J. and Rychel, J.K. 2014. Acupuncture for analgesia in veterinary medicine. Top. Companion Anim. Med. 29, 35–42. Fu, X., Wang, Y.Q. and Wu, G.C. 2006. Involvement of nociception/orphanin FQ and its receptor in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 1078, 212–218. He, J.R., Yu, S.G., Tang, Y. and Illes, P. 2020. Purinergic signaling as a basis of acupuncture-induced analgesia. Purinergic Signal. 16, 297–304. He, T., Zhu, W., Du, S.Q., Yang, J.W., Li, F., Yang, B.F., Shi, G.X. and Liu, C.Z. 2015. Neural mechanisms of acupuncture as revealed by fMRI studies. Auton. Neurosci. 190, 1–9. Kim, D.H., Ryu, Y., Hahm, D.H., Sohm, B.Y., Shim, I., Kwon, O.S., Chang, S., Gwak, Y.S., Kim, M.S., Kim, J.H., Lee, B.H., Jang, E.Y., Zhao, R., Chung, J.M., Yang, C.H. and Kim, H.Y. 2017. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci. Rep. 2017; 79(1):15214; doi:10.1038/s41598-017-14359-z Kim, H., Mawla, I., Lee, J., Gerber, J., Walker, K., Kim, J., Ortiz, A., Chan, S.T., Loggia, M.L., Wasan, A.D., Edwards, R.R., Kong, J., Kaptchuk, T.J, Gollub, R.L., Rosen, B.R. and Napadow, V. 2020. Reduced tactile acuity in chronic low back pain is linked with structural neuroplasticity in primary somatosensory cortex and is modulated by acupuncture therapy. Neuroimage. 217, 116899; doi:10.1016/j.neuroimage.2020.116899 Liang, Y., Qiu, Y., Du, J., Liu, J., Fang, J., Zhu, J. and Fang, J. 2016. Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation. Acupunct. Med. 34, 40–47. Lim, T.K., Ma, Y., Berger, F. and Litscher, G. 2018. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines 5, 1–34. Lin, L., Skakavac, N., Lin, X., Lin, D., Borlongan, M.C., Borlongan, C.V. and Cao, C. 2016. Acupuncture-induced analgesia: the role of microglial inhibition. Cell Transplant. 25, 621–628. Lu, Z., Dong, H., Wang, Q. and Xiong, L. 2015. Perioperative acupuncture modulation: more than anaesthesia. Br. J. Anaesth. 115, 183–193. Lundeberg, T. and Lund, I. 2016. Peripheral components of acupuncture stimulation-their contribution to the specific clinical effects of acupuncture. In: Medical acupuncture: a western scientific approach. Eds., Filshie, J., White, A. and Cummings, M. Philadephia, PA, Elsevier, pp: 22–58. Maeda, Y., Kim, H., Kettner, N., Kim, J., Cina, S., Malatesta, C., Gerber, J., McManus, C., Ong-Sutherland, R., Mezzacappa, P., Libby, A., Mawla, I., Morese, L.R., Kaptchuk, T.J., Audette, J. and Napadow, V. 2017. Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain 140, 914–927. McDonald, J.L., Cripps, A.W. and Smith, P.K. 2015. Mediators, receptors, and signalling pathways in the anti-inflammatory and antihyperalgesic effects of acupuncture. Evid. Based Complement. Alternat. Med. 2015, 975632; doi:10.1155/2015/975632. Pei, P., Liu, L., Zhao, L., Cui, Y., Qu, Z. and Wang, L. 2016. Effect of electroacupuncture pretreatment at GB20 on behavior and the descending pain modulatory system in a rat model of migraine. Acupunct. Med. 34, 127–135. Qiao, L.N., Liu, J.L., Tan, L.H., Yang, H.L., Zhai, X. and Yang, Y.S. 2017. Effect of electroacupuncture on thermal pain threshold and expression of calcitonin-gene related peptide, substance P and ɤ-aminobutyric acid in the cervical dorsal root ganglion of rats with incisional neck pain. Acupunct. Med. 35, 276–283. Quandt, J. and Dewey, C.W. 2019. Pain management and acupuncture. In: Small animal surgery, 5th ed. Eds., Fossum, T.W. Philadelphia, PA: Elsevier, pp: 140–157. Rungsri, P., Trinarong, C., Rojanasthien, S., Xie, H. and Pirunsan, U. 2009. The effectiveness electro-acupuncture on pain threshold in sport horses with back pain. Am. J. Trad. Chin. Vet. Med. 4, 22–26. Sheng-Xing, M.A. 2017. Nitric oxide signaling molecules in acupuncture points: toward mechanisms of acupuncture. Chin. J. Integr. Med. 23, 812–815. Skarda, R.T. and Muir, W.W. 2003. Comparison of electro-acupuncture and butorphanol on respiratory and cardiovascular effects and rectal pain threshold after controlled rectal distention in mares. Am. J. Vet. Res. 6, 137–144. Soligo, M., Nori, S.L., Protto, V., Florenzano, F. and Manni, L. 2013. Acupuncture and neurotrophin modulation. Int. Rev. Neurobiol. 111, 91–124. Takano, T., Chen, X., Luo, F., Fujita, T., Ren, Z., Goldman, N., Zhao, Y., Markman, J.D. and Nedergaard, M. 2012. Traditional acupuncture triggers a local increase in adenosine in human subjects. J. Pain 13, 1215–1223. Theysohn, N., Choi, K.E., Gizewski, E.R., Wen, M., Rampp, T., Gasser, T., Dobos, G.J., Forsting, M. and Musial, F. 2014. Acupuncture-related modulation of pain-associated brain networks during electrical pain stimulation: a functional magnetic resonance imaging study. J. Altern. Complement. Med. 20, 893–900. Wang, X., Ju, S., Chen, S., Gao, W., Ding, J., Wang, G., Cao, H., Tian, H. and Li, X. 2017. Effect of electro-acupuncture on neuroplasticity of spinal cord-transected rats. Med. Sci. Monit. 23, 4241–4251. Xiao, L.Y., Wang, X.R., Ye, Y., Yang, J.W., Cao, Y., Ma, S.M., Li, T.R. and Liu, C.Z. 2018. Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation 21, 762–776. Xie, H. and Chrisman, C. 2009. Equine acupuncture: from ancient art to modern validation. Am. J. Trad. Chin. Vet. Med. 4, 1–4. Xie, H. and Preast, V. 2013. Yin and Yang. In: Traditional Chinese veterinary medicine, 2nd ed. Eds., Xie, H., Preast, V. Reddick, FL: Chi Institute Press, pp: 1–22. Xie, H., Collahan, P.T. and Ott, E.A. 2005. Evaluation of electro-acupuncture treatment of horses with signs of chronic thoracolumbar pain. J. Am. Vet. Med. Assoc. 227, 281–286. Xie, H., Ott, E.A. and Colahan, P. 2009. The effectiveness of electro-acupuncture on experimental lameness in horses. Am. J. Trad. Chin. Vet. Med. 4, 17–29. Xie, H., Ott, E.A., Harkins, J.D., Tobin, T., Colahan, P.T. and Johnson, M. 2001. Influence of electro-acupuncture stimulation on pain threshold in horses and its mode of actions. J. Equine Vet. Sci. 21, 591–600. Xing, J.J., Zeng, B.Y., Li J., Zhuang, Y. and Liang, F.R. 2013. Acupuncture point specificity. Int. Rev. Neurobiol. 111, 49–63. Yen, C.M., Wu, T.C, Hsieh, C.L., Huang, Y.W. and Lin, Y.W. 2019. Distal electroacupuncture at the LI4 acupoint reduces CFA-induced inflammatory pain via the brain TRPV1 signaling pathway. Int. J. Mol. Sci. 20, 1–13. Zhang, R., Lao, L., Ren, K. and Berman, B.M. 2014. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 120, 482–503. Zhao, Z.Q. 2008. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85, 355–375. Zhou, W. and Benharash, P. 2014. Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Meridian Stud. 7, 190–193. | ||
| How to Cite this Article |
| Pubmed Style Dewey CW, Xie H. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Vet J. 2021; 11(2): 203-209. doi:10.5455/OVJ.2021.v11.i2.3 Web Style Dewey CW, Xie H. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. https://www.openveterinaryjournal.com/?mno=49570 [Access: April 26, 2024]. doi:10.5455/OVJ.2021.v11.i2.3 AMA (American Medical Association) Style Dewey CW, Xie H. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Vet J. 2021; 11(2): 203-209. doi:10.5455/OVJ.2021.v11.i2.3 Vancouver/ICMJE Style Dewey CW, Xie H. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Vet J. (2021), [cited April 26, 2024]; 11(2): 203-209. doi:10.5455/OVJ.2021.v11.i2.3 Harvard Style Dewey, C. W. & Xie, . H. (2021) The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Vet J, 11 (2), 203-209. doi:10.5455/OVJ.2021.v11.i2.3 Turabian Style Dewey, Curtis W, and Huisheng Xie. 2021. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Veterinary Journal, 11 (2), 203-209. doi:10.5455/OVJ.2021.v11.i2.3 Chicago Style Dewey, Curtis W, and Huisheng Xie. "The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades." Open Veterinary Journal 11 (2021), 203-209. doi:10.5455/OVJ.2021.v11.i2.3 MLA (The Modern Language Association) Style Dewey, Curtis W, and Huisheng Xie. "The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades." Open Veterinary Journal 11.2 (2021), 203-209. Print. doi:10.5455/OVJ.2021.v11.i2.3 APA (American Psychological Association) Style Dewey, C. W. & Xie, . H. (2021) The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Veterinary Journal, 11 (2), 203-209. doi:10.5455/OVJ.2021.v11.i2.3 |