Open Veterinary Journal, (2021), Vol. 11(3): 401–406
Original Research
10.5455/OVJ.2021.v11.i3.11
Kaliandra honey improves testosterone levels, diameter and epithelial thickness of seminiferous tubule of white rat (Rattus norvegicus) due to malnutrition through stimulation of HSP70
Nefranindy Rahma1, Wurlina Wurlina2, Sri Pantja Madyawati2, Budi Utomo2, Tatik Hernawati2 and Erma Safitri2*
1Practicing Veterinarian,Surabaya, Indonesia
2Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia
*Corresponding Author: Erma Safitri. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: erma-s [at] fkh.unair.ac.id
Submitted: 05/01/2021 Accepted: 29/06/2021 Published: 10/08/2021
© 2021 Open Veterinary Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Abstract
Background: Malnutrition can cause an increase in oxidative stress as it triggers the expression of heat shock protein70 (HSP70), a chaperon molecule that is needed to repair damaged cells within optimal levels. Honey is a source of feed that can stimulate HSP70 expression, which can be given to the malnourished in the animal trial.
Aim: The purpose of this study was to prove that Kaliandra honey can improve testosterone levels, diameter, and epithelial thickness of the seminiferous tubule of rat testes (Rattus norvegicus) due to malnutrition through stimulation of HSP70, which is expressed immunohistochemically.
Methods: This study used 40 male rats, which were divided into four treatment groups: T0 (negative control): normal rats and not given honey; T1 (positive control): malnourished rats and not given honey; T2 (treatment 2): malnourished rats and given 30% Kaliandra honey (v/v) for 10 days; T3 (treatment 3), malnourished rats and given 50% Kaliandra honey (v/v) for 10 days. The condition of malnutrition is carried out by fasting the feed for five consecutive days resulting in damage to the male reproductive organs, especially the testes.
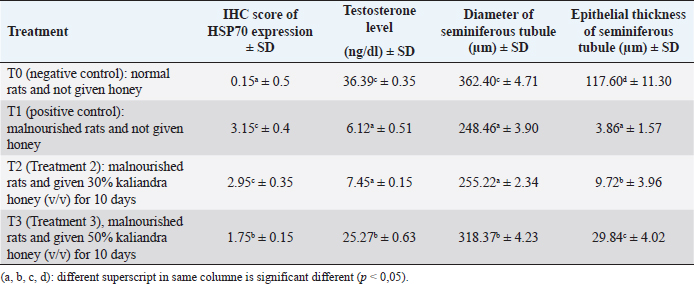
Results: The results showed that Kaliandra honey at a dose of 50% (v/v) had a significant effect in improving testosterone levels, diameter, and epithelial thickness of seminiferous tubule of malnourished male rats through stimulation of HSP70 expression. The HSP70 expression scores by IHC at T0, T1, T2, and T3 were 0.15a ± 0.5, 3.15c ± 0.4, 2.95c ± 0.35, and 1.75b ± 0.15, sequentially. enzyme-linked immunosorbent assay indirect testosterone levels at T0, T1, T2, and T3 (in μg/dl) were 36.39c ± 0.35, 6.12a ± 0.51, 7.45a ± 0.15, 25.27b ± 0.63, sequentially. The diameter and epithelial thickness of the seminiferous tubule of the testes (in μm) in the four treatments T0, T1, T2, and T3 were 362.40c ± 4.71, 248.46a ± 3.90, 255.22a ± 2.34, 318.37b ± 4.23 and 117.60d ± 11.30, 3.86a ± 1.57, 9.72b ± 3.96, 29.84c ± 4.02 sequentially.
Conclusion: The conclusion of the study showed that Kaliandra honey at a dose of 50% (v/v) had a significant effect in improving testosterone levels, diameter, and epithelial thickness of the seminiferous tubule of malnourished rats through stimulation of HSP70, although not significantly the same as negative control (T0).
Keywords: Kalliandra honey, Malnutrition, HSP70, Seminiferous tubule, Testosterone.
Introduction
Reproduction is a process of reproducing a living thing starting from the union of an egg with spermatozoa, thus forming a zygote (Hafez and Hafez, 2010). Internal and external factors influence the success of the reproductive process. Internal factors include organ function and metabolic hormones such as growth hormone and insulin like growth factor, which in turn affect reproductive hormones (Ipsa et al., 2019). One of the important hormones in male reproduction is testosterone, namely, through its role in libido and the spermatogenesis process (Patel et al., 2019). Testosterone also affects the maturation of spermatozoa in the epididymal tract, sexual behavior, and the maintenance and activation of male genital organs (Sullivan and Mieusset, 2016).
External factors include the quality and quantity of spermatozoa, which is influenced by the quality and amount of feed that enters the body (Huetos et al., 2018). Quality feed is food that contains balanced protein, carbohydrates, minerals, and vitamins. An imbalance, such as fasting, will result in malnutrition (Safitri et al., 2016).
Malnutrition can cause an increase in free radicals due to the body's cells experiencing oxidative stress. Oxidative stress in cells can trigger the expression of heat shock protein (HSP), one of which is HSP70 (Sardjito et al., 2018). HSP70 is a chaperon molecule needed to repair damaged cells but with optimal levels (not exceeding the needs of damaged cells). Honey is a source of feed that can stimulate HSP70 expression, which can be given to malnourished mice (Prasetyo and Safitri, 2016). This is because honey contains high level of antioxidants such as flavonoids and phenolic compounds (Pontis et al., 2014). The honey that has high antioxidant potential is Calliandra tree honey (Calliandra callothyrsus). Calliandra tree honey has the highest levels of flavonoids and phenolic compounds compared to other types of monofloral honey such as Rubber tree honey (Hevea brasiliensis) and Kapok tree honey (Ceiba pentandra) (Ustadi et al., 2017).
Based on this background, it is necessary to conduct research and prove that Kaliandra honey can improve testosterone levels, diameter, and thickness of the seminiferous tubule lining of rat testes (Rattus norvegicus) due to malnutrition through stimulation of HSP70 expressed immunohistochemically (IHC).
Materials and Methods
Research place
The research was conducted in the cage for experimental animals, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia. The observation phase of HSP70 expression by IHC and measurement of the diameter and thickness of the epithelial layer by histopathology anatomy of the seminiferous testicular tubules were carried out at the Pathology Laboratory of the Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia. Testosterone levels were measured at the Faculty of Veterinary Medicine Laboratory, Brawijaya University, Malang, Indonesia.
Treatment of experimental animals
40 male rats (R. norvegicus) aged 3 months and weighing 250–300 g were adapted for 7 days, after which they were randomly divided by a simple randomized method. There were four treatments, as follows: T0 (negative control): normal rats and not given honey; T1 (positive control): malnourished rats and not given honey; T2 (treatment 2): malnourished rats and given 30% honey (v/v) (Kaliandra honey, Amanah, Kampung Madu Kediri, East Java, Indonesia) for 10 days; T3 (treatment 3), malnourished rats and given 50% (v/v) honey (Kaliandra honey, Amanah, Kampung Madu Kediri, East Java, Indonesia) for 10 days. The condition of malnutrition is carried out through fasting on feed for 5 days with the result of damage to the male reproductive organs, especially the testes (Safitri et al., 2016).
The honey used in this study was calliandra honey (Kaliandra Honey, Amanah, Kampung Madu Kediri, East Java, Indonesia), obtained from the flower nectar of the Calliandra tree (C. callothyrsus). After treatment, all experimental animals were euthanized by cervical dislocation. Before doing cervical dislocation, blood is drawn first without anti-coagulants, then it is deposited to obtain serum. Furthermore, the testicle is taken by surgery. The obtained testes are then put into a plastic pot containing 10% formalin.
Expression of HSP 70 in testicular tissue by IHC method
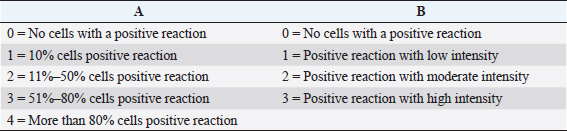
The IHC observation was performed to determine the expression of HSP70. Before going to IHC methods, histological preparation were made by way of an incision, made transversely to obtain testis tissue from paraffin blocks (CAS Number 8002-74-2, Sigma-Aldrich, USA). Further examination was performed through IHC techniques using monoclonal antibodies (H5147-100UL, Sigma-Aldrich, USA). This is done to determine the expression of HSP70. Observations of HSP70 were made using a digital microscope (VHX-7000 series, Keyence, Indonesia) with a magnification of 200 times, and the expression of each variation can be shown by the percentage and intensity of the brownish tissue. Chromogen discoloration at each incision of the tissue was measured by scoring. IHC scoring is semi-quantitative observation, based on cross references between positive percentages of seminiferous tubule (A) with the intensity of color (B) (Safitri et al., 2019). The expression of HSP70 is indicated by the percentages and intensity of tissue with brownish discoloration chromogen in each incision (Table 1).
Measurement of testosterone levels with the enzyme-linked immunosorbent assay (ELISA) indirect method
Before the hormone analysis, the sample was diluted with aquabidestilata (IKA Pharmindo-500 ml, Indonesia) in a ratio of 1:4. Standard solutions of 0.2 ng/ml to 16 ng/ml are prepared. The sample and standard solution prepared were put in each 25 μl duplo into the microplate well (96 well SIAL0596, Sigma-Aldrich, USA). Furthermore, 200 μl of conjugate enzyme solution (Cat. No. 4395/ 4445, Kementec, Denmark) was added to each well and covered with cling film (KF17336, Neuromics, Minnesota), then homogenized by shaking it slowly for 10 seconds. After the incubation, each well was washed with 300 μl of washing solution (Cat No 10010023, ThermoFisher Sci, Singapore) for 3-4 times washing. The next step, included putting 200 μl of substrate solution (Cat. No. 4395/ 4445, Kementec, Denmark) into each well, and then covered with a cling film (KF17336, Neuromics, Minnesota) and incubated for 45 minutes at room temperature. The enzymatic reaction was stopped by adding a 100 μl of stop solution of 0.5 M H2SO4 (Cat No. 34022, ThermoFisher Sci, Singapore) into each well and absorbance was carried out using an ELISA reader (CTK Biotech, Thomas Scientific, Spanyol) at a wavelength of 450 nm (Armansyah et al., 2018).
Table 1. Score of IHC based on multiplication between score positif procentages of seminiferous tubule (A) with intensity of colour (B).
Observation of the epithelial diameter and epithelial thickness of the seminiferous tubule
Observation of the diameter and epithelial thickness of seminiferous tubule of the testis was carried out histopathologically. Observations were made on five testicular slices in histopathological preparations for each replication in one treatment. Each testicular organ slice was randomly selected and then checked under a digital microscope (VHX-7000 series, Keyence, Indonesia).
The testes obtained after washing in a physiological solution (Cat No 10010023, Thermo Fisher Sci, Singapore) were then put into a 10% formalin solution (HT 501128, Merck, Germany) and histopathological preparations were made by staining with hematoxylin eosin (H&E staining) (ab 245885, Abcam, London). The histopathological preparations of the testes were observed under a digital microscope (VHX-7000 series, Keyence, Indonesia) in five different fields of view, with a 200-fold magnification, then measured and recorded the diameter and thickness of the epithelial layer of the seminiferous tubule (Samik et al., 2018).
Statistical analysis
Expressions of HSP70, testosterone levels, diameter and epithelial thickness of seminiferous tubule were statistically analyzed using Statistical Package for the Social Sciences 18 for Windows XP with the level of significance being 0.05 (p=0.05) and the confidence level at 99% (α=0.01). Steps of comparative hypothesis tests are as follows: test data normality with the Kolmogorof–Smirnov test, homogenecity of variance test, analysis of variance factorial, and post-hoc test (least significant difference test) using the Duncan 5%.
Ethical approval
All experimental protocols were reviewed and approved by the Ethical Clearance Commission—Univeristas Airlangga Faculty of Dental Medicine Health Research with number 714/HRECC.FODM/X/2019.
Results
Score IHC of HSP70 in testicular tissue
The data obtained from 40 white male rats (R. norvegicus) with 4 groups and 10 replications are as follows: T0 (negative control): normal rats and not given honey; T1 (positive control): malnourished rats and not given honey; T2 (treatment 2): malnourished rats and given 30% honey (v/v) for 10 days; T3 (treatment 3), malnourished rats and given 50% honey (v/v) for 10 days. The results of this study, in the form of HSP70 expression using immunohistochemical methods. HSP70 is a chaperon molecule needed to repair damaged cells, but at optimal levels (not exceeding the need for cell repair). The HSP70 score for the four treatments can be seen in Figure 1 and Table 2.
Testosterone levels
Based on the Duncan test (5%), it can be seen that the highest testosterone levels (in μg/dl) were obtained at treatment T0 36.39c ± 6.35, which was significantly different (p < 0.05) from the other three treatments (T1, T2 and T3.). The lowest testosterone level was obtained at T1, namely 6.12a ± 1.51, which was not significantly different (p > 0.05) with T2 treatment, namely 7.45a ± 0.15. The level of testosterone at T3 was 25.27b ± 0.63, although it was significantly different (p < 0.05) from T1 and T2, but still could not reach the normal levels like T0. These results can be seen in Table 2.
Diameter of seminiferous tubule
Based on the Duncan test (5%), the highest seminiferous tubule diameter (in μm) was obtained in the T0 treatment, namely 362.40c ± 4.71, which was significantly different (p < 0.05) from the other three treatments (T1, T2 and T3). The lowest seminiferous tubule diameter was obtained at T1, namely 248.46a ± 3.90, which was not significantly different (p > 0.05) with T2 treatment, namely 255.22a ± 2.34. The testosterone level at T3 was 318.37b ± 4.23, although it was significantly different (p < 0.05) from T1 and T2, it still could not reach normal levels as in the negative control group (T0). These results can be seen in Table 2 .
The epithelial thickness of seminiferous tubule
Based on the Duncan test (5%), the highest thickness of the seminiferous tubule layer (in μm) was obtained in the T0 treatment, namely 117.60d ± 11.30, which was significantly different (p < 0.05) from the other three treatments (T1, T2 and T3). The lowest seminiferous tubule diameter was obtained at T1, namely 3.86a ± 1.57, which was also significantly different (p < 0.05) from the other three treatments. In T2 treatment, the thickness of the seminiferous tubule layer increased slightly to 9.72b ± 3.96. The thickness of the epithelial layer of the seminiferous tubule at T3, although there was a significant difference (p < 0.05) compared to T1 and T2, which was 29.84c ± 4.02, but still far from normal thickness as in the negative control group (T0). These results can be seen in Table 2.
Discussion
Based on the results of this study, the form of HSP70 expression using immunohistochemical methods showed an increase in the HSP70 score in treatment 3 (T3). Although this increase was not able to match the negative control (T0), this increase was able to improve the condition of the testicles which were damaged due to malnutrition, such as an increase in testosterone levels as well as the diameter and thickness of the epithelium of the seminiferous tubule HSP70 is a chaperon molecule needed to repair damaged cells, but at optimal levels (not exceeding the need for cell repair). The HSP70 score for the four treatments can be seen in Figure 1 and Table 2.
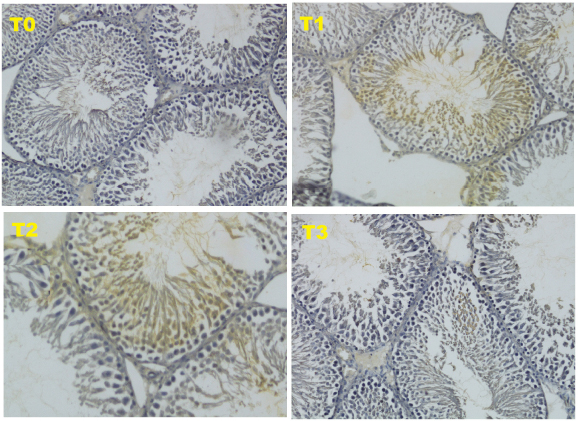
Fig. 1. Expression of HSP70 with IHC method with 200 × magnification.
Table 2. Average score IHC from HSP70 expression, testosterone level, diameter, and seminiferous tubule epithelial thickness of testis.
The HSP70 is an induced protein and will be strongly expressed due to oxidative stress such as reactive oxygen species (ROS). The oxidative stress in this study occurred due to malnutrition. HSP70 will be overexpressed in cells that are damaged (Niu et al., 2006). The expression of HSP70 as a chaperon molecule will stimulate endogenous stem cells to differentiate into progenitor cells from male gamete cells or known as Spermatogonial Stem Cells in mice (Safitri et al., 2016). In this study, the expression of HSP70 in T3 treatment was able to cause an improvement in the diameter and epithelial thickness of the seminiferous tubule of the testis, which was significantly different from the positive control. However, these improvements were still not equal to the negative control.
Stem cells are a special type of undifferentiated cell. Stem cells can be found in almost every type of tissue that makes up the body in multicellular organisms. Stem cells have two unique properties, first is their capacity to renew or regenerate themselves. This causes the stem cells to make a replica of the same cell itself through cell division. The second ability is to differentiate into other cells. Stem cells can develop into various types of mature cells, for example, nerve cells, heart muscle cells, skeletal muscle cells, pancreatic cells (Halim et al., 2010), female gamete cells (Prasetyo and Hetianah, 2017), and male gamete cells. (Safitri et al., 2016).
Table 2 shows that the levels of the highest testosterone levels (μg/dl) were obtained at treatment T0 36.39c ± 6.35, which was significantly different (p < 0.05) from the other three treatments (T1, T2 and T3.). The lowest testosterone level was obtained at T1, namely 6.12a ± 1.51, which was not significantly different (p > 0.05) with T2 treatment, namely 7.45a ± 0.15. The level of testosterone at T3 was 25.27b ± 0.63, although it was significantly different (p < 0.05) from T1 and T2, but still could not reach the normal levels like T0
At the time of malnourished rats will increase the expression of cytochrome P450 (CYP450), especially CYP1A1. The expression of CYP450 results in disruption of the steroidogenesis process in Leydig cells. Inside Leydig cells, there is a receptor mediator called androgen receptor (AR). AR plays a role in mediating the maturation of the steroidogenesis pathway (Wang et al., 2009). However, with the expression of CYP450 there is a bond between CYP450 and AR, resulting in a reduction of AR action on Leydig cells and an increase in ROS due to malnutrition in cells, resulting in death Leydig cells. Decrease in the number of Leydig cells resulting in a decrease in the production of the hormone testosterone, which results in decreased spermatogenesis (Smith and Walker, 2014).
In this study (T3), 50% (v/v) of Kaliandra honey was given and can increase the hormone testosterone through gonadotropin activity due to the influence of pantothenic acid contained in honey. The presence of pantothenic acid is also necessary for converting choline to acetylcholine, a neurotransmitter that plays a role in memory function, mental development, and the reproductive system. Pantothenic acid is also a catalyst that regulates the production and release of adrenal hormones such as adrenocorticothropic hormone (ACTH) (Walji, 2001). ACTH will later induce the anterior pituitary to produce gonadotropic hormones that increase male gonadal activity (Hafez and Hafez, 2010).
The administration of 50% (v/v) honey, which functioned as an anti-oxidant in this study, also significantly affected the diameter and thickness of the seminiferous tubule epithelium. This is in accordance with research (Noh et al., 2020), which states that antioxidant compounds have a major effect in overcoming oxidative stress, which is the cause of seminiferous tubule damage. One of the ingredients in honey as an antioxidant compound is pantothenic acid, which works through hormonal regulation on adrenal steroid secretion (Jaroenporn et al., 2008) and then through hypothalamus hormonal secretion to affects target cells in the seminiferous tubule so that there is the repair of the tissue, that have previously been damaged due to malnutrition (Safitri et al., 2016).
The seminiferous tubule is lined by a special stratified and complex epithelium called the germinal or seminiferous epithelium. The basal portion of the epithelium is lined with fibrous connective tissue, with an innermost layer containing myoid cells flattened and resembling smooth muscle. The epithelium of the seminiferous tubules consists of supporting or sustentacular cells (sertoli cells) and proliferative cells of the spermatogenic lineage that can be differentiated morphologically into spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids, and spermatozoa (Mescher, 2012). This seminiferous tubule is the factory for the production of spermatozoa cells. Therefore, the measurement of the diameter and thickness of the epithelial of the seminiferous tubules needs to be done so that it can be determined whether giving Kaliandra honey in this study can improve the size of the seminiferous tubules that have been damaged due to malnutrition.
Based on observations and analyzes conducted in the four treatment groups with 10 replications, it was shown that Kaliandra honey at a dose of 50% (v/v) had a significant effect in improving testosterone levels, diameter, and epithelial thickness of seminiferous tubules of testicular rats that were malnourished through stimulation of HSP70 although not significantly the same as negative control (T0).
Acknowledgments
The authors would like to thank the Dean of Faculty of Veterinary Medicine, Universitas Airlangga and Universitas Brawijaya for providing the facilities for this research.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors contribution
Nefranindy Rahma and Erma Safitri conceived the study, handling of the animal treatment and wrote the paper. Budi Utomo performed the statistical analysis. Wurlina and Sri Pantja Madyawati observed the Testosteron level with ELISA method and HSP70 score with immunohistochemical method. Tatik Hernawati observed the diameter and epithelial thickness of the seminiferous tubule of the testes with histopathological Anatomy.
References
Armansyah, T., Barat, E.R.P., Handini, C.V.R. and Siregar, T.N. 2018. Concentration and motility of spermatozoa and testosterone level of kacang goat after seminal vesicle extract administration. Open. Vet. J. 8(4), 406.
Hafez,.E.S.E. and Hafez, B. 2010. Reproduction in farm animal, 7th ed. Philadelphia, PA: Lippincott Williams and Wilkins.
Halim, D., Murty, H., Sandra, F., Boediono, A., Djuwantono, T. and Setiawan B. 2010. Stem Cell dasar teori and aplikasi klinis, 1st ed. Indonesia, Erlangga.
Huetos, A.S., Esteban, N.R., Tomas, N.B., Vizmanos, B., Bullo, M., and Salvado, J.S. 2018. The effect of nutrients and dietary supplements on sperm quality parameters: a systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. 9(6), 833–848.
Ipsa, E., Cruzat, V.F., Kagize, J.N., Yovich, J.L. and Keane, K.N. 2019. Growth hormone and insuline like growth factor action in reproductive tissue. Front. Endocrinol. 10, 777.
Jaroenporn, S., Yamamoto, T., Itabashi A., Nakamura, K., Azumano, I., Watanabe, G. and Taya, K. 2008. Effects of pantothenic acid supplementation on adrenal steroid secretion from male rats. Biologic. Pharm. Bullet. 31(6), 1205–1208.
Mescher, A.L. 2012. Histologi dasar junqueira teks dan atlas, 14th ed. Jakarta, Indonesia: EGC.
Noh, S., Go, A., Kim, D.B., Park, M., Jeon, H.W. and Kim, B. 2020. Role of antioxidant natural products in management of infertility: a review of their medicinal potential. Antiox. (Basel). 9(10), 957–961.
Niu, P., Liu, L., Gong, Z., Tan, H., Wang, F., Yuan, J., Feng, Y., Wei, O., Tanguay, R.M. and Wu, T. 2006. Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell. Stress. Chap. 11(2), 162–169.
Patel, A.S., Leong J.Y., Ramos, L. and Ramasamy R. 2019. Testosterone is a contraceptive and should not be used in men who desire fertility. World. Mens. Health. 37(1), 45–54.
Pontis, I.A., da-Costa, L.A.M.A., da-Silva, S.J.R. and Flach, A. 2014. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Tech. 34(1), 69–73.
Prasetyo, R.H. and Safitri, E. 2016. Effects of honey to mobilize endogenous stem cells in efforts intestinal and ovarian tissue regeneration in rats with protein energy malnutrition. Asian. Pac. J. Reprod. 5(3), 198–203.
Safitri, E., Utama, S., Widiyatno, T.V., Sandhika, W. and Prasetyo, R.H. 2016. Auto-regeneration of mice testicle seminiferous tubules due to malnutrition based on stem cells mobilization using honey. Asian. Pac. J. Reprod. 5(1), 30–34.
Safitri, E., Widiyatno, T.V., Prasetyo, R,H. dan Sandika, W. 2019. Effectivity of honey to regenerate the production of testosterone by induction of endogenous stem cells of rat with low libido due to malnutrition. Biocell. 43, 235–239.
Prasetyo, R.H. and Hestianah, E.P. 2017. Honey can repair damage of liver tissue due to protein energy malnutrition through induction of endogenous stem cells. Vet. World. 10(6), 711–715.
Samik, A., Mulyati, S., Hernawati, T. and Safitri, E. 2018. Effect Of aluminum silicate on the spermatozoa, plasma membrane and seminiferous tubules of mice exposed to Fusarium Graminearum (Sordariomycetes: Hypocreales: Nectriaceae). Philip. J. Vet. Med. 55(SI), 59–66.
Sardjito, T., Srianto, P., Nidom, C.A., Mustofa, I. and Safitri, E. 2018. Effect of heat shock protein (HSP) in post thaw Baluran bull semen. Indian. Vet. J. 95(10), 9–10.
Smith, L.B. and Walker, W.H. 2014. The regulation of spermatogenesis by androgens. Semin. Cell. Dev. Biol. 30, 2–13.
Sullivan, R. and Mieusset, R. 2016. The human epididymis: its function in sperm maturation. Hum. Reprod. Update. 22(5), 574–87.
Ustadi, U., Radiati, L.E. and Thohari, I. 2017. Bioactive components of rubber tree honey (Hevea brasiliensis) and Calliandra (Calliandra callothyrsus) and Kapok Honey (Ceiba pentandra). J. Ilm. Teknol. Hasil Tern. 12(2), 97–102.
Walji, H. 2001. Terapi Lebah. Jakarta, Indonesia: Prestasi Pustaka.
Wang, R.S., Yeh, S., Tzeng, C.R. and Chang, C. 2009. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell specific androgen receptor knockout mice. Endocr. Rev. 30(2), 119–132.











