| Original Article | ||
Open Vet J. 2023; 13(3): 297-306 Open Veterinary Journal, (2023), Vol. 13(3): 297–306 Original Research Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament diseaseFlorencia Sollier Podestá* and Daniela Fabiana Izquierdo CaquíasSmall Animal Surgery and Anesthesia Unit, Department of Clinics and Veterinary Hospital, Faculty of Veterinary Medicine, Universidad de la República, Montevideo, Uruguay *Corresponding Author: Florencia Sollier Podestá. Small Animal Surgery and Anesthesia Unit, Department of Clinics and Veterinary Hospital, Faculty of Veterinary Medicine, Universidad de la República, Montevideo, Uruguay. Email: florencia.sollier [at] gmail.com. Submitted: 08/12/2022 Accepted: 10/02/2023 Published: 09/03/2023 © 2023 Open Veterinary Journal
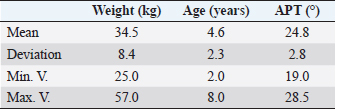
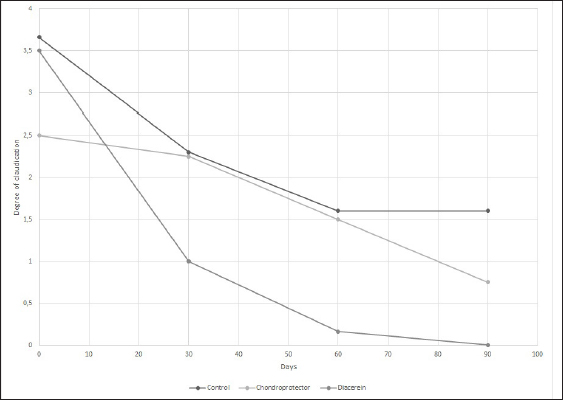
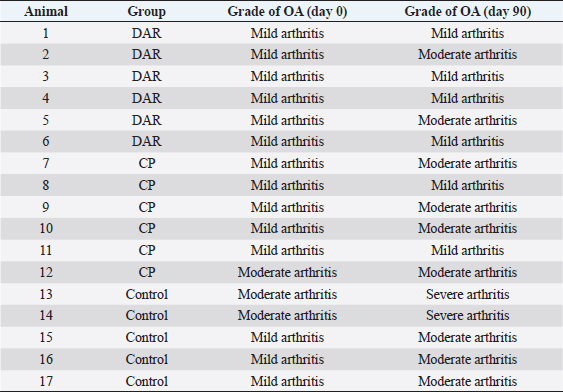
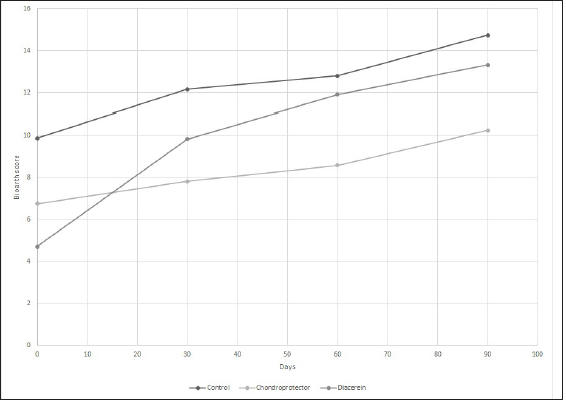
AbstractBackground: Cranial cruciate ligament disease is one of the leading causes of pelvic limb claudication in canines and osteoarthritis in the stifle joint. Historically, studies have focused on surgical options to improve the stability of the stifle joint, although none of the techniques described in the literature prevents the development of osteoarthritis. Aim: This study aimed at proving the presence of osteoarthritis at the time of diagnosis of cranial cruciate ligament rupture, as well as evaluating the benefits of administering diacerein (DAR) or chondroprotective coadjuvants to the extracapsular fabelo-tibial technique. Methods: Seventeen dogs aged between 2 and 8, weighing more than 25 kg, with no predilection for breed or sex, were operated on using this technique. These were divided into three groups: DAR, Chondroprotector (CP), and Control. The animals were treated for 90 days and controlled clinically, radiologically, and using multidimensional scales for pain and quality of life. The statistical analysis used was descriptive and through non-parametric tests. Results: All patients had some degree of osteoarthritis at the beginning of the study associated with the presence of pain. The treated groups improved the claudication scores; however, the changes were significant for the DAR group. The pain score improved in all animals, including those in the Control group; however, the differences were significant only in the treated groups. On the other hand, no significant differences were detected in the radiological studies, so it would be convenient to perform this study over more than 90 days. Conclusion: The surgical treatment accompanied by drugs that act on the degradation of articular cartilage has better clinical results. Keywords: Cruciate ligament, Degenerative disease, Dogs, Joint regeneration, Stifle joint. IntroductionCranial cruciate ligament disease is the most common degenerative condition of the stifle joint in the dog (Perrone et al., 2018). The degenerative process is present in the early stages, before the clinical development of joint instability (Yarnall et al., 2019). Although it is a complex genetic disease with moderate heritability, it is considered a multifactorial pathology, with genetic and risk factors such as breed, age, gender, reproductive status, and weight (Whitehair et al., 1993; Duval et al., 1999). For this reason, treatment should not only focus on providing joint stability through surgery but also on the adequate management of chronic pain and progressive degeneration caused by osteoarthritis. Among the pharmacological options accompanying surgical stabilization are slow-acting drugs, such as chondroprotective and diacerein (DAR) (Beale, 2004). These decrease symptomatology and degeneration with a wide margin of safety (Reginster et al., 2001). Although a wide variety of studies can be found, the results remain controversial due to the lack of prospective studies with a reasonable number of cases, control groups, and strictly controlled methodology. DAR, an alkaloid with a low molecular weight anthraquinone structure (Martel-Pelletier and Pelletier, 2010), acts at the cytokine level by inhibiting IL1-β production and activity (Dougados et al., 2001; Permuy et al., 2015) causing a decrease in caspase-3 (related to a reduction in the level of programmed cell death), and a decrease in nitric oxide synthase and nitric oxide production (Pelletier et al., 2003). It also acts by reducing the phagocytic migration of macrophages (Yaron et al., 1999) and inhibiting the secretion of metalloproteinases (MMPs) (Tamura et al., 2002; Mongil et al., 2006). In the case of DAR, there is little research on animals, but the existing ones are promising (Carney, 1996; Nganvongpanit et al., 2014). This study aimed to evaluate the effect of the administration of chondroprotective and DAR in the postoperative period on the progression of osteoarthritis, physical activity (claudication and muscular atrophy), and quality of life. Materials and MethodsStudy designThe prospective study was carried out at the Veterinary Hospital Center of the Small Animal Clinic and Surgery Unit of the Faculty of Veterinary Medicine (UdelaR) between 2017 and 2019. Patients: inclusion and exclusion criteriaCanines of both sexes, aged between 2 and 8 years old, with a body weight between 25 and 57 kg, and cranial cruciate ligament disease were included. Animals with a history of previous surgical interventions in the joint to be treated or with other associated joint pathologies were omitted. No pregnant or lactating females participated in the study, either. Clinical evaluationBefore entering the study, after the general objective assessment, blood samples were taken to assess the state of health through a complete blood count and blood biochemistry (kidney and liver function). Subsequently, in the orthopedic evaluation, the following items were recorded: body weight, claudication score (from 0 to 4 according to support and muscular atrophy), tibial compression test response, presence of seat test, medial reinforcement, and crepitation. The orthopedic clinical examination was performed on the day of the first consultation (T0) and on days 7, 15, 30 (T1), 60 (T2), and 90 (T3) postoperatively. The same person in the same environment carried out these procedures. Surgical procedureAll animals were operated on by the same surgeon using the extracapsular fabelo-tibial technique. They were premedicated with acepromazine (0.05 mg/kg) and morphine IM (0.5 mg/kg); induction was performed with propofol IV (maximum dose: 5 mg/kg) and maintenance of anesthesia with isoflurane in oxygen by a semi-closed circular valve circuit. Amoxicillin (22 mg/kg) was administered 30 minutes before the surgery, to which were added IV: dipyrone (25 mg/kg), ketoprofen (1 mg/kg), and fentanyl (5 µg/kg). The patients were positioned in dorsal decubitus and shaved from the distal region of the tarsus to the proximal part of the femur, which was subsequently prepared with 2% chlorhexidine, 70% isopropyl alcohol, and 5% povidone-iodine. The approach to the knee was through a craniolateral incision from the distal femoral region to the mid-proximal part of the tibia. A medial arthrotomy was performed to assess the degree of osteoarthritis in the femoral condyles and the condition of the medial meniscus, which, in the event of injury, underwent meniscectomy. The anchor points for the fabellotibial suture were, caudally, to the femoro-fabellar ligament surrounding the lateral sesamoid, and cranially, a hole made through the tibial crest at the quasi-isometric point, two-thirds of the way from the long digital extensor tendon to the crest, at the level of the attachment of the patellar tendon to the tibial crest (Roe et al., 2008). In the postoperative period, all patients received tramadol (5 mg/kg every 8 hours for 2 days) and dipyrone (25 mg/kg every 12 hours for 4 days). Applying cold to the surgical wound site and restricted physical activity was also recommended. In addition, the groups were randomly assigned: DAR, Chondroprotectors, or Control. The animals belonging to the DAR group received Artrodar (Artrodar, TRB Pharma S.A. Laboratory, Buenos Aires, Argentina) at a dose of 2 mg/kg once a day with food, and the animals belonging to the CP group received Artrin (Artrin, Brouwer Laboratory, Buenos Aires, Argentina) 1 tablet every 24 hours. Both drugs were administered during the 90 days of the study. The Control group was not medicated. Guardians were asked to report any changes or events in their dogs once the suggested medication was started. They were clinically evaluated at the agreed follow-ups and by telephone. Radiologic studyThe radiologic study was performed on days 0, 30, 60, and 90 in the Imaging Department of the Veterinary Hospital of the Faculty of Veterinary Medicine. The animals were sedated with morphine 0.5 mg/kg and acepromazine 0.05 mg/kg IM. Two images were taken of the stifle joint: latero-medial and craniocaudal. The images were taken on the same equipment (Vetter-Rems 100, Kodak DirectView digitizer, Classic CRCarestream System), and the technique used was an average of 0.05 mAs and 65 kv. The images were evaluated by three radiologists unrelated to the study based on the Bioarth scale, ignoring the identity of the patients or the group they belonged to (Sánchez Carmona et al., 2006). Multidimensional scalingFrom day 0 to day 90, guardians completed the Brown scale weekly (Brown et al., 2009) to assess chronic pain and the Helsinki scale (Hielm-Bjorkman et al., 2009) to determine the quality of life. Statistical analysisStatistical analysis was based on a descriptive analysis of the sample using measures of central tendency: mean and median, and dispersion: standard deviation, minimum value, and maximum value. For the qualitative variables, relative frequency tables were made. Graphs were also made according to the nature of the variables (quantitative or qualitative). The radiologists' scores for the Bioarth scale were evaluated and plotted using measures of central tendency. The Friedman test was used to evaluate changes over time, corrected by the Wilcoxon signed-rank test for pair comparisons. The Kruskal-Wallis rank-sum test was used to evaluate differences between groups. The claudication score over time was evaluated using the Friedman test and corrected using the Wilcoxon signed-rank test for pair comparisons. The Kruskal-Wallis rank-sum test was used to evaluate differences between groups. For the chronic pain and quality of life scales, the Friedman test was performed for each treatment group, and the post-hoc Wilcoxon signed-rank test was used to evaluate the different scores over time. Likewise, the Wilcoxon rank sum test was used to evaluate the time differences for each treatment group. Values of (p < 0.05 were considered significant. The data was analyzed using the RStudio Environment (version 4.0.0, Copyright (C) 2020). Ethical approvalBoth the consultations and surgical interventions, as well as the clinical and radiological follow-up, were carried out in the Veterinary Hospital Center of the Small Animal Clinic and Surgery UdelaR, with the prior approval of the Ethics Commission of the Faculty of Veterinary Medicine (CEUA) (Ethics Commission on Animal Use). ResultsA total of 17 patients completed the study, nine females and eight males; the distribution in the study groups was randomized, and the final allocation was: DAR (6 animals), CP (6 animals), and Control (5 animals). Table 1 details the descriptive analysis for weight, age, and tibial plateau angle (APT). In addition, 13 of the 17 animals presented meniscus tears. Clinical examination: claudication scoreIn all three groups, the claudication score tended to decrease over time. The effect of time on the decrease in claudication was consistent for the groups treated with CP (p-value=0.02) or DAR (p-value=0.001) but not for the Control group (p-value=0.06). Differences within treatment groups over time were not significant. However, significant differences were detected between the three groups at times 0 (p-value=0.05), 60 (p-value=0.01), and 90 (p-value=0.0079). The DAR group was significantly different from the other two groups at both 60 and 90 days (p-value < 0.05) (Fig. 1). Radiologic evaluationThere were differences in the assessment using the Bioarth scale for the radiologists since one of them rated the images with higher values for all the study groups. Therefore, in addition to the mean and standard deviation, the median of the data was taken into account for data analysis and subsequent graphical representation. All the patients at the beginning of the study (Day 0) presented some degree of osteoarthritis; several animals of the three groups also increased the degree (Table 2). Although changes in the osteoarthritis score are observed over time in the Control group (p-value=0.03), the DAR group (p-value=0.0004), and the CP group (p-value=0.029), these differences do not were significant (p-value > 5) (Fig. 2). As for the differences between study groups, they were not significant (p-value > 0.05). Table 1. General data of the studied group: mean, deviation, minimum value (min. V.), and maximum value (max. V.).
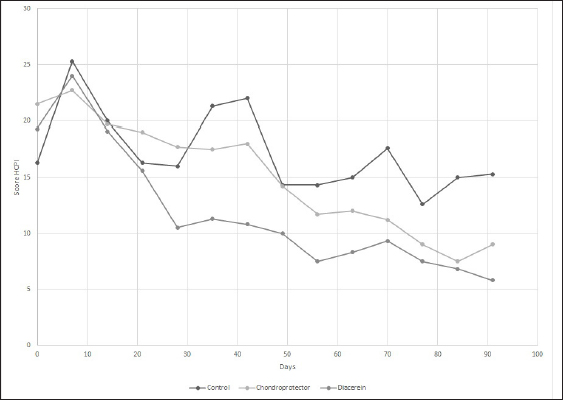
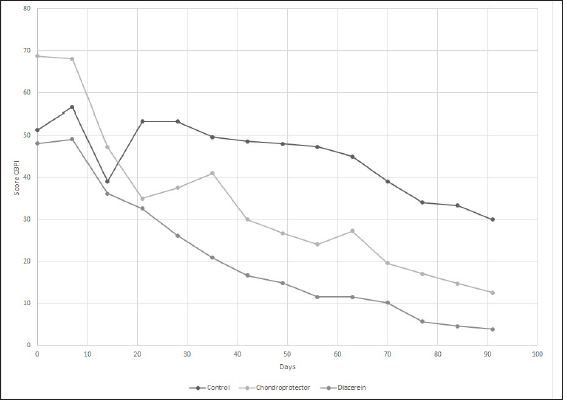
Evaluation of chronic pain and quality of lifeHelsinki Chronic Pain Index (HCPI) The variations for each animal and each group can be observed in Figure 3. Overall, for the three treatment groups, the Helsinki scale showed consistent variation throughout the study (p-value 0.05). Regarding the differences between the groups, significant differences were observed at 28 days (p-value=0.05679), but no differences were observed when the post-hoc test was performed. If we consider the ranges over time, the decrease was only significant in the DAR group, with a 46% decrease in the HCPI value at 28 days of treatment (p-value=0.01). In the CP group, the reduction in the score was significant after 56 days (p-value < 0.05). In the case of the Control group, the values remained constant without finding significant differences (p-value=0.1) Canine brief pain inventory (CBPI) The variations for each animal and each group are shown in Figure 4, respectively. Overall, for the three treatment groups, the Brown scale showed consistent variation throughout the study (p-value < 0.05). As for the differences between the groups, significant differences were observed at 56 days (p-value=0.02) and 91 days (p-value=0.01), but when the post hoc test was performed, no differences were observed. If we consider the ranges over time, the decrease was significant in the DAR group as of day 28, which showed a decline of more than 30% compared to day 0 of the study (p-value=0.02). The decrease is also significant from day 28 to 56 (p-value=0.05). The CP group reached a significant reduction at 91 days (p-value=0.03). In the Control group, no significant differences were observed at 91 days of the study (p-value=0.1). Canine brief pain inventory, the overall impression At time 0, 69% of the animals rated their quality of life as “reasonable,” 23% as “poor,” and one patient as “very good.” At the end of the study, in the DAR group, 66% of the animals rated their quality of life as “very good” and the remaining 33% as “excellent.” In the CP group, 100% rated it as “very good.” Finally, in the Control group, two animals rated it as “good” and one as “very good.” Complications of the surgical technique and treatment safetyAfter the extracapsular fabelo-tibial technique, no major complications were observed. Five animals presented mild seroma in the first postoperative days. Blood count and biochemistry values did not show variations at 91 days of treatment with CP. No patient presented adverse effects after administration. Blood count and biochemistry values did not show variations at 91 days of treatment with DAR. One patient presented a change in stool consistency, slightly softer than usual.
Fig. 1. Mean claudication score of the study groups. The behavior of the control group, CP, and diacerein is shown. Table 2. Osteoarthritis progression for each animal and treatment according to the Bioarth scale.
DiscussionThere is now consensus that the degenerative process precedes cranial cruciate ligament rupture (CCLR), where persistent synovitis and the development of inflammatory-based arthritis are likely factors promoting degenerative ligament rupture (Fujita et al., 2006; Muir, 2010). In our study, no patient had a history of trauma, and all showed some degree of osteoarthritis (OA) at the time of CCLR diagnosis. In addition, the affected breeds were large, the mean age was 4.6 years, and the female-male distribution was even, which is consistent with other authors (Grierson et al., 2011).
Fig. 2. Scoring for the degree and progression of osteoarthritis according to the Bioarth scale. The curves represent the average median values of the observers for the control group, CP, and diacerein.
Fig. 3. Evaluation of quality of life according to the HCPI. The curves represent the means of the values for the control group, CP, and diacerein.
Fig. 4. Evaluation of quality of life according to the CBPI. The curves represent the means of the values for the control group, CP, and diacerein. The purpose of treatment, in addition to stabilization, is the long-term maintenance of joint function, as well as pain reduction and treatment of existing meniscal damage. The medial meniscus, due to its attachment to the medial collateral ligament, has less mobility and, therefore, a higher rate of injury, which over time, stimulates the development of OA (Flo, 1990; Pozzi and Cook, 2010). In our study, 13 of 17 patients underwent partial meniscectomy, coinciding with the 75.86% reported by other authors (Ferrigno et al., 2012; Dal-Bo et al., 2014). Regarding surgical treatment, the extracapsular fabelo-tibial technique is based on periarticular fibrosis produced in the long term (Kowalesky et al., 2012). Its results vary depending on technical features such as tibial anchor point (Roe et al. 2008) or nylon suture tension (Dunn et al., 2012) and depending on animal characteristics such as weight, physical activity, and age (Casale and McCarthy 2009). It is also the technique where OA advances the fastest; in one study, it was determined that animals treated with extracapsular suturing are 5.78 times more likely to have a more significant difference in their postoperative OA score than dogs treated with TPLO (Lazar et al., 2005). The extracapsular technique presents the best results in terms of claudication 6 months after surgery (Alzate Gómez and Tamayo Arango, 2004; Conzemius et al., 2005). In the present case, although the claudication score tended to decrease over time in the three study groups (DAR, CP, and Control), only the DAR treatment showed significant differences at 60 days, which leads us to suspect that stabilization accompanied by adjuvant therapy improves support functionality in less time. None of the currently available techniques can restore the normal kinematics of the stifle joint after CCLR, which is related to late meniscal injury, cartilage damage, and OA progression, leading to chronic pain. Mölsä et al. (2013) found long-term chronic pain in approximately 30% of dogs after surgery for CCLR. All the patients in the study presented some degree of pain at time 0 and, as we could observe, surgical stabilization without adjuvant treatment was not enough to reduce pain scores since the Control group (without treatment) did not present significant differences, neither in the scales nor in claudication, three months after surgery. This is evidence that the treatment of cruciate ligament disease should go hand in hand with treating the degenerative process secondary to it, which seeks to reduce pain and increase limb function, improving quality of life (Aragon et al., 2007). DAR shows the maximum clinical effect between one and three months after the start of treatment (Nguyen et al., 1994; Martel-Pelletier and Pelletier, 2010) and a transporter effect that determines its long-term permanence. Our results coincide with the onset of action since, at 30 days, improvements in clinical and pain scores are observed. However, due to the duration of the study, we cannot evaluate its effects in a period longer than 90 days. Several studies evaluate whether DAR modifies OA progression, but they use induced OA models and evaluate morphological changes by histology and arthroscopy (Carney, 1996; Brandt et al., 1997; Smith et al., 1999). One study group established that DAR administered daily for 6 months at 50 mg/dog or 100 mg/dog does not prevent pathophysiological changes in OA. However, the study population could be considered too heterogeneous as they included animals of various weights and ages with hip, elbow, or knee OA without distinguishing the cause (Nganvongpavit et al., 2014). Another study (Di Sevo, 2017), which included dogs with hip OA that were administered DAR at 2 mg/kg per day for 90 days, did not find the progression of the disease using the Bioarth scale, however, they highlighted the importance of repeating the test over a more extended period, to detect if this trend continues. Similarly, in this study, in the group treated with DAR, only two patients progressed from mild to moderate OA; however, these differences were insignificant. Regarding the clinical examination, the group treated with DAR showed consistent improvements at 60 and 90 days, reaching a score of 0 at the end of treatment. Likewise, Nganvongpanit et al. (2014) administered DAR at 50 and 100 mg/dog, in dogs with hip OA, with significant improvements after three months of treatment. In our study, as in another region (Di sevo, 2017), pain reduction was evidenced clinically and in the multidimensional scales. At 28 days of treatment, there was a 46% reduction in the HCPI value, indicating a favorable treatment outcome since a 30% decrease is considered sufficient to confirm pain reduction. The same applies to the CBPI, which at the end of the study reached values close to 0. Regarding adverse effects, like Smith et al. (1999), Brandt et al. (1997), and Nganvonpavit et al. (2014), we found that it is safe to administer DAR for 90 days because of its safety margin since no alterations were found in renal or hepatic function or the blood count. However, the n of this study is insufficient to conclude in this regard. In dogs, dark urine and diarrhea, and to a lesser extent, vomiting, have been reported (Carney, 1996; Brandt et al., 1997; Nganvongpanit et al., 2014). In humans, the causes of diarrhea and dark urine may be due to the chemical structure of DAR and rhein, which are derivatives of anthraquinone, a laxative agent. The darkening of urine is caused by chemical reactions occurring in an acidic medium, directly related to the anthraquinone structure of the molecule (Nicolas et al., 1998). In the case of chondroprotective, there is a lack of sensitive analytical methods that can quantify these agents in biological matrices, added to many commercial products available on the market (Comblain et al., 2015). Regarding the influence on the progression of OA, while one study found no differences after administration, possibly attributed to a study period shorter than that recommended to expect any effect (180 days) (Bonastre Ráfales, 2002), others revealed less progression in dogs that received CP compared to placebo dogs (Velasco et al., 2009). In this study, four of the animals receiving chondroprotective progressed to moderate OA, yet these differences were insignificant. To discuss this in greater depth, it would be desirable for the studies to be consistent in their methodology. In the physical assessment, some authors find positive effects on claudication scores, physical performance, degree of muscle atrophy, and return to function at 60 days (Arias et al., 2007; Velasco et al., 2009; Bonastre Ráfales, 2012). In our study, although there was a tendency to decrease, we did not find significant improvements, which agrees with Moreau et al. (2003), who, in addition to the subjective clinical evaluation, used a force platform to measure stride objectively. These differences between studies could be attributed to insufficient doses or administration time since there is evidence that only at 5 months an increase of glycosaminoglycans can be detected in the synovial fluid (Johnson et al., 2001). However, we detected improvements in the multidimensional scales, with a significant decrease in the HCPI value at 56 days of the study and in the CBPI at 90 days. In this context, Scott et al. (2017) observed an improvement in pain severity and interference scores in the treated groups and the control group that received a placebo. This caused them to adjudicate a placebo effect on the guardians when performing the CBPI. Other authors do not find such benefits in its administration (Alves et al., 2017). In our study, all patients achieved a perfect score, while the results of Alves et al. (2017) were somewhat lower. This could be because they included OA secondary to different causes (not only to CCLR). Therefore, we can assume that we found better results due to surgical treatment added to the use of articular cartilage protectors. Regarding side effects, as in several studies (Moreau et al., 2003; Alves et al., 2017; Scott et al., 2017), in this study, no patient showed a significant change in blood count and blood biochemistry values, although some hemostatic and gastrointestinal alterations have been reported (McNamara et al., 1996; Mc Carty et al., 2007; Scott et al., 2017). One of the limitations of our study was that the dogs in the Control group were neither medicated nor received a placebo. This could interfere especially with differences in scores on multidimensional scales by guardians. Furthermore, since radiographic changes do not necessarily correspond to the degree of clinical signs, and clinical examination using the claudication and muscle atrophy scale can be considered semi-objective, it would be convenient to have the analysis of the force plate as an objective method to be able to conclude. ConclusionAll patients with cranial cruciate ligament disease had osteoarthritis with evidence of chronic pain at diagnosis before surgical stabilization. Surgical stabilization by the extracapsular fabello-tibial technique, as the sole treatment, is insufficient to reduce pain. The administration of DAR at a dose of 2 mg/kg per day for 90 days was proven effective in controlling clinical signs and improving quality of life through a decrease in chronic pain without triggering side effects or alterations in blood parameters. The administration of the combination of glycosaminoglycan (GA), chondroitin sulfate (CS), and manganese for 90 days tends to improve claudication scores and significantly improves chronic pain scores without being associated with adverse effects. Conflict of interestThe Authors declare that there is no conflict of interest. ReferencesAlves, J.C., Santos, A.M. and Jorge, P.I. 2017. Effect of an oral joint supplement when compared to carprofen in the management of hip osteoarthritis in working dogs. top. Companion Anim. Med. 32(4), 126–129. Alzate Gómez, G.J. and Tamayo Arango, L.J. 2004. Comparación de la técnica de sutura supracondilar con la técnica modificada DeAngelis para la corrección de ruptura del ligamento cruzado anterior en perros, Universidad de Antioquia, Facultad de Ciencias Agrarias, Escuela de Medicina Veterinaria, Medellín, Colombia. Aragon, C.L., Hofmeister, E.H. and Budsberg, S. 2007. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 230(4), 514–521. Arias, S.S.A., Faria, R.C.M. and Gonçalves, M.E. 2007. Desempeño clínico de perros con enfermedad articular degenerativa de rodilla tratados con ácido hialurónico y sulfato de condroitina. Vet. Méx. 38(3), 331–345. Beale, B. 2004. Use of neutraceuticals and chondroprotectants in osteoarthritic dogs and cats. Vet. Clin. North Am. J. Small Anim. Pract. 34(1), 271–289. Brandt, K.D., Smith, G., Yong Kang, S.Y., Myers, S., O'connor, B. and Albrecht, M. 1997. Effects of diacerhein in an.accelerated canine model of osteoarthritis. Osteoarthr. Cartil. 5(6), 438–449. Brown, D.C., Boston, R., Coyne, J.C. and Farrar, J.T. 2009. A novel approach to the use of animals in studies of pain: validation of the canine brief pain inventory in canine bone cancer. Pain Med. 10(1), 133–142. Bonastre Ráfales, C. 2012. Estudio clínico de los cambios osteoartrósicos de la rodilla inestable del perro por rotura del ligamento cruzado anterior (LCA) tras el tratamiento por osteotomía niveladora del platillo tibial (TPLO) con y sin condroprotectores. Universidad de Extremadura, Facultad de Veterinaria de Cáceres, España. Carney, S.L. 1996. Effect of diacetyl rhein on the development of experimental osteoarthritis. A biochemical investigation. Osteoarthr. Cartil. 4(4), 251–261. Casale, S.A. and McCarthy, R.J. 2009. Complications associated with lateral fabellotibial suture surgery for cranial cruciate ligament injury in dogs: 363 cases (1997-2005). J. Am. Vet. Med. Assoc. 234(2), 229–235. Conzemius, M.G., Evans, R.B., Besancon, F., Gordon, W.J., Horstman, C.L., Hoefle, E.D., Nieves, M.A. and Wagner, S.D. 2005. Effect of surgical technique on limb function after surgery for rupture of the cranial cruciate ligament in dogs. J. Am. Vet. Med. Assoc. 226(2), 232–236. Comblain, F., Serisier, S., Barthelemy, N., Balligand, M. and Henrotin, Y. 2015. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J. Vet. Pharmacol. Therap. 39(1), 1–15. Dal-Bo, I., Ferrigno, C.R., Izquierdo Caquías, D.F., Della Nina, M.I., Ferreira, M.P., Valente de Figueiredo, A.V., Oliveira Cavalcanti, R.A., do Santos, J.F. and Ferraz, V.C. 2014. Correlação entre ruptura de ligamento cruzado cranial e lesão de menisco medial em cães. Cienc. Rural. 44(8), 1426–1430. Di Sevo, V. 2017. Uso De La Diacereína En Caninos Con Osteoartrosis: Estudio Clínico y Serológico. Universidad De La República, Facultad De Veterinaria, Montevideo, Uruguay. Dougados, M., Nguyen, M., Berdah, L., Mazieres, B., Vignon, E. and Lequesne, M. 2001. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis. ECHODIAH, a three-year, placebo-controlled trial. Arthritis Rheum. 44(11), 2539–2547. Dunn, A.L., Buffa, E.A., Marchevsky, A.M., Heller, J., Moores, A.P. and Farrell, M. 2012. Inter and intra-operator variability associated with extracapsular suture tensioning. An ex vivo study. Vet. Comp. Orthop. Traumatol. 25(1), 472–477. Duval, J.M., Budsberg, S.C., Flo, G.L. and Sammarco, J.L. 1999. Breed, sex, and body weight as risk factors for rupture of the cranial cruciate ligament in young dogs. J. Am. Vet. Med. Assoc. 215(6), 811–814. Ferrigno, C.R.A., Caquias, D.F.I., Nina, M.I.D., Cunha, O.D., Ito, K.C., Mariani, T.C., Ferraz, V.C.D.M. and Cotes, L. 2012. Ruptura de menisco associada à ruptura de ligamento cruzado cranial em cães. Braz. J. Vet. Res. Anim. Sci. 49(4), 301–306. Flo, G.L. 1990. Meniscectomy. In Current techniques in small animal surgery, 3rd ed. Ed., Bojrab, M.J. Philadelphia, PA: LeaandFebiger, pp: 694–700. Fujita, Y., Hara, Y., Nezu, Y., Schulz, K.S. and Tagawa, M. 2006. Proinflammatory cytokine activities, matrix metalloproteinase-3 activity, and sulfated glycosaminoglycan content in synovial fluid of dogs with naturally acquired cranial cruciate ligament rupture. Vet. Surg. 35(1), 369–376. Grierson, J., Asher, L. and Grainger, K. 2011. An investigation into risk factors for bilateral canine cruciate ligament rupture. Vet. Comp. Orthop. Traumatol. 24(3), 192–196. Hielm-Björkman, A.K., Rita, H. and Tulamo, R.M. 2009. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am. J. Vet. Res. 70(6), 727–734. Johnson, K.A., Hulse, D.A., Hart, R.C., Kochevar, D. and Chu, Q. 2001. Effects of an orally administered mixture of chondroitin sulfate, glucosamine hydrochloride and manganese ascorbate on synovial fluid chondroitin sulfate 3B3 and 7D4 epitope in a canine cruciate ligament transection model of osteoarthritis. Osteoarthr. Cartil. 9(1), 14–21. Kowalesky, M.P., Boudrieau, R.J. and Pozzi, A. 2012. Stifle joint. In Veterinary Surgery Small Animal. Eds., Tobias, K.M. and Johnston, S.A. Bengaluru, India: Elsevier, pp: 906–998. Lazar, T.B., Berry, C.R., Dehaan, J.J., Peck, J.N. and Correa, M. 2005. Long-term radiographic comparison of tibial plateau leveling osteotomy versus extracapsular stabilization for cranial cruciate ligament rupture in the dog. Vet. Surg. 34(2), 133–141. McCarthy, G., O’Donovan, J., Jones, B., McAllister, H., Seed, M. and Mooney, C. 2007. Randomised double-blind, positive-controlled trial to assess the efficacy of 49 glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet. J. 174(1), 54–61. McNamara, P.S., Barr, S.C. and Erb, H.N. 1996. Hematologic, hemostatic, and biochemical effects in dogs receiving an oral chondroprotective agent for thirty days. Am. J. Vet. Res. 57(9), 1390–1394. Martel-Pelletier, J. and Pelletier, J.P. 2010. Effects of diacerein at the molecular level in the oateoarthritis disease process. Ther. Adv. Musculoskelet. Dis. 2(2), 95–104. Mölsä, S.H., Hielm-Björkman, A.K. and Laitinen-Vapaavuori, Q.M. 2013. Use of an owner questionnaire to evaluate long-term surgical outcome and chronic pain after cranial cruciate ligament repair in dogs: 253 cases (2004–2006). J. Am. Vet. Med. Assoc. 243(5), 689–695. Mongil, E., Sánchez, I., Torre, F., Callejo, A. and Arizaga, A. 2006. Fármacos de acción lenta (Sysadoa) en el tratamiento de la osteoartrosis. Rev. Soc. Esp. Dolor. 13(7), 485–496. Moreau, M., Dupuis, J., Bonneau, N.H. and Desnoyer, M. 2003. Clinical evaluation of a nutraceutical, carprofen and meloxicam forthetreatment of dogs with osteoarthritis. Vet. Rec. 152(11), 323–329. Muir, P. 2010. Advances in canine cruciate ligament. Wiley-Blackwell, Iowa, USA, pp: 289. Nganvongpanit, K., Boonsri, B., Sripratak, T., Markmee, P. and Kongtawelert, P. 2014. Clinical Study on the effects of diacerein and diacerein combined with chondroitin sulfate on canine hip osteoarthritis. Kafkas. Univ. Vet. Fak. Derg. 20(3), 383–392. Nguyen, M., Dougados, M., Berdah, L. and Amor, B. 1994. Diacerhein in the treatment of osteoarthritis of the hip. Arth. Rheu. 37(4), 529–536. Nicolas, P., Tod, M., Padoin, C. and Petitjean, O. 1998. Clinical pharmacokinetics of diacerein. Clin. Pharmacokinet. 35(5), 347–359. Pelletier, J.P., Mineau, F., Boileau, C. and Martel-Pelletier, J. 2003. Diacerein reduces the level of cartilage chondrocyte DNA fragmentation and death in experimental dog osteoarthritic cartilage at the same time that it inhibits caspase-3 and inducible nitric oxide synthase. Clin. Exp. Rheumatol. 21(2), 171–177. Permuy, M., Guede, D., López-Peña, M., Munoz, F., Caeiro, J.R. and GonzalezCantalapiedra, A. 2015. Effects of diacerein on cartilage and subchondral bone in early stages of osteoarthritis in a rabbit model. BMC Vet. Res. 2(11), 143. Perrone, G., Murray, M. and Vavken, P. 2018. Regenerative medicine and cranial cruciate ligament repair. In Advances in the canine cranial cruciate ligament. Ed., Muir, P. Ames, IA: Wiley-Blackwell, pp: 371–377. Pozzi, A. and Kim, S.E. 2010. Biomechanics of the normal and cranial cruciate ligament deficient stifle. In Advances in the canine cranial cruciate ligament. Ed., Muir P. Ames, IA: Wiley-Blackwell, pp: 37–42. Reginster, J.Y., Deroisy, R., Rovati, L.C., Lee, R.L., Lejeune, E., Bruyere, O., Giacovelli, G., Henrotin, Y., Dacre, J.E. and Reichert, C.G. 2001. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357(9252), 251–256. Roe, S.C., Kue, J. and Gemma, J. 2008. Isometry of potential suture attachment sites for the cranial cruciate ligament deficient canine stifle. Vet. Comp. Orthop. Traumatol. 21(3), 215–220. Sánchez Carmona, A., Agut, A., Chico, A., Closa, J.M., Rial, J. and Velasco, A. 2006. Desarrollo de una escala de valoración radiológica del grado de osteoartrosis para las articulaciones de la rodilla y el codo en el perro—Escala “Bioarth”. Clín. Vet. de Pequeños Anim. 26(3), 269–275. Scott, R.M., Evans, R. and Conzemius, M.G. 2017. Efficacy of an oral nutraceutical for the treatment of canine osteo arthritis a double-blind, randomized, placebo-controlled prospective clinical trial. Vet. Comp. Orthop. Traumatol. 30(1), 318–323. Smith, G.N., Myers, S.L., Brandt, K.D., Mickler, E. and Albrecht, M. 1999. Diacerhein treatment reduces the severity of osteoarthritis in the canine cruciate-deficiency model of osteoarthritis. Arth. Rheu. 42(3), 545–554. Tamura, T., Shirai, T., Kosaka, N., Ohomori, K. and Takafumi, N. 2002. Pharmacological studies of diacerein in animal models of inflammation, arthritis and bone resorption. Euro. J. Pharmacol. 448(1), 81–87. Velasco, A., Sellés, M., Chico, A. and Bonet, S. 2009. Evaluación de la eficacia postquirúrgica del condroitín sulfato (condrovet®) en perros con artrosis de rodilla secundaria a rotura del ligamiento cruzado anterior. Clin. Vet. Peq. An. 29(2), 103–108. Whitehair, J.G., Vasseur, P.B. and Willits, N.H. 1993. Epidemiology of cranial cruciate ligament rupture in dogs. J. Am. Vet. Med. Assoc. 203, 1016–1019. Yarnall, B.W., Chamberlain, C.S., Hao, Z. and Muir, P. 2019. Proinflammatory polarization of stifle synovial macrophages in dogs with cruciate ligament rupture. Vet. Surg. 48(6), 1005–1012. Yaron, M., Shirazi, I. and Yaron, I. 1999. Anti-interleukin-1 effects of diacerein and rhein in human osteoarthritic synovial tissue and cartilage cultures. Osteoarthr. Cartil. 7(3), 272–280. | ||
| How to Cite this Article |
| Pubmed Style Podestá FS, Caquías DFI. Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Vet J. 2023; 13(3): 297-306. doi:10.5455/OVJ.2023.v13.i3.6 Web Style Podestá FS, Caquías DFI. Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. https://www.openveterinaryjournal.com/?mno=27000 [Access: July 11, 2025]. doi:10.5455/OVJ.2023.v13.i3.6 AMA (American Medical Association) Style Podestá FS, Caquías DFI. Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Vet J. 2023; 13(3): 297-306. doi:10.5455/OVJ.2023.v13.i3.6 Vancouver/ICMJE Style Podestá FS, Caquías DFI. Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Vet J. (2023), [cited July 11, 2025]; 13(3): 297-306. doi:10.5455/OVJ.2023.v13.i3.6 Harvard Style Podestá, F. S. & Caquías, . D. F. I. (2023) Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Vet J, 13 (3), 297-306. doi:10.5455/OVJ.2023.v13.i3.6 Turabian Style Podestá, Florencia Sollier, and Daniela Fabiena Izquierdo Caquías. 2023. Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Veterinary Journal, 13 (3), 297-306. doi:10.5455/OVJ.2023.v13.i3.6 Chicago Style Podestá, Florencia Sollier, and Daniela Fabiena Izquierdo Caquías. "Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease." Open Veterinary Journal 13 (2023), 297-306. doi:10.5455/OVJ.2023.v13.i3.6 MLA (The Modern Language Association) Style Podestá, Florencia Sollier, and Daniela Fabiena Izquierdo Caquías. "Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease." Open Veterinary Journal 13.3 (2023), 297-306. Print. doi:10.5455/OVJ.2023.v13.i3.6 APA (American Psychological Association) Style Podestá, F. S. & Caquías, . D. F. I. (2023) Postoperative efficacy of chondroprotectors and diacerein in dogs with osteoarthritis secondary to cranial cruciate ligament disease. Open Veterinary Journal, 13 (3), 297-306. doi:10.5455/OVJ.2023.v13.i3.6 |