| Review Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 12-18 Review Article A review of current knowledge on avian Newcastle infection in commercial poultry in the Kingdom of Saudi ArabiaMohammed Al-Rasheed1,2*1Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al Hofuf, Saudi Arabia 2Avian Research Center, King Faisal University, Al Hofuf, Saudi Arabia *Corresponding Author: Mohammed Al-Rasheed. Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al Hofuf, Saudi Arabia. Email: malrasheed [at] kfu.edu.sa Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
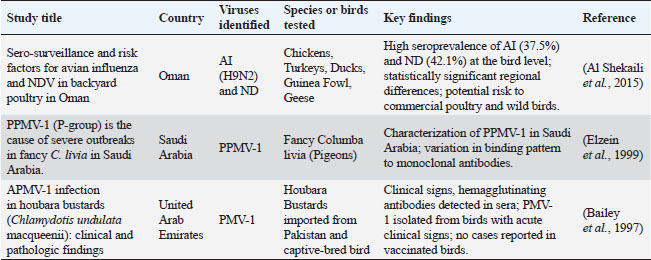
ABSTRACTNewcastle disease (ND) is a tremendously contagious avian infection with extensive monetary ramifications for the chicken zone. To reduce the effect of ND on the Saudi rooster enterprise, our analysis emphasizes the necessity of genotype-particular vaccinations, elevated surveillance, public recognition campaigns, and stepped-forward biosecurity. Data show that one-of-a-kind bird species, outdoor flocks, and nearby differences in susceptibility are all vulnerable. The pathogenesis consists of tropism in the respiratory and gastrointestinal structures and some genotypes boom virulence. Laboratory diagnostics use reverse transcription-polymerase chain reaction, sequencing, and serotyping among different strategies. Vital records are supplied through immune responses and serological trying out. Vaccination campaigns, biosecurity protocols, and emergency preparedness are all covered in prevention and manipulation techniques. Notably, co-circulating genotypes and disparities in immunization regulations worry Saudi Arabia. The effect of ND in Saudi Arabia is tested in this paper, with precise attention paid to immunological reaction, pathogenesis, susceptibility elements, laboratory analysis, and preventative and manipulation measures. Saudi Arabia can shield its bird region and beef up its defences against Newcastle’s ailment, enforcing those hints into its policies. Keywords: Avian paramyxovirus, Newcastle disease virus, Pathology, Diagnosis, Vaccination. IntroductionNewcastle disease (ND), sometimes called avian ND, is a highly contagious virus that poses a significant threat to the global poultry industry (Alexander and Senne, 2003). ND poses a severe threat to poultry populations due to its ability to spread rapidly and economically in the considerable loss caused by the virus (Leslie, 2000; Alders, 2014). Although the virus affects a variety of birds, chickens are most affected, so the poultry industry should prioritize (Miller and Torchetti, 2014). Like many other countries, Saudi Arabia recognized the importance of ND control and management roles. Newcastle disease virus (NDV), commonly known as paramyxovirus-1 (PMV-1), is a common virus in the genus Avulavirus that causes epidemics in poultry and other poultry species and belongs to the family Paramyxoviridae (https://talk.ictvonline.org/taxonomy/) (Jeong et al., 2018). High levels (velogenic), including the most virulent velogenic strains, which generally cause high mortality. The diversity of NDV strains highlights the importance of better understanding the virus to effectively limit its impact on poultry populations (Brown and Bevins, 2017). Direct contact between susceptible and diseased birds is the primary method of transmission of ND. Contaminated clothes, water, feed, and equipment are other possible transmission routes. Furthermore, farmed poultry might contract the virus from wild birds that act as carriers (Miller et al., 2013; Suarez et al., 2020). ND can present with a range of clinical manifestations, including signs of the neurological, respiratory, and digestive systems, depending on the virus’s virulence. High death rates characterize severe cases despite the wide range of clinical symptom presentation. ND must be promptly diagnosed and treated with the proper management techniques, including immunization, isolating afflicted birds, and strict biosecurity measures. It is advised to consult a veterinarian or the local animal health authorities in cases where ND is suspected of receiving an accurate diagnosis and instructions on how to put control measures in place (Brown and Bevins, 2017). Newcastle disease has significantly affected Saudi Arabia’s commercial chicken production. Poultry producers have experienced significant financial losses due to outbreaks, decreased egg production and higher death rates (Almubarak, 2019). These ramifications influence food security and the accessibility of poultry goods on the local market in addition to the poultry industry’s financial viability. To slow the spread of ND, the Kingdom of Saudi Arabia has combined vaccination campaigns, quarantine guidelines, and biosecurity measures. Notwithstanding these endeavours, certain obstacles endure, such as the possibility of the virus evolving into a more pathogenic variant. Monitoring and surveillance are necessary to identify and contain future epidemics quickly. The Kingdom of Saudi Arabia works with regional and international organizations to exchange best practices and insights in managing ND. Given that wild and farmed birds can spread NDV, it is essential to comprehend the dynamics of the virus in the area. Saudi Arabia’s poultry sector, especially in the Eastern Region, has grown significantly in recent years, producing billions of eggs and millions of broiler birds. An investigation into the function of NDV in this context was conducted in the Eastern Region of Saudi Arabia between 2012 and 2014. In order to provide light on the dynamics of the disease in the area, this survey attempted to describe the circulating NDV strains (Almubarak, 2019). It is crucial to comprehend the variety of NDV strains in Saudi Arabia and how they affect the chicken sector. This information can help manage and prevent Newcastle disease in chicken populations by informing vaccination plans, biosecurity precautions, and response procedures. We examine the survey’s findings and debates in the following parts, providing insight into the NDV strains that are now circulating in Saudi Arabia’s Eastern Region. Overview of the diseaseBeyond just chickens, ND is a highly contagious viral virus that affects many different species of birds. NDV is categorized as an orthoavulavirus, and one of its noteworthy traits is that it may infect various bird species (Kaleta and Baldauf, 1988). This makes it a severe worry regarding poultry’s health and the preservation of birds. The wide range of avian species that are infected with NDV includes both wild and domesticated birds. Newcastle disease can harm pigeons, ducks, turkeys, geese, pheasants, quails, and even certain raptor species, although study and preventative efforts primarily target hens (McMullin, 2020). NDV infections can also affect migratory birds, shorebirds, and wild ducks. NDV can operate as a reservoir for the virus and is frequently seen circulating in populations of wild birds. The fact that NDV can infect such a wide variety of bird species highlights how widespread the virus is. It affects wild bird populations and the larger ecological context, not just the production of chickens. The management and control of NDV are more critical due to the interaction between wild and farmed birds and the possibility of NDV mutations. Furthermore, the effects of ND vary depending on the type of bird it affects. Various species may present with different clinical manifestations, degrees of vulnerability, and consequences based on variables like the virulence of the NDV strain, the kind of host, and habitat. Because of this, comprehending and treating ND is a difficult task with many facets that call for an all-encompassing strategy that considers the needs of both domestic and wild bird populations. The virusThe NDV, a virus pathogen with unique features that set it out as a fatal threat in the poultry industry, is categorically identified as the cause of ND. NDV is categorized as an Avulavirus and a Paramyxoviridae family member. The ability of this virus to display a broad spectrum of virulence levels among its strains is unique and essential to comprehend the variety of clinical symptoms linked to outbreaks of ND. The virulence of NDV strains ranges from low (lentogenic) to moderate (mesogenic) and, at the highest pathogenicity level, high (velogenic). This spectrum allows the strains to be identified from one another. The potential for velogenic strains to generate catastrophic outbreaks with significant mortality rates in poultry populations makes them particularly concerning among these pathogens (Almubarak, 2019) The genetic properties of the NDV strains, especially in areas like the virus’s fusion protein cleavage site (FPCS), are primarily responsible for the virulence variances. The presence of numerous amino acids at the FPCS is a characteristic of velogenic strains. This allows for quick and effective viral replication, which spreads throughout the host and eventually results in severe clinical symptoms. The severity of the NDV strain that caused the outbreak is closely linked to the variety of clinical signs of ND. Sneezing and coughing are common clinical symptoms of mild respiratory infections caused by lentogenic strains of the disease (Dortmans et al., 2010). On the other hand, when velogenic strains are present, the illness manifests very quickly and with more severe symptoms. Respiratory distress (sneezing, coughing, nasal discharge), digestive problems (diarrhea, decreased appetite), nervous system abnormalities (torticollis, paralysis), and a notable decrease in egg production in laying hens are some examples of these clinically severe indications (Bertran et al., 2017). Moreover, significant death rates are typical when velogenic strains cause the outbreak. The fact that NDV strains exhibit such a broad range of virulence highlights the variety of clinical symptoms and the content of severity observed during ND outbreaks. This variance is a significant obstacle to ND diagnosis, treatment, and control. Because clinical indications can range in severity from mild and unnoticeable to highly virulent and life-threatening, it is critical that veterinarians and poultry health authorities accurately detect outbreaks and take swift action to contain them. Modes of transmissionND is highly contagious and can spread in several ways. The most frequent method of transmission is direct contact between vulnerable and infected birds. The virus can quickly spread throughout flocks due to its presence in bodily fluids, including feces and respiratory secretions. NDV can also spread through tainted food, drink, clothing, equipment, and human contact. Proper biosecurity protocols are essential to stop the virus from entering poultry operations. An important factor in the epidemiology of ND is wild birds. These birds can potentially be carriers of NDV, storing it and possibly passing it on to domestic poultry. Because it can spread the virus to new areas, interactions between wild and domesticated bird species make disease control efforts more difficult (Awan et al., 1994). Clinical signs and lesionsThe virulence of the NDV strain causing the ailment influences the degree to which a particular symptom appears. ND symptoms can take many distinct forms. The complexity of ND is partly attributed to the variation in its clinical presentations. Numerous organ system clinical symptoms frequently accompany ND outbreaks. In less severe cases of ND, respiratory symptoms such as coughing, sneezing, and nasal discharge are often present. Conversely, birds infected with less virulent strains of NDV may exhibit gastrointestinal symptoms such as diarrhea and decreased appetite (Sutton et al., 2013). Velogenic NDV strains are linked to the worst clinical manifestations. In certain situations, birds may exhibit neurological symptoms such as paralysis, torticollis, and neck twisting. These nervous system symptoms show the extremely pathogenic characteristics of velogenic NDV strains. The decline in egg production for laying hens is a noteworthy and concerning indicator. An abrupt and discernible decrease in egg production can be a preliminary marker of ND in a flock. Necrosis and haemorrhage of the laryngeal tonsils in the cranial portion of the trachea are consistently observed in chickens with the virulent viscerotropic (Wakamatsu et al., 2006). During egg yolk peritonitis, atrophied follicles and a deteriorated oviduct may occur in birds in lay (Bwala et al., 2012). With virulent viscerotropic isolates, necrosis and hemorrhagic lesions in lymphoid-dependent regions of the gut (lymphoid tissue of lower eyelid, cecal tonsils, Peyer’s patches) of infected chickens and turkeys are prevalent (McMullin, 2020). It is critical to recognize that there are significant differences in the clinical spectrum of ND. Sneezing and coughing are two common respiratory signs that sick birds may exhibit. On the other hand, velogenic strains can cause the quick onset of severe clinical symptoms, which can lead to a high mortality rate in infected birds. This diverse spectrum of clinical manifestations highlights how intricate ND is. It also highlights the significance of timely and precise diagnosis and the implementation of suitable control measures catered to the unique features of every outbreak. Prompt intervention is essential to reduce the effects of this extremely contagious and potentially fatal avian disease (Awan et al., 1994). DistributionsTable 1 shows an overview of the superiority of PMV-1 in some Middle Eastern international locations. Table 1. Gives an overview of the superiority of PMV-1 in some Middle Eastern international locations. The table presents the combined infection charges in domestic and wild birds in Saudi Arabia, Oman and the United Arab Emirates due to exceptional multi-winged paramyxovirus (APMV) serotypes. Some of these strains are APMV-1, APMV-2, APMV-4, and pigeon paramyxovirus-1 (PPMV-1).
Economic impact on poultryND is well known for its ability to cause the poultry industry to suffer significant financial losses. Wide-ranging and complex are the effects of ND epidemics. Poultry producers suffer from decreased egg production, elevated mortality rates, and immediate financial losses during ND outbreaks. These effects have broader economic repercussions for food security and the accessibility of poultry products on the domestic market, extending beyond the poultry industry. Moreover, NDV can change, producing increasingly aggressive strains that could make treating the illness even more difficult (Alexander and Senne, 2003). Therefore, it is essential to maintain constant surveillance, monitoring, and quick action in the event of an epidemic to protect chicken populations and the poultry industry from the negative consequences of ND. ND’s highly contagious nature puts it at the top of the list of poultry-related diseases. Appropriate control and prevention measures must be implemented to reduce its effects and safeguard chicken populations. Implementing quarantine protocols, vaccination campaigns, and biosecurity measures are essential parts of the multidisciplinary strategy needed to slow the development of ND (Rehan et al., 2019). ND in Saudi ArabiaNDs are the most prevalent form of bird infection, and outbreaks have happened in Saudi Arabia and other nations worldwide. The disease has severely hurt the poultry industry of Saudi Arabia, for it has so many negative impacts. The following particulars pertain to the incidence and consequences of ND in Saudi Arabia. Prevalence and impactND has continued to pose a concern to Saudi Arabia, with isolated outbreaks happening in various parts of the country (El-Zein, 1986; Al-Ali et al., 2018; Almubarak, 2019). These outbreaks, which can be moderate to severe, can wipe out whole poultry flocks, including turkeys and chickens. Significant trade restrictions on poultry goods and decreased poultry production have economic effects. ND also erodes customer trust in chicken products, which impacts the overall stability and profitability of the poultry sector. To lessen these difficulties and protect public health and the chicken business, the Saudi Arabian government has launched vaccination programs, implemented biosecurity protocols, and collaborated with other countries to control and prevent the disease. Transmission and control challengesSaudi Arabia has several noteworthy issues regarding ND transmission and control. First, the high density of poultry in the nation, which is made up of many backyard and commercial flocks—creates an environment where communities are close to one another, which raises the possibility of disease transmission. Second, since wild birds can transport and spread the virus, the Kingdom is more vulnerable to introducing the NDV due to its location along important migratory bird routes. Finally, vaccination campaigns and biosecurity protocols have a significant role in the efficacy of ND management (Bertran et al., 2017). It is possible that certain chicken producers may not adhere to or execute these suggested protocols, which could cause difficulties in managing the virus’s transmission. Improving ND prevention and management in Saudi Arabia requires addressing several issues ( Almubarak, 2019). Saudi government measuresThe Saudi Arabian government has taken proactive steps to control and prevent ND. Their tactics include large-scale immunization campaigns to immunize a significant percentage of the chicken population. Furthermore, the government actively encourages and enforces biosecurity measures in commercial poultry farms. These procedures include strict access controls to farms, proper carcass disposal, and the upkeep of exacting hygienic standards (Al-Ali et al., 2018). In addition, implementing thorough quarantine guidelines for imported chicken and poultry products is essential to halting the spread of the NDV within the nation. Together, these initiatives demonstrate the government’s dedication to reducing the effects of ND and preserving Saudi Arabia’s poultry sector. International collaborationSaudi Arabia leads international collaboration to control and monitor ND. Nationally and internationally renowned organizations like the World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO) have ties since they all support the exchange of knowledge, best practices, and information concerning the significance of the dream. This commitment involves implementing stringent sanitary laws for the worldwide trade and certification of disease-free zones for chicken products. Saudi Arabia’s geographical proximity to other Gulf nations highlights the significance of regional collaboration in halting the spread of communicable or non-communicable diseases and other transboundary animal illnesses (Almubarak, 2019; Clemmons et al., 2021). International organizations’ functionInternational organizations, especially the World Organisation for Animal Health (OIE), greatly aid the coordination of worldwide efforts to monitor and prevent animal diseases. The OIE sets international standards and guidelines for disease management since it is the premier intergovernmental organization devoted to animal health and welfare. It gives participating nations a forum to work together to combat zoonoses and animal illnesses, promoting trade between countries while lowering the danger of disease. By encouraging openness, confidence, and collaboration among its more than 180 member nations, the OIE improves animal health worldwide. Recognizing the interdependence of human, animal, and environmental health, the OIE adopts a One Health strategy in collaboration with other international organizations such as the FAO and WHO. These organizations are essential to preserving health when disease transcends national boundaries. The existence of mixed infections of different avian paramyxovirus (APMV) serotypes highlights the complexity of APMV epidemiology in the Middle East (Suarez et al., 2020). This region includes Saudi Arabia, which implies a higher risk of exposure to several APMV serotypes (Almubarak, 2019). The Oman study highlights how commonplace APMV-1 and APMV-4 are in backyard chickens, suggesting that these viruses are everywhere (Al Shekaili et al., 2015). This information is critical for the poultry business in Saudi Arabia since it may impact vaccination plans, biosecurity protocols, and surveillance initiatives aimed at mitigating the risk of numerous APMV serotypes. This review data highlights the necessity of comprehensive control and prevention measures for ND in Saudi Arabia, considering the variety of APMVs in the area. Susceptibility in Saudi ArabiaND is a severe hazard to poultry in Saudi Arabia and is influenced by several factors. Various backyard and commercial flocks define the poultry landscape of the country. Backyard flocks are especially susceptible to the introduction of NDV, possibly by wild birds or other carriers, because they frequently lack biosecurity precautions and immunization regimens (Munir et al., 2012). On the other hand, commercial farms follow tight vaccination schedules and biosecurity precautions, which lower susceptibility. Due to the country’s distinct biodiversity and migratory bird patterns, different NDV genotypes are introduced, which increases exposure, particularly in areas where poultry and wild birds coexist. Additionally, regional variances like humidity and variations in vaccination methods affect poultry susceptibility to ND (Samour, 2014). Pathogenesis and pathology in Saudi ArabiaThe pathogenesis and pathology of NDV in Saudi Arabia provide unique insights while mainly following the worldwide pattern. Most NDV strains found in the area have a tropism for the gastrointestinal and respiratory systems, which can lead to distinctive digestive lesions and clinical indications of respiratory distress. Notably, strains that cause neurological symptoms and lesions are neurotropic, essential for comprehending ND’s clinical manifestations. It is also clear that NDV genotypes have an impact. Typically found in Saudi Arabia, strains with genotype VII.1.1 are known for their heightened virulence, which can lead to more severe respiratory and digestive system problems and more excellent mortality rates in infected birds (Almubarak, 2019; Sultan et al., 2021). Laboratory diagnosis, isolation, and identificationND is diagnosed, isolated, and identified in laboratories in Saudi Arabia per recognized protocols. These diagnostic endeavours involve several crucial methods. To detect NDV genetic material, reverse transcription-polymerase chain reaction is utilized. This method provides a way to verify infection and evaluate the genotype of the virus, which in turn identifies potential vaccine mismatches. An essential first step in characterizing and assessing NDV pathogenicity is isolating the virus from field samples. A network of diagnostic laboratories is kept up to date specifically for this purpose in Saudi Arabia. Hemagglutination and hemagglutination inhibition (HI) assays are essential serotyping techniques that help differentiate NDV from other avian illnesses and provide accurate serological identification (Suarez et al., 2020). Additionally, post-mortem histopathology analyses are crucial for understanding the particular lesions linked to ND in chickens (Mao et al., 2022). Serological tests and immune responseIn Saudi Arabia, serological tests are indispensable for comprehending the immune response to ND. HI testing is a standard method for assessing immune function following vaccination (Peeters et al., 2001). It provides crucial information on vaccine protection and helps determine its effectiveness. Furthermore, enzyme-linked immunosorbent tests play an essential role in the detection of antibodies in the serum (Eterradossi, 1992), providing a reliable tool for monitoring poultry in areas where the environment is resistant to the diseases, particularly in areas with a diversity of NDV genotypes, specific genotype testing can yield critical insights into the immune response and the efficacy of vaccines, ensuring a targeted approach to ND control in Saudi Arabia. Prevention and controlND prevention and control in Saudi Arabia employ a multimodal strategy. In commercial poultry farms, vaccination regimens are robust and frequently delivered, emphasizing customizing vaccines to the prevalent genotypes of NDV to maximize effectiveness. It is crucial to prioritize biosecurity measures to prevent the introduction and spread of viruses, especially in backyard flocks. During an outbreak, administering emergency vaccinations is an essential control measure, mainly if genotype-specific vaccines are accessible (Mao et al., 2022). Monitoring and managing ND outbreaks requires cooperation between veterinary professionals, government officials, and chicken farmers. These revelations shed light on Saudi Arabia’s difficulties and tactics in fighting this economically critical bird illness. Biosecurity in poultry farmingEffective biosecurity measures are necessary in Saudi Arabian chicken production to prevent ND and other infectious diseases. Controlled entry to the farm with complete decontamination, the isolation and quarantine of newly arrived birds, regular cleaning and disinfection, rodent and pest control, and the exclusion of problems and wild birds are essential procedures. Emphasizing cleanliness and illness prevention, personnel training is critical. Maintaining the flock’s immunity requires frequent vaccination schedules in addition to biosecurity. Together, these all-encompassing precautions protect the chicken business by lowering the possibility of ND outbreaks and financial losses (Almubarak, 2019; Mao et al., 2022). ImmunizationVaccination is the primary means of controlling ND in Saudi Arabia’s chicken industry. Vaccines are used, either inactivated or live attenuated, depending on the individual farm’s needs. The vaccination schedule is carefully prepared, with different vaccinations for layers, broilers, and primary and booster shots. The effectiveness of vaccines is ensured by ongoing evaluation and monitoring. Given the variety of NDV genotypes, vaccinations targeted to a particular genotype are thought to improve immunity. Prioritizing quality control and keeping thorough immunization records are crucial (Yadin, 1981). Emergency vaccination is a quick response tactic to stop the spread of illness in outbreak settings. Robust biosecurity protocols and a well-executed immunization campaign are essential for protecting the chicken business and reducing ND-related losses (Almubarak, 2019). DiscussionThe literature analysis highlights several patterns, discrepancies, and debates about ND in Saudi Arabia. The Middle East also tends to have mixed infections with different APMV serotypes, which could add to the region’s complicated ND epidemiology (Roy, 2012). There are apparent gaps in the research, especially regarding specifics on how common certain pathogens are in Saudi Arabia. Although the backyard poultry industry is highlighted, information regarding commercial poultry farms is minimal. Furthermore, little is known about the genetic diversity and evolutionary history of NDV strains in Saudi Arabia. The ramifications of dual APMV infections are controversial since they may make ND control difficult. It is still up for debate to what degree these coexisting diseases affect the spread of disease and the efficacy of vaccinations. ND significantly affects Saudi Arabia’s poultry sector, especially backyard flocks. This industry is especially vulnerable to ND due to a lack of immunisation, and the high seroprevalence rates highlight the virus’s continued spread. This creates unique difficulties for the management and surveillance of diseases. Saudi Arabia’s strategy for ND management, which emphasizes immunization and biosecurity, is in line with international plans. Enhancing surveillance efforts, especially for the genetic characterization of circulating NDV strains, might still be possible. Increased cooperation with surrounding nations may also be beneficial in addressing the transboundary character of the illness. In summary, ND presents a significant risk to Saudi Arabia’s poultry sector, and successfully mitigating its effects requires a multifaceted strategy. ConclusionND could severely affect private and commercial flocks, seriously threatening Saudi Arabia’s chicken sector. Due to its varied poultry industry, regional variances, and the co-circulation of various NDV genotypes, the nation faces particular issues. The literature evaluation emphasizes the need for genotype-specific vaccinations and surveillance gaps. In many ways, including immunization and biosecurity protocols, Saudi Arabia’s control and prevention efforts are similar to those of other countries. To address genotype variety, however, improvements can be made in strengthening surveillance, raising awareness, and developing vaccination plans. By tackling these issues, Saudi Arabia can more effectively shield its poultry sector from the financial effects of ND. AcknowledgmentI want to express my sincere gratitude to the Deanship of Scientific Research at King Faisal University for their financial support through Project Number (GRANT4588). This support was instrumental in conducting the research and developing this manuscript. Conflict of interestThe author claims to have no conflicts of interest. ReferencesAl-Ali, A., El-Sabagh, I., Mohamed, M., Alluwaimi, A. and Arif, I. 2018. Molecular characterization of common respiratory viral infections in broilers in Al-Hassa, Eastern Province, Saudi Arabia. Thai. J. Vet. Med. 48(2), 235–245. Alders, R.G. 2014. Making Newcastle disease vaccines available at village level. Vet. Rec. 174(20), 502–503. Alexander, D.J. and Senne, D.A. 2003. Newcastle disease. Dis. Poult. 11(1), 64–87. Almubarak, A.I. 2019. Molecular and biological characterization of some circulating strains of Newcastle disease virus in broiler chickens from Eastern Saudi Arabia in 2012-2014. Vet. World. 12(10), 1668. Al Shekaili, T., Clough, H., Ganapathy, K. and Baylis, M. 2015. Sero-surveillance and risk factors for avian influenza and Newcastle disease virus in backyard poultry in Oman. Prev. Vet. Med. 122(1–2), 145–153. Awan, M.A., Otte, M.J. and James, A.D. 1994. The epidemiology of Newcastle disease in rural poultry: a review. Avian. Pathol. 23(3), 405–423. Bailey, T.A., Nicholls, P.K., Wernery, U., Samour, J., Cooper, J.E. and O’Leary, M.T. 1997. Avian paramyxovirus type 1 infection in Houbara bustards (Chlamydotis undulata macqueenii): clinical and pathologic findings. J. Zoo. Wildl. Med. 28(3), 325–330. Bertran, K., Susta, L. and Miller, P.J. 2017. Chapter 51—avian influenza virus and Newcastle disease virus. In Egg innovations and strategies for improvements, Chapter 51. Ed., Hester, P.Y. San Diego, CA: Academic Press, pp: 547–559. Brown, V.R. and Bevins, S.N. 2017. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet. Res. 48, 1–15. Bwala, D.G., Clift, S., Duncan, N.M., Bisschop, S.P. and Oludayo, F.F. 2012. Determination of the distribution of lentogenic vaccine and virulent Newcastle disease virus antigen in the oviduct of SPF and commercial hen using immunohistochemistry. Res. Vet. Sci. 93(1), 520–528. Clemmons, E.A., Alfson, K.J. and Dutton, J.W. 2021. Transboundary animal diseases, an overview of 17 diseases with potential for global spread and serious consequences. Animals 11(7), 2039. Dortmans, J.C., Fuller, C.M., Aldous, E.W., Rottier, P.J. and Peeters, B.P. 2010. Two genetically closely related pigeon paramyxovirus type 1 (PPMV-1) variants with identical velogenic fusion protein cleavage sites but with strongly contrasting virulence. Vet. Microbiol. 143(2–4), 139–144. El-Zein, A. 1986. Characterization of a velogenic Newcastle disease virus isolated from broilers in Saudi Arabia. Avian. Dis. 30(4), 825–828. Elzein, E.A., Manvell, R., Alexander, D. and Alafaleq, A.I. 1999. Pigeon paramyxovirus-1 (P-group) as the cause of severe outbreaks in fancy Columba livia in Saudi Arabia. J. Vet. Med. Series. B. 46(10), 689–692. Eterradossi, N. 1992. Discrepancies in turkey rhinotracheitis ELISA results using different antigens. Vet. Rec. 131, 563–564. Jeong, J., Kim, Y., An, I., Wang, S.J., Kim, Y., Lee, H.J., Choi, K.S., Im, S.P., Min, W., Oem, J.K. and Jheong, W. 2018. Complete genome sequence of a novel avian paramyxovirus isolated from wild birds in South Korea. Arch. Virol. 163(1), 223–227. Kaleta, E.F. and Baldauf, C. 1988. Newcastle disease in free-living and pet birds. In Newcastle disease. Developments in veterinary virology. Ed., Alexander, D.J. Boston, MA: Springer, pp: 197–246. Leslie, J. 2000. Newcastle disease: outbreak losses and control policy costs. Vet. Rec. 146(21), 603–606. Mao, Q., Ma, S., Schrickel, P.L., Zhao, P., Wang, J., Zhang, Y., Li, S. and Wang C. 2022. Review detection of Newcastle disease virus. Front. Vet. Sci. 9, 936251. McMullin, P.F. 2020. Diseases of poultry 14th edition: David E. Swayne, Martine Boulianne, Catherine M. Logue, Larry R. McDougald, Venugopal Nair, David L. Suarez, Sjaak de Wit, Tom Grimes, Deirdre Johnson, Michelle Kromm, Teguh Yodiantara Prajitno, Ian Rubinoff & Guillermo Zavala (Eds.), Hoboken, NJ: John Wiley & Sons, pp: 1451. Avian. Pathol. 49(5), 526. Miller, P.J., Afonso, C.L., El Attrache, J., Dorsey, K.M., Courtney, S.C., Guo, Z. and Kapczynski, D.R. 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Develop. Comp. Immunol. 41(4), 505–513. Miller, P.J. and Torchetti, M.K. 2014. Newcastle disease virus detection and differentiation from avian influenza. Methods. Mol. Biol. 1161, 235–239. Munir, M., Abbas, M., Khan, M.T., Zohari, S. and Berg, M. 2012. Genomic and biological characterization of a velogenic Newcastle disease virus isolated from a healthy backyard poultry flock in 2010. Virol. J. 9, 46. Peeters, B.P., de Leeuw, O.S., Verstegen, I., Koch, G. and Gielkens, A.L. 2001. Generation of a recombinant chimeric Newcastle disease virus vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine 19(13-14), 1616–1627. Rehan, M., Aslam, A., Khan, M.R., Abid, M., Hussain, S., Umber, J., Anjum, A. and Hussain, A. 2019. Potential economic impact of Newcastle disease virus isolated from wild birds on commercial poultry industry of Pakistan: a review. Hosts. Viruses. 6(1), 1–15. Roy, P. 2012. Diagnosis and control of Newcastle disease in developing countries. World’s. Poult. Sci. J. 68(4), 693–706. Samour, J. 2014. Newcastle disease in captive falcons in the Middle East: a review of clinical and pathologic findings. J. Avian. Med. Surg. 28(1), 1–5. Suarez, D.L., Miller, P.J., Koch, G., Mundt, E. and Rautenschlein, S. 2020. Newcastle disease, other avian paramyxoviruses, and avian metapneumovirus infections. In Diseases of poultry. Eds., Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., Prajitno, T.Y., Rubinoff, I. and Zavala, G. Hoboken, NJ: John Wiley & Sons, Inc., pp: 109–166. Sultan, H.A., Elfeil, W.K., Nour, A.A., Tantawy, L., Kamel, E.G., Eed, E.M., El Askary, A. and Talaat, S. 2021. Efficacy of the Newcastle disease virus genotype VII. 1.1-matched vaccines in commercial broilers. Vaccines (Basel) 10(1), 29. Sutton, D., Aldous, E.W., Warren, C.J., Fuller, C.M., Alexander, D.J. and Brown, I.H. 2013. Inactivation of the infectivity of two highly pathogenic avian influenza viruses and a virulent Newcastle disease virus by ultraviolet radiation. Avian. Pathol. 42(6), 566–568. Wakamatsu, N., King, D.J., Kapczynski, D.R., Seal, B.S. and Brown C.C. 2006. Experimental pathogenesis for chickens, turkeys, and pigeons of exotic Newcastle disease virus from an outbreak in California during 2002-2003. Vet. Pathol. 43(6), 925–933. Yadin, H. 1981. Aerosol vaccination against Newcastle disease: factors affecting the serological response in chickens. Avian. Pathol. 10(3), 329–341. | ||
| How to Cite this Article |
| Pubmed Style Mohammed Al-Rasheed. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 12-18. doi:10.5455/OVJ.2024.v14.i1.2 Web Style Mohammed Al-Rasheed. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. https://www.openveterinaryjournal.com/?mno=176974 [Access: July 09, 2025]. doi:10.5455/OVJ.2024.v14.i1.2 AMA (American Medical Association) Style Mohammed Al-Rasheed. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 12-18. doi:10.5455/OVJ.2024.v14.i1.2 Vancouver/ICMJE Style Mohammed Al-Rasheed. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Vet J. (2024), [cited July 09, 2025]; 14((1) (Zagazig Veterinary Conference)): 12-18. doi:10.5455/OVJ.2024.v14.i1.2 Harvard Style Mohammed Al-Rasheed (2024) A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 12-18. doi:10.5455/OVJ.2024.v14.i1.2 Turabian Style Mohammed Al-Rasheed. 2024. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 12-18. doi:10.5455/OVJ.2024.v14.i1.2 Chicago Style Mohammed Al-Rasheed. "A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia." Open Veterinary Journal 14 (2024), 12-18. doi:10.5455/OVJ.2024.v14.i1.2 MLA (The Modern Language Association) Style Mohammed Al-Rasheed. "A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 12-18. Print. doi:10.5455/OVJ.2024.v14.i1.2 APA (American Psychological Association) Style Mohammed Al-Rasheed (2024) A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 12-18. doi:10.5455/OVJ.2024.v14.i1.2 |