| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 225-241 Original Research Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular dockingSawsan S. Elbasuni1*, Hanan A. A. Taie2, Samah M. Abdel Gawad3, Reda E. L. Kamar4, Hala El Daous5, Marwa Darweish6, Mai O. Nada7, Walaa F. SaadEldin8 and Marwa I. Abdel Haleem11Department of Avian and Rabbit Diseases, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 2Plant Biochemistry Department, National Research Centre, Dokki, Giza, Egypt 3Department of Parasitology, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 4Department of Histology, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 5Department of Hygiene and Veterinary Management, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 6Department of Pathology, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 7Department of Veterinary Pharmacology, Animal Health Research Institute-Benha Branch, Agriculture Research Center (ARC), Benha, Egypt 8Educational Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Egypt *Corresponding Author: Sawsan S. Elbasuni. Departmessnt of Avian and Rabbit Diseases, Faculty of Veterinary Medicine, Benha University, Benha, Egypt. Email: Sowsan.mohamed [at] fvtm.bu.edu.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
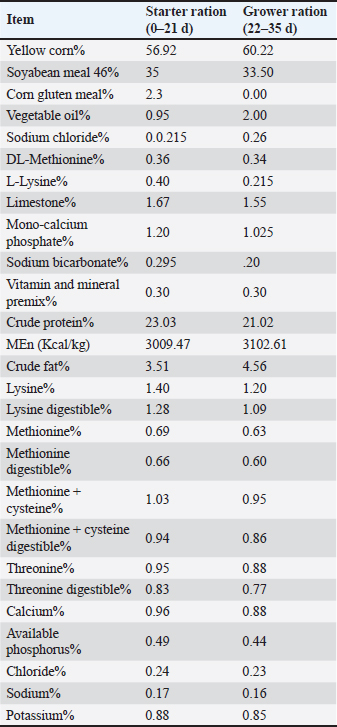
ABSTRACTBackground: Coccidiosis is one of the most economically significant poultry diseases worldwide, caused by the pathogenic Eimeria species, and is characterized by decreased weight gain (WG) and failure to grow due to malabsorption, low feed conversion rate, bloody diarrhea, and dehydration. Aim: This study investigated the effectiveness of licorice root extract (LRE) in controlling cecal coccidiosis to determine whether its combination with maduramicin could help alleviate the pathological, biochemical, and histopathological effects of cecal coccidiosis in Sasso broiler chicks. Methods: A total of 125 one-day-old Sasso broiler chicks were categorized into five equal groups (n=25), each consisting of five replicates (n=5 per replicate). G1-LE received a basal diet supplemented with LRE (3 g/kg); G2-ME received a basal diet containing maduramycin (0.5 g/kg); and G3-LME received a basal diet containing LRE and maduramicin together with the same rates. G4-E (positive control) and G5-N (negative control) received no additives in their feed. Birds in groups (G1-4) were challenged on day 14 of the experiment by orally intercropping a 1 ml suspension of Eimeria tenella sporulated oocysts. Results: Groups of birds fed on LRE and maduramicin separately or together appeared to be in good condition where no deaths or clinical abnormalities were observed, based on the analysis of clinicopathological examination. Compared with the G4-E positive control, the dropping scoring and oocyst shedding of groups G1-LE, G2-ME, and G3-LME along the 10th-day post-challenge (dpc), as well as macroscopic and microscopic lesions scoring at the 7th dpc, was considerably lower. The dual supplementation use of LRE and maduramicin in G3-LME's reduced the harmful effects of coccidian, which appeared only as a mononuclear cellular infiltration and a small number of oocysts invading the intestinal glands. Molecular docking revealed that LRE and maduramicin interacted with E. tenella DNA polymerase, E. tenella apical membrane antigen 1, and microneme protein binding sites resulting in reduced E. tenella replication and invasion. Conclusion: The inclusion of LRE and maduramicin, individually or in combination, in the diet might effectively mitigate the detrimental effects of coccidiosis. Keywords: Chicken, Coccidiosis, Glycyrrhiza glabra, Licorice, Maduramicin. IntroductionThe annual cost of coccidiosis to the poultry industry is estimated at £10.4 billion (Hussain, et al., 2017; Blake et al., 2020) making it one of the most economically significant poultry illnesses worldwide. The prevalence of coccidian infection in commercial poultry production ranges from 5% to 70% in developing countries, and Egypt is one of those countries (Du and Hu, 2004; Al-Gawad et al., 2012; Abbas et al., 2017). Seven different species of the obligate intracellular parasite Eimeria produce coccidiosis in chickens, leading to widespread illness and death (Berezin et al., 2010; Moryani et al., 2021). Clinical manifestations of coccidiosis, are caused by exposure to the pathogenic sporulated Eimeria species oocysts in the environment (Sundar et al., 2017; Kalkal et al., 2021). Free radical oxidants are produced by the host’s cellular immunological response because of Eimeria entry into intestinal cells at various locations (Allen et al., 1998) but pathogenic oxidative stress and disruption of the ecological redox balance may result from exposure to excessive levels of infection (Georgieva et al., 2006). A natural chemical substance called maduramicin helps manage coccidiosis, by blocking ion transport channels and disrupting the osmotic balance of the parasite. Drug resistance and negative effects on both beneficial organisms and human consumers from drug residues in the environment or in chicken by-products are two drawbacks of the widespread use of these chemical agents in chicken farm feed and/or water to combat coccidiosis (Williams, 1998; Peek and Landman, 2011; Kadykalo et al., 2018). In recent years, medicinal plants have been used to fight coccidiosis by lowering stress levels and relieving oxidative stress, resulting in improved nutrient uptake, overall health, and increased yield (Awais et al., 2018; Kadykalo et al., 2018; Pop et al., 2019). Licorice or Glycyrrhiza glabra (G. glabra), has been used medicinally for centuries, and its numerous active components including saponin, triterpenes, flavonoids, coumarins, sugars, starch, amino acids, choline, tannins, phytosterols, ascorbic acid, and others give it a wide range of biological and pharmaceutical applications. Extracts of G. glabra have been tested in vitro at varying concentrations for their purported role in controlling cecal coccidiosis, where the number of sporulated and unsporulated oocysts was significantly reduced. In a dose-dependent manner, G. glabra extracts alone or in combination with other substances have been shown to have therapeutic and preventative benefits on single and combined infections of several Eimeria species (Pop et al., 2019; Hussain et al., 2022; Ghafouri et al., 2023). The objective of this study is to investigate the efficacy of herbal remedies, specifically G. glabra, in the management of cecal coccidiosis. In addition, we aimed to determine whether combining these herbal remedies with chemotherapeutic agents such as maduramicin could help mitigate the pathological, biochemical, and histopathological effects of coccidiosis in Sasso broiler chicks. Integrating this supplementary value enables the application of traditional medicine knowledge in clinical contexts. Material and MethodsBirds, and managementA total of 125 one-day-old Sasso broiler chicks were procured from a local hatchery in Egypt. At the Faculty of Veterinary Medicine, Benha University, the chicks were kept in clean and sanitized conditions. The birds were housed in a floor system using the standard management procedures (Abdel Haleem et al., 2019). There were enough feeders and waterers for each compartment. From 14 to 24 days of age, plastic sheets were placed over the bedding material to allow dropping scoring and collection for oocyst counting. All chicks were fed a broiler starter ration for three weeks, then a broiler grower ration until the 35th day. Ration formulation was in accordance with the guidelines outlined in the NRC (1994) (Table 1). Table 1. Basal diet composition and its chemical analysis.
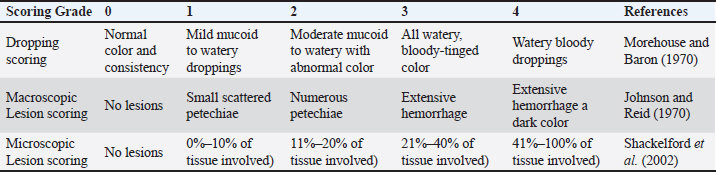
Experimental designThe chicks were randomly divided into five distinct groups (n=25 per group), each consisting of five replicates (n=5 per replicate). The first group (G1-LE) received a basal diet supplemented with licorice root powder extract at a dosage of 3 g/kg (Rashidi et al., 2020), the second group (G2-ME) was provided with the basal diet supplemented with maduramicin (Atco-pharma, Egypt) at a dosage of 0.5 g/kg. Finally, the third group (G3-LME) was given the basal diet supplemented with a combination of licorice root extract (LRE) and maduramicin throughout the experiment. The control positive group (G4-E) and control negative group (G5-N) did not receive any medications in their feed and the G5-N group maintained in isolation from the experimental area. Birds in groups (G1-4) were subjected to a challenge on the 14th day of the experiment. This challenge involved intercropping a 1 ml (5 × 104 oocysts) suspension of Eimeria tenella sporulated oocysts (Pop et al., 2019), while G5-N received 1 ml of normal saline solution intracrop. Eimeria tenella oocystsThe oocysts of E. tenella were obtained from a commercial broiler flock that experienced cecal coccidiosis in Qalyubia Governorate, Egypt. The oocysts were isolated using a combination of sieve and sedimentation methods (Longstaffe, 1984). The identification of oocysts was conducted based on their morphological properties (Al-Gawad et al., 2012). The viability of the oocysts was verified through the production of a distinctive manifestation of cecal coccidiosis in experimental chicks (Swayne et al., 2020). The suspension containing fresh sporulated oocysts was preserved in a refrigerator (4°C) using a 2.5% potassium dichromate solution until use. The quantification and microscopic identification of sporulated oocysts in the culture were performed on the fourteenth day of the experiment directly before challenge. To minimize manufactured errors and improve the reproducibility of experiments, we have opted to designate a sample size of five avian subjects for each test as mentioned in the statistical analysis. Licorice root extract preparation and phytochemical analysisThe powdered aqueous ethanolic extract (70%) of licorice root (LRE) was purchased from Al-Abji Factory, Cairo, Egypt, for the extraction of vegetables and essential oils. The Folin-Ciocalteu procedure was used to determine the total phenol (TP) and total tannin (TT) content and results were expressed in milligrams of gallic acid equivalent (GAE) per gram of dry extract weight (Cheel et al., 2007). The total flavonoid content was conducted (Ordoñez et al., 2006). The quantification of overall flavonoid content was conducted by ascertaining the quercetin equivalent through the utilization of a calibration curve. The total saponin content was measured calorimetrically (Makkar et al., 1997). With minor modifications, total terpenoids were estimated and results were expressed in milligrams of linalool (LE) per gram of dry sample (Koleva et al., 2002). Clinicopathological assessmentsBody performance parameterFeed intake (FI), body weight (BW), weight gain (WG), and feed conversion ratio (FCR) were estimated at day 10 post-challenge (dpc) (Abdel Haleem et al., 2019), as well as mortalities. All birds were weighed before and after the challenge, and their clinical symptoms were monitored daily. In cases where deaths were recorded, autopsies were performed. To evaluate the efficacy of different interventions, the anticoccidial index (ACI) was calculated using the following parameters according to De Pablos et al. (2010). Dropping scoringBird droppings 10 dpc were graded on a scale of 0 to 4 according to consistency and the presence of mucus and/or blood (Morehouse and Baron, 1970; De Pablos et al., 2010) Oocyst shedding (oocysts per gram) (OPG)Fresh fecal samples (n= 5 per group) were collected daily from the covered plastic lid starting at 3–10 dpc. Fecal samples were individually placed in sealed plastic bags homogenized, and subsequently stored at 4°C. OPG were counted using the McMaster counting chamber. This method has also been used to determine the oocyst index in different groups, compared to the OPG of the control positive group (Dommels et al., 2007). Pathological assessmentsOn the 10th dpc, five birds from each group were randomly selected and euthanized by neck dislocation. To alleviate any potential distress experienced by the birds, sodium pentobarbital (50 mg/kg) was provided via intraperitoneal injection before this particular stage to detect macroscopic changes in the cecum and scored from 0 to 4 using Johnson and Reid (1970) (Table 2). Ceca were preserved in 10% formalin and routinely processed according to the method of Bancroft et al. (1996), as well as, scoring according to the nature and extent of the lesion and recurrence, (Shackelford et al., 2002) (Table 2), which was used to calculate the histological injury score (HIS) and lesion index (Dommels et al., 2007). Biochemical parametersBlood samples (n= 5 per group) were collected at 14, 21, and 35 days of age from the right jugular vein of the neck using sterile plane blood collecting tubes, and the serum was centrifuged out of the blood samples and stored at −20°C for biochemical analysis. Biomarkers of oxidative stress (Biodiagnostic kit’s guidelines), such as serum malondialdehyde (MDA) were determined using a colorimetric technique (Kei, 1978), serum total superoxide dismutase (T-SOD) (Nishikimi et al., 1972), and catalase enzyme (Aebi, 1984). Lipid profile was evaluated as follows: total cholesterol (Richmond, 1973), high-density lipoprotein cholesterol (HDL-C) (Lopes-Virella et al., 1977), low-density lipoprotein cholesterol (LDL-C) (Wieland and Seidel, 1983), and triacylglycerol (Fossati and Prencipe, 1982), and liver function was monitored by measuring serum aspartate transferase (AST) and alanine transferase (ALT) (Young, 1997). Meanwhile, creatinine and urea were measured (Tovar et al., 2002). Table 2. Grading scores of Clinicopathological changes.
Table 3. Active compounds of the LRE.
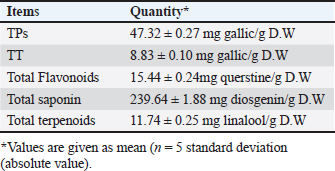
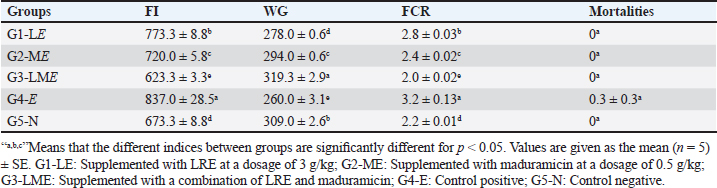
Molecular dockingThree-dimensional (3D) structures of E. tenella DNA polymerase, E. tenella apical membrane antigen 1 (EtAMA1), and E. tenella microneme protein 3 (EtMIC3) proteins were retrieved from the AlphaFold Protein Structure Database (https://www.uniprot.org/). In addition, the 3D structure of maduramicin was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), while the 3D structures of licorice bioactive compounds were retrieved from the LOTUS database (https://lotus.naturalproducts.net/). Protein preparation, protein-ligands interactions, and visualization were performed using Molecular Operating Environment software (MOE 2022.02, Chemical Computing Group, Montreal, QC, Canada). Statistical analysisDifferences between groups were analyzed using One-Way ANOVA and Tukey’s multiple comparison Post hoc tests (Duncan, 1955), and statistical analysis was performed using the statistical software package SPSS for Windows (version 20.0; SPSS Inc., Chicago, IL, USA). Statistical significance between mean values was set at p < 0.05. A sample of at least five in each group and a total sample of 25 chicks (divided into five groups) are required to estimate an effect size=1.029 (Hussain et al., 2022) and a normal mortality rate (dropout) of 10% with a significance level of 5%, which will provide 90% power. The sample size is calculated using G-Power Software (3.0.10). Ethical approvalThe Animal Welfare Committee of the Faculty of Veterinary Medicine, Benha University (BUFVTM 04-02-22), granted approval for all operations performed in this study, which included bird handling and maintenance. ResultPhytochemical analysisSeveral bioactive components were found in a chemical investigation of LRE. Table 3 displays the main components, TPs 47.32 ± 0.27 mg gallic/g, total flavonoids 15.44 ± 0.24 mg quercetin/g, and TTs 8.83 ± 0.10 mg gallic/g. The total terpenoids in the sample were 11.740.25 mg linalool/g, while the total saponin content was 239.641.88 mg diosgenin/g. The relatively high content of total saponin in the investigated extract was documented as 239.64 ± 1.88 mg diosgenin/g, while total terpenoids were 11.74 ± 0.25 mg linalool/g. Clinicopathological assessmentsIn the current study, the G3-LME group showed the most significant decrease in FI and FCR while registering the highest performance compared to other groups, at the same time, it had the highest average WG during the challenge period, and in contrast, G4-E had the lowest WG and highest FI and FCR. In terms of dropping scoring, oocyst output, FCR, WG, cecal damage, and ACI values, they were more potent in G3-LME compared to groups fed LRE and maduramicin individually (Table 4). Depression, ruffled feathers, lethargy, and bloody diarrhea were frequently seen in G4-E-challenged birds. Other gross cecal changes included enlargement and discoloration, numerous mucosal hemorrhages, and sausage-like bloody contents (Fig. 1). In addition, histological analysis showed that loss of intestinal villi, degeneration of intestinal glands laden with coccidian oocysts, mononuclear cell infiltrate, and hemorrhages all contributed to the deterioration of the cecal architecture. Therefore, as indicated in Tables 5–7 and Figures 1–3, it was evident that the mean oocyst shedding and dropping scores, macroscopic, and microscopic lesions in this group recorded the highest values. Table 4. Effect of LRE and maduramicin alone or in combination On the performance of Sasso broilers challenged with Eimeria tenella.
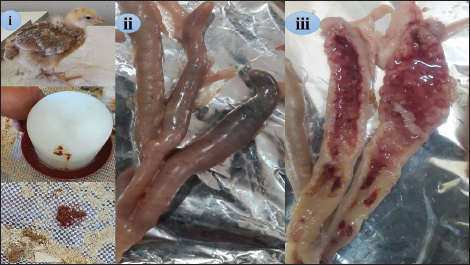
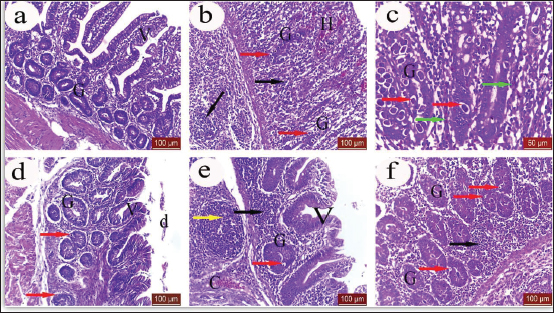
Fig. 1. Clinical and pathological findings of the cecal coccidiosis in Sasso chickens observing (i) depressed bird with ruffled feather and bloody droppings, (ii) enlarged and sausage appearance ceca, and (iii) cecal core with bloody contents. Birds fed LRE and/ or maduramicin appear to be healthy; no deaths or clinical abnormalities were observed, according to the analysis of clinicopathological markers. Compared with G4-E, the dropping scoring and oocyst shedding of groups G1-LE, G2-ME, and G3-LME along the 10th dpc, as well as the macroscopic and microscopic lesions scored at the 7th dpc, was considerably reduced (Table 5). The cecum of birds in G1-LE showed modest desquamation of the intestinal villi epithelial cells and reduced oocyst invasion of the intestinal glands (Fig. 2d). The intestinal blood vessels of birds in group G2-ME showed substantial mononuclear cellular infiltration, either diffuse or cluster-aggregated congestion, as well as a modest oocyst invasion (Fig. 2e). A mononuclear cellular infiltration and a small number of oocysts invading the intestinal glands were recorded in group G3-LME (Fig. 2f).
Fig. 2. Effect of LRE and maduramicin alone or in combination on the histopathological findings in the cecal tissues of Sasso chickens challenged with E. tenella oocysts observing (a) cecum of the control negative group showing normal intestinal villi (V) and intestinal glands (G), (H & E stain, X 20 lens). (b) Cecum of the control positive groups showed atrophied intestinal glands (G), hemorrhage (H), mononuclear cellular infiltration (black arrow) and oocyst (red arrow). (H & E stain, X 20 lens). (c) Higher magnification of showed atrophied intestinal glands (G), oocyst (red arrow) R.B. Cs in congested blood capillaries (green arrow). (H & E stain, X 40 lens). (d) cecum of birds fed on the licorice treated diet showed intestinal villi (V) with desquamated epithelial cells (d), intestinal glands (G) and some oocyst invading intestinal glands (red arrow). (H & E stain, X 20 lens). (e) Cecum of maduramicin treated groups showed normal intestinal villi (V), intestinal glands (G) with some oocyst (red arrow), congestion of blood vessels (C), mononuclear cellular infiltration either diffuse (black arrow) or nodular (yellow arrow). (H & E stain, X 20 lens). (f) Cecum of combined treated groups with licorice and maduramicin displayed little affected intestinal glands which invaded by low number of oocyst (red arrow), diffuse cellular infiltration (black arrow). (H & E stain, X 20 lens).
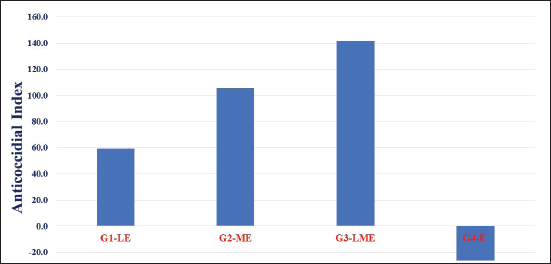
Fig. 3. The ACI value was lower than 120 which means “lack of anticoccidial activity,” at 120–140 “mild effective,” at 140–160 “moderate effective” and at higher than 160 “marked effective.” The ACI is calculated according to the equation of subscripting for the sum of the lesion index (LI) and oocyst index (OI) from the sum for the percentages of survival and relative gain weight %S + %RGW) − (LI + OI) (De Pablos et al. 2010), where LI is the lesion index as the HIS multiplied by 10 and OI is the oocyst index as (OPG output of each experimental group/OPG output of the infected untreated control) × 100.
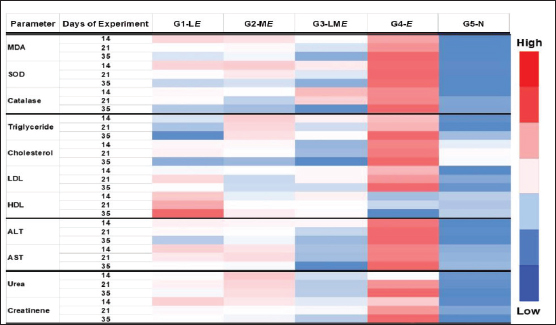
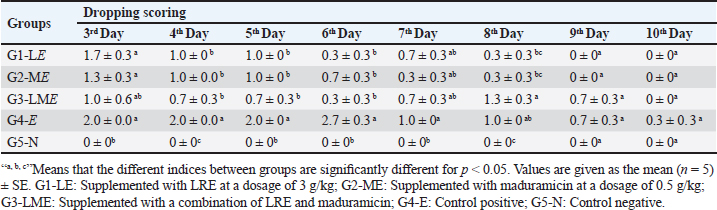
Fig. 4. Heat map showing the effect of LRE and maduramicin alone or in combination on mean values for antioxidant and lipid profiles and liver and kidney function tests for different experimental groups. Biochemical parametersEffects of individual and combined doses of LRE (3 g/kg) and maduramicin (0.5 g/kg) on oxidative stress biomarkers of E. tenella-challenged birds were recorded at 14, 21, and 35 days of the experiment (Fig. 4). After E. tenella infection, MDA, SOD, and catalase were higher than the negative control. The trial showed increased antioxidant enzymes in G3-LME after 35 days, with MDA, SOD, and catalase levels of 3.09 ± 0.05, 3.10 ± 0.03 (nmol/ml), 158.38 ± 0.88, 164.11 ± 0.31 (U/mg), and 16.64 ± 0.25, 16.19 ± 0.22 (U/mg) for G1-LE and G2-ME The MDA, SOD, and catalase levels in G3-LME was close to the control negative group (2.38 ± 0.04, 141.56 ± 1.01, and 15.06 ± 0.67). The G4-E had significantly higher total cholesterol (202.65 ± 1.19mg/dl), triacylglycerol (110.07 ± 0.18 mg/dl), and LDL-C (58.88 ± 0.61 mg/dl), but lower HDL-C (56.44 ± 0.37 mg/dl) at 35 days. Throughout the trial period, the lipid profile of the challenged broilers was gradually improved in G1-LE, G2-ME, and G3-LME. Total cholesterol was increased in G1-LE and G2-ME (158.77 ± 0.86 and 160.70 ± 0.46 mg/dl, respectively), but the combined treatment G3-LME showed the largest increase (152.51 ± 0.75 mg/dl) compared to the negative control at 35 days of age. The greatest elevation in HDL-C was observed in G1-LE (76.84 ± 0.56 mg/dl) followed by G2-ME (66.11 ± 0.79 mg/dl) and G3-LME (65.12 ± 0.37 mg/dl). Triacylglycerol levels were slightly different between the infected and feed-treated groups (Fig. 4). Following the E. tenella challenge, AST and ALT levels increased directly as recorded in the positive control G4-E group (161.890.69 and 76.330.18 (U/l), respectively). In contrast, the highest drop in both enzymes was listed in the G3-LME group, reaching 133.53 ± 0.42 and 60.48 ± 0.47 (U/l), respectively, while the G5-N group was found to have values of 139.65 ± 0.18 and 55.66 ± 0.42 (U/l). During several periods of the experiment, G1-LE and G2-ME showed a decrease in liver enzymes throughout the experiment, reaching a significant decrease on the 35th day of the experiment (Fig. 4). Table 5. Effect of LRE and maduramicin alone or in combination on the dropping scores of E. tenella challenged groups along 10 days post-challenge.
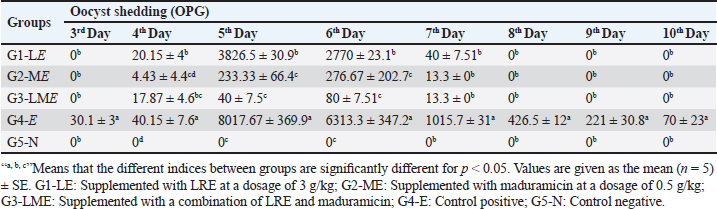
Table 6. Effect of LRE and maduramicin alone or in combination on the oocyst shedding for ten days post challenged with E. tenella.
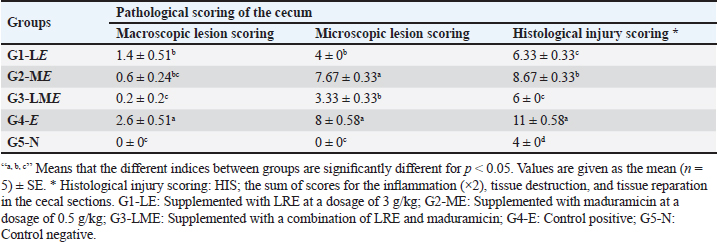
Table 7. Effect of LRE and maduramicin alone or in combination on the pathological change scores in the cecum of the Sasso chicken for 7 days post-challenge with E. tenella.
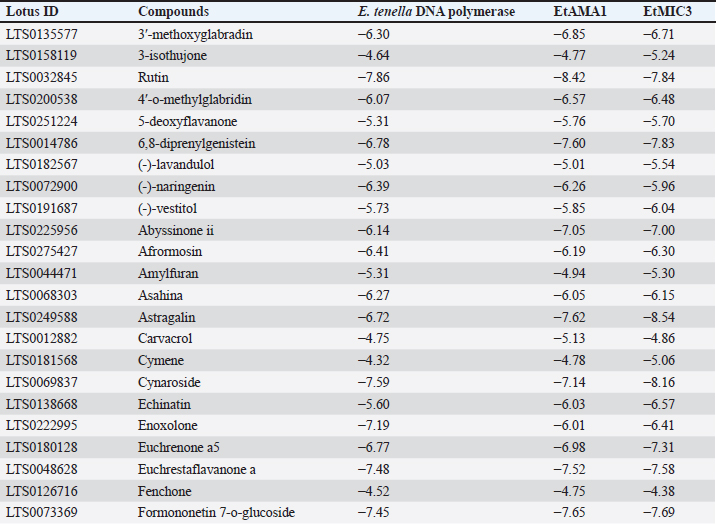
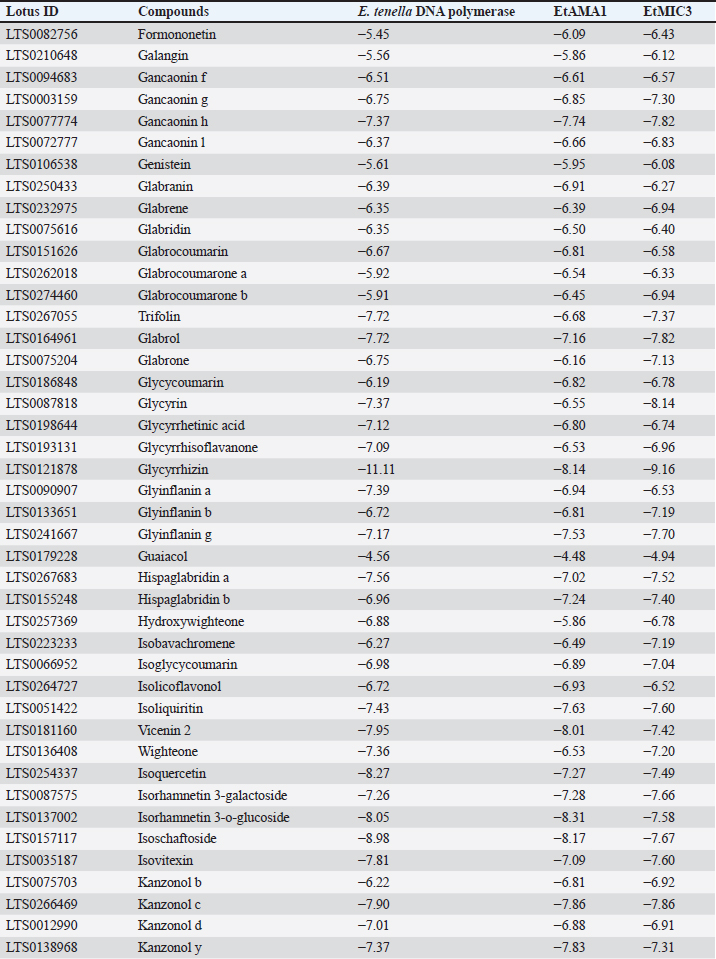
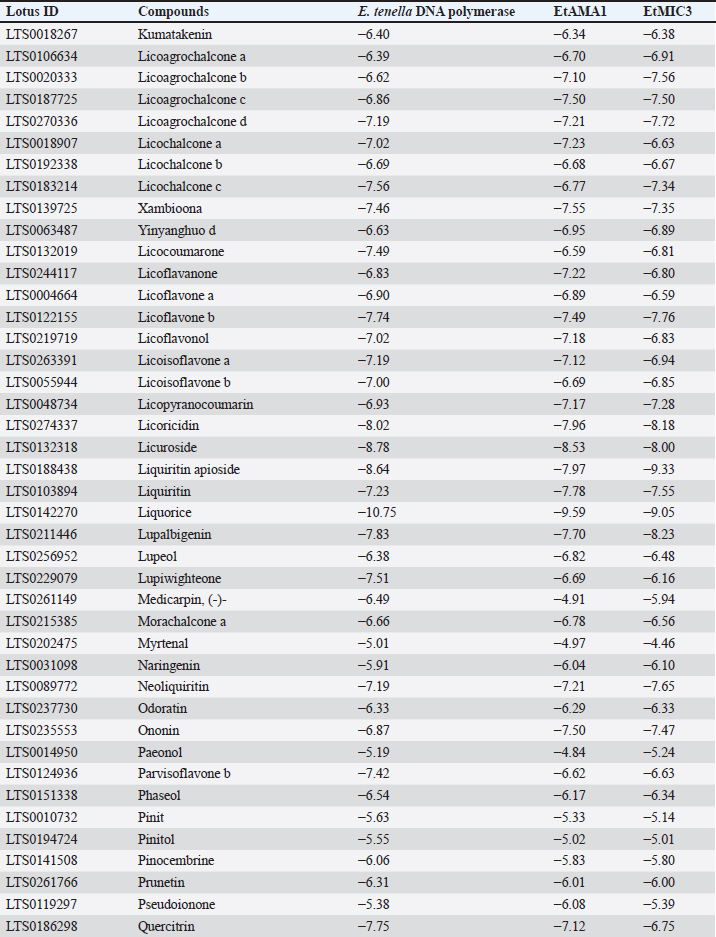
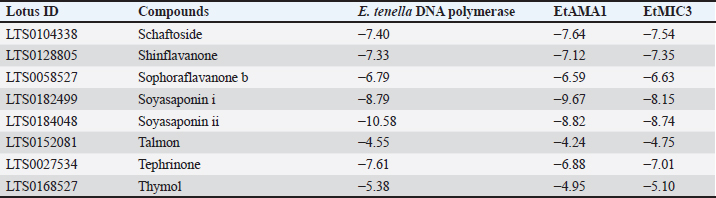
Table 8. Molecular docking scores of licorice’ bioactive compounds and E. tenella DNA polymerase, E. tenella apical membrane antigen 1 (EtAMA1), and EtMIC3 binding sites.
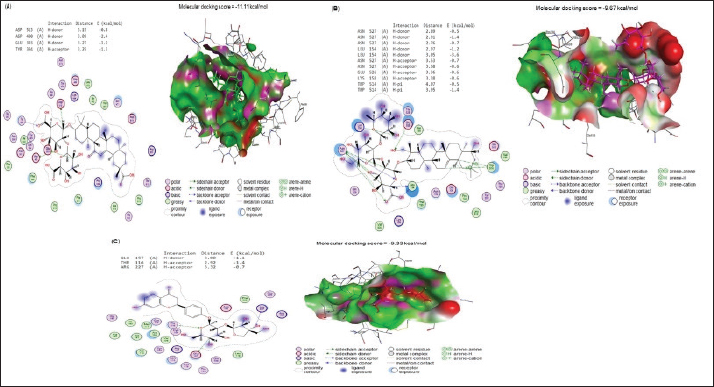
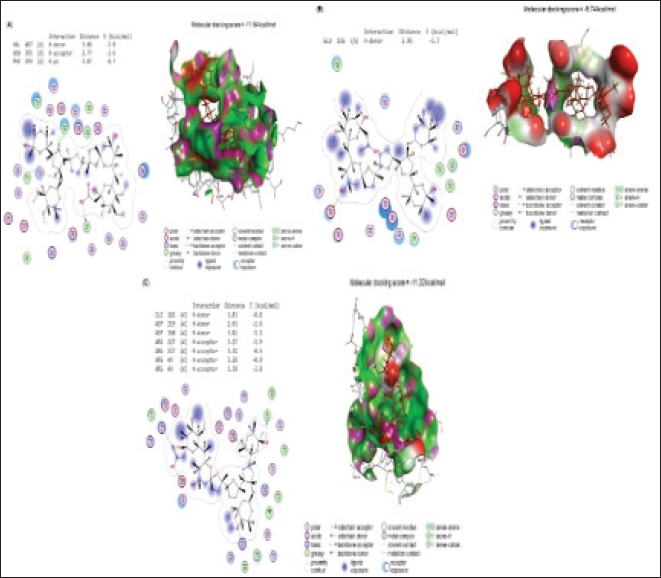
Fig. 5. (A) Molecular interaction of glycyrrhizin with E. tenella DNA polymerase binding site, (B) molecular interaction of soyasaponin i with E. tenella apical membrane antigen 1 (EtAMA1) binding site, and (C) molecular interaction of liquiritin apioside EtMIC3 binding site. The positive control group had greater serum urea and creatinine concentrations than the negative control group at all experimental time points (14, 21, and 35 days). All examined feed regimens improved kidney functioning by lowering serum urea levels. The highest reduction in serum urea was noticed in G3-LME, followed by G1-LE and G2-ME which came in second and third place, with 7.26 ± 0.12 and 8.09 ± 0.17 mg/dl, respectively. The G1-LE, G2-ME, and G3-LME showed significantly lower serum creatinine values (0.55 ± 0.03, 0.54 ± 0.02 and 0.50 ± 0.01 mg/dl), respectively (Fig. 4). Molecular dockingMolecular docking interactions of licorice’s bioactive compounds are represented in Table 8 and Figure 5. Glycyrrhizin interacted with E. tenella DNA polymerase binding site by energy of −11.11 kcal/mol with ASP513 (H-donor), ASP400 (H-donor), GLU303 (H-donor), and TYR394 (H-acceptor) (Fig. 5A). −9.67 kcal/mol is the molecular docking score of soya saponin with EtAMA1 binding site interaction through H-donor (ASN527 and LEU154), H-acceptor (ASN527, GLU526, and LYS158), and H-pi (TRP514) (Fig. 5B). In addition, liquiritin apioside bound with EtMIC3 binding site with energy value of −9.33 kcal/mol through interaction with GLU157 (H-donor), THR116 (H-acceptor), and ARG227 (H-acceptor) (Fig. 5C). Maduramicin interacted with E. tenella DNA polymerase binding site with an energy of −11.54 kcal/mol through interaction with ILE183 (H-donor), ASP219 (H-donor), ASP160 (H-acceptor), ARG227 (H-acceptor), and ARG44 (H-acceptor), as presented in Figure 6A. In addition, maduramicin bound with GLU136 (H-donor) residue in EtAMA1 binding site with energy value of −8.74 kcal/mol (Fig. 6B). By energy value of −11.22 kcal/mol, maduramicin interacted with EtMIC3 binding site by H-donor (ILE183, ASP219, and ASP160) and H-acceptor (ARG227 and ARG44) (Fig. 6C).
Fig. 6. (A) Molecular interaction of maduramicin with E. tenella DNA polymerase binding site, (B) molecular interaction of maduramicin with E. tenella apical membrane antigen 1 (EtAMA1) binding site, and (C) molecular interaction of maduramicin with EtMIC3 binding site. DiscussionAvian coccidiosis, a cosmopolitan protozoal infection, has been linked to significant financial losses in the poultry sector (Moryani et al., 2021). To address the negative effects of anticoccidial feed additives on poultry diets on bird health; a new trend has emerged in natural alternatives due to their high safety rates and positive impacts. Licorice is one of the oldest and most well-known medicinal plants that have anticoccidial activities on several Eimeria species (Pop et al., 2019; Hussain et al., 2022; Ghafouri et al., 2023)anticoccidials are generally used as feed additives. However, the frequent usage has given rise to the occurrence of resistant strains to available anticoccidial drugs. Botanicals may work as substitutes to anticoccidial drugs. The current research was designed to evaluate the efficacy of aqueous methanol extracts of Glycyrrhiza glabra (licorice. Hence, in this study, the clinical, biochemical, and histopathological effects of LRE alone or in combination with maduramicin were evaluated in Sasso broilers infected with E. tenella. The chemical examination of the LRE in this study showed numerous crucial secondary metabolites. These included phenols, flavonoids, tannins, saponins, and terpenoids. Similarly, a large body of research has stated that phenolic compounds and flavonoids as well as saponins and terpenoids found in LRE are abundant (Rodino et al., 2015; Soliman and El-Genaidy, 2021). Many medicinal effects have been attributed to plants rich in phenols, flavonoids, and tannins (Rajpurohit et al., 2017), this could explain why it has therapeutic effects. According to the findings of the current study, the infected Sasso chicks in G4-E gained less weight with high FCR and ruffled feathers in comparison to other groups. It is backed up by reports indicating that coccidian infection reduces the bird performance (Logan et al., 1993; Hashmi et al., 1994; Tipu et al., 2002). These findings could be attributed to the parasite’s mechanism of destroying the absorptive mucosal surface and competing for micronutrients which in turn results in a metabolic disturbance and detrimental effect on nutrition utilization (Ali et al., 2019) Birds in G3-LME had superior clinicopathological scores than the other infected groups in terms of FCR and BW with decreased OPG count, severity of cecal lesions at 7th dpc, and absence of bloody secretion. Similar claims were made about the efficacy of adding LRE or maduramicin to feed on reducing symptoms of coccidiosis (Williams, 1998; Peek and Landman, 2011; Kadykalo et al., 2018; Pop et al., 2019; Hussain et al., 2022; Ghafouri et al., 2023). Despite this, the moderate ACI was recorded in the G3-LME with a value of 141.8, while the ACI in G1-LE and G2-ME was 59.1 and 105.5, respectively, being insufficient. The therapeutic and prophylactic effect of licorice extracts alone or in combination with other compounds has been demonstrated in a dose-dependent manner in broilers against single and mixed infections of different Eimeria species by oral administration, and the treatments have a positive effect on the intestinal lesions, oocyst outputs, and performance parameters (Pop et al., 2019; Hussain et al., 2022; Ghafouri et al., 2023). These results could be attributed to the effect of the phenolic contents of licorice and the chemical elements of maduramicin which increases intestinal mucosal secretion and block ion transport channels, respectively, which weakens the parasite’s osmotic balance and leads to the impairment in the vital processes of coccidian, and finally, their death (Sikkema et al., 1995; Aly et al., 2005; Arczewska-Wlosek et al., 2012). Regarding antioxidant biomarkers, linear trends were seen between coccidiosis and the clear elevation of MDA, SOD, and catalase enzymes. Indicators of infection-induced oxidative stress were gradually reduced by supplementing the feed with LRE alone or combined with maduramicin. Active secondary metabolites of licorice extract may be responsible for reducing the negative consequences of coccidiosis infection, as flavonoids (isoflavonoids and liquiritin), glycyrrhizic acid, liquiritigenin, triterpenes (glycyrrhizin), and saponins in licorice root have been reported to have antioxidant activity (Vlaisavljević et al., 2018). The present results were consistent with previous work which stated that polyphenol administration in chicken drinking water has been shown to improve antioxidant processes. Similar to previous data reported by Pabón et al. (2003) on the onset of oxidative stress in parasitic diseases, Georgieva et al. (2006) suggested that the increased blood MDA concentrations in 20-day-old broiler chickens infected with E. tenella may be attributable to the occurrence of oxidative stress with increased s production, leading to lipid peroxidation. Moreover, sick chicks in the study had higher blood CAT and lower SOD concentrations compared to controls. Following oxidative stress, an increase in CAT levels has been suggested to disrupt the ecological oxidative balance. Serum MDA concentrations as well as the actions of ALP, AST, ALT, and GGT are frequently utilized as markers of oxidative stress and tissue damage. According to some previous findings, Mahmoodzadeh et al. (2017) and Pastorino et al. (2018) suggested that the active components of licorice, such as flavonoids, saponins, sugars, coumarins, amino acids, starch, tannins, phytosterols, choline, and vitamins, may be correlated to the improved immunity and oxidative states in birds. In the current study, decreased HDL-C with increased serum triacylglycerol and total cholesterol content were indicators of the adverse effects of coccidiosis on broilers. All treatments improved the lipid profile of broiler serum, including total cholesterol, HDL-C, LDL-C, and triacylglycerols, and to some extent mitigated the negative effects of coccidiosis infection. Compared to the control group, feeding birds LRE significantly reduced total cholesterol and enhanced HDL-C. The obtained results were in harmony with a previous study that reported that licorice consumption gradually decreased the levels of total cholesterol, LDL-C, and triacylglycerols in a dose-dependent way (Aghdam et al., 2018). Moreover, a prior investigation verified that feeding birds diets with licorice powder reduced cholesterol, triacylglycerols, and low-density lipoprotein (Fuhrman et al., 2002; Sharifi et al., 2013). The challenged birds in G3-LME showed improvement in lipid profile, which may be attributed to the synergistic effects between LRE and maduramicin. The positive impacts of LRE alone or in combination with maduramicin could be attributed to licorice’s ability to reduce LDL oxidation while inhibiting lipid peroxidation, lipoxygenase, and cyclooxygenase enzyme activity (Fuhrman et al., 2002). Increased release of cholesterol, bile acids, neutral sterols, and hepatic bile acid concentration are thought to be responsible for the cholesterol-lowering effects of licorice (Sharifi et al., 2013). In addition, licorice’s active ingredient, saponin, can block the development of lipid peroxides, increase the rate of cholesterol conversion into bile acids, and accelerate the hepatic clearance of cholesterol. The current acquired results showed that daily use of licorice powder, either as a solo supplement or when combined with maduramicin, had a favorable impact on both the aminotransferase enzymes (AST and ALT) and kidney functions (urea and creatinine). LRE and maduramicin together had a positive synergistic effect on AST, ALT, urea, and creatinine. All bioactive secondary metabolites, which are believed to be the main cause of antioxidant, anti-inflammatory, anticancer, and so on effects, may contribute to the effects of licorice extract (Vlaisavljević et al., 2018). The hepatoprotective effect of licorice relates to an improvement in antioxidant defense and anti-inflammatory response, according to prior research on serum ALT and AST activity and triacylglycerols. The outcomes were consistent with studies by Abd-Al-Sattar (2016), which established that aspartate aminotransferase activity, which was raised by CCl4 in rats to generate acute hepatotoxicity, is inhibited by supplementation with aqueous methanolic extracts of G. glabra. Moreover, oral LRE administration reduced the levels of kidney function parameters (urea, uric acid, and creatinine) in comparison to the control group. In addition, another report evaluated the anti-nephritis activity of glabradin, a pyramid of lavan isolated from G. glabra, after oral administration in glomerular disease-challenged mice, and their results showed a significant decline in the amount of urinary protein excretion, blood urea nitrogen, and serum creatinine levels (Fukai et al., 1998). Molecular docking assessment revealed that licorice’s bioactive compound and maduramicin effectively bind to the binding site of DNA polymerase of E. tenella, EtAMA1, and EtMIC leading to the reduction of E. tenella replication and invasion because EtAMA1 and EtMIC3 facilitate its invasion to the host cells (Jiang et al., 2012; Chen et al., 2021). ConclusionHerbal medicine is effective in treating and preventing several parasitic infections. Herbal remedies show promise as a viable option for treating coccidiosis. Despite the wealth of data available on traditional medicine, little has been done to understand and promote its usage in clinical settings, particularly in conjunction with modern medications that have anticoccidial qualities. In this research, LRE was found to be effective against coccidiosis in chickens when administered simultaneously with maduramicin. We base our recommendation on the bioactive components found in licorice, which include phenols, flavonoids, tannins, saponins, and terpenoids and are believed to be the main reason for licorice’s medicinal benefits. AcknowledgmentsProf. Dr. Ali H. El-Far, Department of Biochemistry, Faculty of Veterinary Medicine, Damanhur University, Egypt, is sincerely thanked for his efforts in molecular docking. Moreover, the authors would like to extend sincere appreciation to Ph. Basma Nabil Mohamed (pharmacist at the Egyptian Ministry of Health), for her valuable aid in calculating the size of experimental groups and samples. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe authors did not receive support from any organization for the submitted work. Author contributionsConceptualization: Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, Samah M. Abdel Gawad, Reda E.L. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa Fathy SaadEldin; Methodology: Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, Samah M. Abdel Gawad, Reda E.L. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa Fathy SaadEldin; Resources: Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, Samah M. Abdel Gawad, Reda E.L. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa Fathy SaadEldin. All authors participated in writing the manuscript: Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, Samah M. Abdel Gawad, Reda E.L. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa Fathy SaadEldin; Review and editing: Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, Hala El Daous. Preparation of all tested diets: Marwa I. Abdel Haleem, Sawsan S. Elbasuni. All authors drafted and approved the final version of the manuscript. Sawsan S. Elbasuni, Marwa I. Abdel Haleem, Hanan A.A. Taie, and Hala El Daous carried out the statistical analysis. Data availabilityData are available on request.ss ReferencesAbbas, A., Iqbal, Z., Abbas, R.Z., Khan, M.K., Khan, J.A., Sindhu, Z. ud D., Mahmood, M.S. and Saleemi, M.K. 2017. In vivo anticoccidial effects of Beta vulgaris (sugar beet) in broiler chickens. Microb. Pathog. 111, 139–144. Abd-Al-Sattar S.L. 2016. Hepatoprotective effect of Glycyrrhiza Glabra L. extracts against carbon tetrachloride-induced acute liver damage in rats. Int. J. Vet. Sci. Med. Res. 1, 1–8. Abdel Haleem, M., Hassan, A. and Moustafa, M. 2019. Effect of using synbiotics and essential oils on performance parameters and immune response of necrotic enteritis challenged broiler chicks. Benha Vet. Med. J. 36, 384–391. Aebi, H. 1984. Catalase in vitro. Methods in enzymology, 105th ed., Cambridge, MA: Academic Press, pp: 121–126. Aghdam Shahryar, H., Ahmadzadeh, A. and Nobakht, A. 2018. Effects of different levels of Licorice (Glycyrrhiza glabra) medicinal plant powder on performance, egg quality and some of serum biochemical parameters in laying hens. Iran. J. Appl. Anim. Sci. 8, 19–124. Al-Gawad, A.A., Mahdy, O.A., Olfat A., El-Massry, A.A.N., Aida A.N. and Al-Aziz, M.S.A. 2012. Studies on coccidia of Egyptian balady breed chickens. Life Sci. J. 9, 568–576. Ali, M., Chand, N., Khan, R.U., Naz, S. and Gul, S. 2019. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 47, 79–84. Allen, P.C., Danforth, H.D. and Augustine, P.C. 1998. Dietary modulation of avian coccidiosis. Int. J. Parasitol. 28, 1131–1140. Aly, A.M., Al-Alousi, L. and Salem, H.A. 2005. Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS Pharm. Sci. Tech. 6, E74–E82. Arczewska-Włosek, A. and Świątkiewicz, S. 2012. The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J. Anim. Feed Sci. 21, 133–142. Awais, M.M., Akhtar, M., Anwar, M.I. and Khaliq, K. 2018. Evaluation of Saccharum officinarum L. bagasse-derived polysaccharides as native immunomodulatory and anticoccidial agents in broilers. Vet. Parasitol. 249, 74–81. Bancroft, J.D., Stevens, A. and Turner, D.R. 1996. Theory and practice of histopathological techniques. Ed., Livingstone, C. Fourth Ed. New York, London, San Francisco, Tokyo. Berezin, V.E., Bogoyavlenskyi, A.P., Khudiakova, S.S., Alexuk, P.G., Omirtaeva, E.S., Zaitceva, I.A., Tustikbaeva, G.B., Barfield, R.C. and Fetterer, R.H. 2010. Immunostimulatory complexes containing Eimeria tenella antigens and low toxicity plant saponins induce antibody response and provide protection from challenge in broiler chickens. Vet. Parasitol. 167, 28–35. Blake, D.P., Knox, J., Dehaeck, B., Huntington, B., Rathinam, T., Ravipati, V., Ayoade, S., Gilbert, W., Adebambo, A.O., Jatau, I.D., Raman, M., Parker, D., Rushton, J. and Tomley, F.M. 2020. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 51, 115. Cheel, J., Theoduloz, C., Rodríguez, J.A., Caligari, P.D.S. and Schmeda-Hirschmann, G. 2007. Free radical scavenging activity and phenolic content in achenes and thalamus from Fragaria chiloensis ssp. chiloensis, F. vesca and F. x ananassa cv. Chandler. Food Chem. 102, 36–44. Chen, W., Ma, C., Li, G., Jia, Z., Yang, X., Pan, X. and Ma, D. 2021. Specific EtMIC3-binding peptides inhibit Eimeria tenella sporozoites entry into host cells. Vet. Res. 52(1), 24. De Pablos, L.M., Dos Santos, M.F.B., Montero, E., Garcia-Granados, A., Parra, A. and Osuna, A. 2010. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol. Res. 107, 601–604. Dommels, Y.E.M., Butts, C.A., Zhu, S., Davy, M., Martell, S., Hedderley, D., Barnett, M.P.G., McNabb, W.C. and Roy, N.C. 2007. Characterization of intestinal inflammation and identification of related gene expression changes in mdr1a-/-mice. Genes Nutr. 2, 209–223. Du, A. and Hu, S. 2004. Effects of a herbal complex against Eimeria tenella infection in chickens. J. Vet. Med. Series B: Infect. Dis. Vet. Public Health 51, 194–197. Duncan, D.R. 1955. Multiple range and multiple F tests. Biometrics, 11, 1–42. Fossati, P. and Prencipe, L. 1982. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 28, 2077–2080. Fuhrman, B., Volkova, N., Kaplan, M., Presser, D., Attias, J., Hayek, T. and Aviram, M.W. 2002. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: Increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutr. 18, 268–273. Fukai, T., Cai, B.S., Maruno, K., Miyakawa, Y., Konishi, M. and Nomura, T. 1998. An isoprenylated flavanone from Glycyrrhiza glabra and rec-assay of licorice phenols. Phytochem. 49, 2005–2013. Georgieva, N.V., Koinarski, V. and Gadjeva, V. 2006. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 172, 488–492. Ghafouri, S.A., Ghaniei, A., Tamannaei, A.E.T., Sadr, S., Charbgoo, A., Ghiassi, S. and Abuali, M. 2023. Evaluation of therapeutic effects of an herbal mixture (Echinacea purpurea and Glycyrrhiza glabra) for treatment of clinical coccidiosis in broilers. Vet. Med. Sci. 9, 829–836. Hashmi, H., Issot, N. and Maqbool, A. 1994. Experimental induction of coccidiosis in broiler chicks with Eimeria tenella and comparative efficacy of different prophylactic measures against the disease. J. Anim. Health Prod. 14, 55–63. Hussain, K., Alsayeqh, A.F., Abbas, A., Abbas, R.Z., Rehman, A., Zaib, W., Ur Rehman, T. and Mahmood, M.S. 2022. Potential of Glycyrrhiza glabra (Licorice) extract an alternative biochemical and therapeutic agent against coccidiosis in broiler chickens. Kafkas Universitesi Veteriner Fakultesi Dergisi, 28, 585–591. Hussain, K., Iqbal, Z., Abbas, R.Z., Khan, M.K. and Saleemi, M.K. 2017. Immunomodulatory activity of Glycyrrhiza glabra extract against mixed Eimeria infection in chickens. Int. J. Agric. Biol. 19, 928–932. Jiang, L., Lin, J., Han, H., Dong, H., Zhao, Q., Zhu, S. and Huang, B. 2012. Identification and characterization of Eimeria tenella apical membrane antigen-1 (AMA1). PloS One, 7(7), e41115. Johnson, J. and Reid, W.M. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exper. Parasitol. 28, 30–36. Kadykalo, S., Roberts, T., Thompson, M., Wilson, J., Lang, M. and Espeisse, O. 2018. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 51, 304–310. Kalkal, H., Kumar, P. and Vohra, S. 2021. Advances in control strategies and vaccine development of coccidiosis in poultry. Pharm. Innov. J. 10, 1084–1090. Kei, S. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta, 90, 37–43. Koleva, I.I., Van Beek, T.A., Linssen, J.P.H., De Groot, A. and Evstatieva, L.N. 2002. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Wiley Online Library 13, 8–17. Logan, N.B., McKenzie, M.E., Conway, D.P., Chappel, L.R. and Hammet, N.C. 1993. Anticoccidial efficacy of semduramicin. 2. Evaluation against field isolates including comparisons with salinomycin, maduramicin, and monensin in battery tests. Poult. Sci. 72(11), 2058–2063. Longstaffe, J.A. 1984. Helminths, arthropods and protozoa of domesticated animals (7th edition). Transact. Royal Soc. Trop. Med. Hyg. 78, 329. Lopes-Virella, M.F., Stone, P., Ellis, S. and Colwell, J.A. 1977. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 23, 882–884. Mahmoodzadeh, Y., Mazani, M. and Rezagholizadeh, L. 2017. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 4, 455–462. Makkar, H.P.S., Becker, K., Abel, H. and Pawelzik, E. 1997. Nutrient contents, rumen protein degradability and antinutritional factors in some colour- and white-flowering cultivars of Vicia faba beans. J. Sci. Food Agric. 75, 511–520. Morehouse, N.F. and Baron, R.R. 1970. Coccidiosis: Evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exper. Parasitol. 28, 25–29. Moryani, A.A., Rajput, N., Naeem, M., Shah, A.H. and Jahejo, A.R. 2021. Screening of the herbs and evaluation of their combined effects on the health and immunity of coccidiosis challenged broiler chickens. Pakistan Vet. J. 41, 228–234. Nishikimi, M., Appaji Rao, N. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. NRC. 1994. Nutrient requirements of poultry (Ninth Ed.). Washington, DC: National Academy Press. Ordoñez, A.A.L., Gomez, J.D., Vattuone, M.A. and Isla, M.I. 2006. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 97, 452–458. Pabón, A., Carmona, J., Burgos, L.C. and Blair, S. 2003. Oxidative stress in patients with non-complicated malaria. Clin. Biochem. 36, 71–78. Pastorino, G., Cornara, L., Soares, S., Rodrigues, F., Beatriz, M. and Oliveira, P.P. 2018. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Wiley Online Library, 32, 2323–2339. Peek, H.W. and Landman, W.J.M. 2011. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Quart. 31, 143–161. Pop, L.M., Varga, E., Coroian, M., Nedisan, M.E., Mircean, V., Dumitrache, M.O., Farczádi, L., Fülöp, I., Croitoru, M.D., Fazakas, M. and Gyorke, A. 2019. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasit. Vectors 2019, 12. Rajpurohit, A., Nayak, D.S., Patil, S., Mahadevan, K. 2017. In vitro antioxidant, antimicrobial and admet study of novel furan/benzofuran c-2 coupled quinoline hybrids. Int. J. Pharm. Pharm. Sci. 19, 144–153. Rashidi, N., Khatibjoo, A., Taherpour, K., Akbari-Gharaei, M. and Shirzadi, H. 2020. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B1. Poult. Sci. 99, 5896–5906. Richmond, W. 1973. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 19, 1350–1356. Rodino, S., Butu, A., Butu, M. and Cornea, P.C. 2015. Comparative studies on antibacterial activity of licorice, elderberry and dandelion. Digest J. Nanomat. Biostruct. 10, 947–955. Shackelford, C., Long, G., Wolf, J., Okerberg, C. and Herbert, R. 2002. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 30, 93–96. Sharifi, S., Khorsandi, S., Khadem, A., Salehi, A. and Moslehi, H. 2013. The effect of four medicinal plants on the performance, blood biochemical traits and ileal microflora of broiler chicks. Veterinarski Arhiv. 83, 69–80. Sikkema, J., de Bont, J.A. and Poolman, B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59, 201–222. Soliman, N. and El-Genaidy, M. 2021. Toxicological and histological effects of licorice Glycyrrhiza glabra L., roots aqueous extract on mediterranean fruit fly, ceratitis capitata wied. (Diptera: Tephritidae) Under Laboratory Conditions. J. Plant Protect. Pathol. 12, 553–562. Sundar, S.T.B., Harikrishnan, T.J., Latha, B.R., Chundra, G.S. and Kumar, T.M.A.S. 2017. Anticoccidial drug resistance in chicken coccidiosis and promising solutions : a review. J. Entomol. Zool. Stud. 5, 1526–1529. Swayne, D.E., Boulianne, M., Logue, C.M., Mcdougald, L.R., Nair, V. and Suarez, D.L. 2020. Diseases of Poultry, 14th Edition mycoplasma. Tipu, M.A., Pasha, T.N. and Ali, Z. 2002. Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers. Int. J. Poult. Sci. 1, 91–93. Tovar, D., Zambonino, J., Cahu, C., Gatesoupe, F., Vázquez-Juárez, R. and Lésel, R. 2002. Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae. Aquacul. 204, 113–123. Vlaisavljević, S., Šibul, F., Sinka, I., Zupko, I., Ocsovszki, I. and Jovanović-Šanta, S. 2018. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Indust. Crops Prod. 112, 217–224. Wieland, H. and Seidel, D. 1983. A simple specific method for precipitation of low density lipoproteins. J. Lipid Res. 24, 904–909. Williams, R.B. 1998. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Parasitol. 28, 1089–1098. Young, D.S. 1997. Effects of drugs on clinical Laboratory tests. Ann. Clin. Biochem. Int. J. Lab. Med. 34, 579–581. | ||
| How to Cite this Article |
| Pubmed Style Elbasuni SS, Taie HA, Gawad SMA, Kamar RE, Daous HE, Darweish M, Nada MO, Saadeldin WF, Haleem MIA. Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 225-241. doi:10.5455/OVJ.2024.v14.i1.20 Web Style Elbasuni SS, Taie HA, Gawad SMA, Kamar RE, Daous HE, Darweish M, Nada MO, Saadeldin WF, Haleem MIA. Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. https://www.openveterinaryjournal.com/?mno=176969 [Access: July 11, 2025]. doi:10.5455/OVJ.2024.v14.i1.20 AMA (American Medical Association) Style Elbasuni SS, Taie HA, Gawad SMA, Kamar RE, Daous HE, Darweish M, Nada MO, Saadeldin WF, Haleem MIA. Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 225-241. doi:10.5455/OVJ.2024.v14.i1.20 Vancouver/ICMJE Style Elbasuni SS, Taie HA, Gawad SMA, Kamar RE, Daous HE, Darweish M, Nada MO, Saadeldin WF, Haleem MIA. Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Vet J. (2024), [cited July 11, 2025]; 14((1) (Zagazig Veterinary Conference)): 225-241. doi:10.5455/OVJ.2024.v14.i1.20 Harvard Style Elbasuni, S. S., Taie, . H. A., Gawad, . S. M. A., Kamar, . R. E., Daous, . H. E., Darweish, . M., Nada, . M. O., Saadeldin, . W. F. & Haleem, . M. I. A. (2024) Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 225-241. doi:10.5455/OVJ.2024.v14.i1.20 Turabian Style Elbasuni, Sawsan S., Hanan A.a. Taie, Samah M. Abdel Gawad, Reda E.l. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa F. Saadeldin, and Marwa I. Abdel Haleem. 2024. Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 225-241. doi:10.5455/OVJ.2024.v14.i1.20 Chicago Style Elbasuni, Sawsan S., Hanan A.a. Taie, Samah M. Abdel Gawad, Reda E.l. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa F. Saadeldin, and Marwa I. Abdel Haleem. "Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking." Open Veterinary Journal 14 (2024), 225-241. doi:10.5455/OVJ.2024.v14.i1.20 MLA (The Modern Language Association) Style Elbasuni, Sawsan S., Hanan A.a. Taie, Samah M. Abdel Gawad, Reda E.l. Kamar, Hala El Daous, Marwa Darweish, Mai O. Nada, Walaa F. Saadeldin, and Marwa I. Abdel Haleem. "Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 225-241. Print. doi:10.5455/OVJ.2024.v14.i1.20 APA (American Psychological Association) Style Elbasuni, S. S., Taie, . H. A., Gawad, . S. M. A., Kamar, . R. E., Daous, . H. E., Darweish, . M., Nada, . M. O., Saadeldin, . W. F. & Haleem, . M. I. A. (2024) Efficacy of dietary supplements of Glycyrrhiza glabra (Licorice) and maduramicin alone or in combination with Eimeria tenella infected chicks: A clinical study and molecular docking. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 225-241. doi:10.5455/OVJ.2024.v14.i1.20 |