| Research Article | ||
Open Vet J. 2023; 13(10): 1366-1378 Open Veterinary Journal, (2023), Vol. 13(10): 1366–1378 Original Research Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United KingdomAndrea Kashani-Carver1,*, Conor O’Halloran2, Emma Scurrell3, Heidi Featherstone4 Felipe Ferreira de Freitas5 and Robert Lowe11Optivet Referrals, Havant, UK 2Royal (Dick) School of Veterinary Studies and the Roslin Institute, University of Edinburgh, Easter Bush Campus, UK 3Cytopath Ltd., Ledbury, Herefordshire, UK 4The Ralph Veterinary Referral Centre, Marlow, Buckinghamshire, UK 5EDF Energy UK, Brighton and Hove, Hove, UK *Corresponding Author: Andrea Kashani-Carver. Optivet Referrals, 3 Downley Road, Havant, UK. Email: Andrea [at] Optivet.com Submitted: 27/07/2023 Accepted: 27/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
ABSTRACTBackground: Only 27 cases of equine conjunctival haemangiosarcoma have been reported in the literature over the past 37 years. Out of these, 22% of cases were lost to follow-up, 52% were euthanized, and 26% survived. A scarcity of cases and information is available for this rarely seen conjunctival tumour. Aim: To describe the clinical features, management, and outcome of conjunctival hemangiosarcoma in seven horses in the UK. Methods: Optivet medical records were reviewed for equine cases seen or advised on with a histopathological diagnosis of conjunctival haemangiosarcoma between January 2013 and March 2023. Medical records were accessed for details of signalment, history, management, and follow-up. Histopathology was used to confirm the diagnosis of haemangiosarcoma and assess the surgical margins. Immunohistochemistry was performed in a minority of cases with poorly differentiated solid tumours to support vascular lineage. Results: Seven eyes from seven horses (five geldings and two mares) with a mean age of 16 years and median of 18 years (range 10–21 years) met the criteria. Serosanguinous discharge was seen in six eyes. All eyes were managed surgically; 4 by exenteration and 3 by conjunctivectomy/keratectomy. Adjunctive cryotherapy was performed in two eyes. Metastatic disease in the ipsilateral parotid salivary gland, confirmed with histopathology, was seen in one horse. Surgical margins were clear in all but one eye. Solar elastosis was noted in five eyes. All horses were healthy at the last follow-up (0.2–5 years, mean 2.9 years, and median 2 years). Conclusion: Equine conjunctival haemangiosarcoma is rare. Serosanguinous ocular discharge is a common clinical sign. Early surgical excision is highly effective. Solar elastosis is a common histopathological feature, suggesting a role for UV-light in the pathogenesis. Keywords: Haemangiosarcoma, Solar elastosis, Horse, Vascular tumour, Ocular tumour. IntroductionPrimary tumours of vascular origin arise from the endothelium of blood or lymph vessels and can be benign or malignant. The equine eye is rarely affected, and both adnexal and intraocular forms have been reported (Hargis et al., 1978; Bolton et al., 1990). Primary conjunctival vascular tumours have been reported infrequently in several species including the cat (Multari et al., 2002; Pirie and Dubielzig, 2006), dog (Liapis and Genovese, 2004), horse (Vestre et al., 1982; Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018), cow (Sutton and McLennan, 1982; Ruggles et al., 1992), giant panda (Lopez et al., 1996), and humans (Honavar and Manjandavida, 2015). They originate from the endothelium of blood vessels within the conjunctiva associated with the limbus, the medial and lateral canthus, the third eyelid (TEL) and, rarely, from the cornea. The single report of primary corneal haemangiosarcoma postulated an origin from corneal neovascularization (Lavach and Severin, 1977; Vestre et al., 1982; Hacker et al., 1986; Crawley et al., 1987; Bolton et al., 1990). They are classified histologically as benign (haemangioma) or malignant (haemangiosarcoma) (Knottenbelt et al., 2015). Dogs are the most commonly affected species (Hargis et al., 1978; Pirie et al., 2006), and the lateral bulbar conjunctiva and the leading edge of the TEL are the most frequent locations (Hargis et al., 1978; Peiffer et al., 1978; Peiffer and Terrell, 1978; Pirie et al., 2006). Definitive diagnosis requires histological examination following biopsy (incisional or excisional), and the treatment of choice is surgical excision (Pirie et al., 2006). The prognosis in dogs is good; there is a risk of local recurrence, but metastatic disease has not been reported (Hargis et al., 1978; Peiffer et al., 1978; Peiffer and Terrell, 1978). The most common ocular and adnexal tumour in horses is squamous cell carcinoma (SCC) (Lavach and Severin, 1977; Gearhart et al., 2007; Scherrer et al., 2018); it is also the second most common cutaneous tumour (Sundberg et al., 1977). Other tumours that present in the equine conjunctiva include papilloma, melanoma, mastocytoma, basal cell carcinoma, schwannoma, adenoma and adenocarcinoma, haemangioma, and haemangiosarcoma. Older horses are predisposed (Sansom et al., 2006; Scherrer et al., 2018), and a serosanguinous ocular discharge is the main clinical feature (Sansom et al., 2006; Scherrer et al., 2018). Treatments have included surgical excision, enucleation, exenteration, surgical debulking with adjunctive therapies, radiation alone and euthanasia (Moore et al., 1986; Sansom et al., 2006; Scherrer et al., 2018). Adjunctive therapies included β-irradiation with strontium90, iridium191, brachytherapy, and cryotherapy (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006). Haemangiosarcoma is rare, with only 27 cases reported over almost four decades since 1986 (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006, Gearhart et al., 2007; Wegge et al., 2010; Arenas-Gamboa and Mansell, 2011; Pinn et al., 2011; Scherrer et al., 2018). From these 27 cases, six were lost to follow-up (Arenas-Gamboa and Mansell, 2011), 14 were euthanized (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Scherrer et al., 2018), and seven survived (Sansom et al., 2006; Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018). The seven surviving cases were all reported in more recent years (2006 onwards) when the aggressive nature of ocular and periocular haemangiosarcomas was becoming more apparent (Sansom et al., 2006; Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018) and treatment options were advancing. Local recurrence and confirmed metastatic disease to the ipsilateral retropharyngeal lymph node has been reported in one horse (Gearhart et al., 2007); however, further follow-up was scarcely seen in the literature, the most commonly reported outcome was euthanasia without post-mortem (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Pinn et al., 2011; Scherrer et al., 2018). Irrespective of the management of the cases, the poor outcome remained unchanged (Gearhart et al., 2007; Scherrer et al., 2018). The aim of this study is to describe seven cases of equine conjunctival haemangiosarcoma in the UK in terms of clinical presentation, management, and outcome. Materials and MethodsStudy populationOptivet Referrals medical records were searched for equine cases seen or advised on with a histopathological diagnosis of conjunctival haemangiosarcoma between January 2013 and March 2023. Signalment, history, eye affected, duration of clinical signs, management, and follow-up were recorded. Phone calls to referring veterinary surgeons and owners were used to gather information pertaining to the current health status of the horse and its recurrence. Histopathological evaluation and immunohistochemistry protocolAll tissue samples were fixed in 10% neutral buffered formalin and submitted for histopathological examination. Routine 4 µm hematoxylin and eosin (H&E)-stained sections were examined by a board-certified pathologist. Immunohistochemistry (IHC) was performed in poorly differentiated solid tumours to support vascular lineage as a requirement for further tumour identification. IHC was performed retrospectively in all cases where it had not been performed as part of the initial histopathological examination to investigate for concordance and to assess the value of immunohistochemical staining in the diagnosis of vascular tumours. Von Willebrand Factor (vWF, a vascular endothelial marker) was applied in all cases; vimentin (general mesenchymal marker) and cytokeratin (general epithelial marker) were applied in two cases. The samples were initially pre-treated; this involved each sample being heated to 97oC, incubated for 20 minutes at 97oC and then cooled to 65oC. Following this, IHC was performed using the following specific antibodies: – vWF—Polyclonal Rabbit: Anti-Human von Willebrand Factor. Dilution 1:6400 – Cytokeratin—Monoclonal Mouse : Anti-Human Cytokeratin clone—AE1/AE3 – Vimentin—Monoclonal Mouse : Anti-Vimentin clone—V9. Dilution 1:1000 IHC was performed using the Dako Autostainer Link 48 (Agilent, California, USA) using the streptavidin-biotin immunoperoxidase technique (Warren and Summers, 2007). Ophthalmic examinationAll horses underwent a full ophthalmic examination of both eyes, performed by a board-certified ophthalmologist or ophthalmology resident. Examination included slit lamp biomicroscopy (Kowa SL-15; Kowa; Japan), indirect ophthalmoscopy (Heine; USA), applanation tonometry (Tonopen Vet; Reichert, USA) (after the application of topical anaesthesia with 0.5% proxymetacaine hydrochloride (Bausch + Lomb; Kingston, UK)), and fluorescein stain (Bausch + Lomb; Kingston, UK). Routine neuro-ophthalmic tests were assessed (palpebral reflex, menace response, dazzle reflex and direct/indirect pupillary light reflexes). Sedation protocolSurgical procedures were performed under standing sedation with intravenous detomidine at 30 mcg/kg (Domosedan, Vetoquinol, Towcester, UK) and butorphanol at 0.5 mg/kg (Torbugesic, Zoetis, Leatherhead, UK). Nerve blocksSurgery of the TEL was facilitated by local infiltration of 1–2 ml mepivacaine hydrochloride (Intra-Epicaine, Dechra, Northwich, UK) using a 25G 5/8” needle. Akinesia and anaesthesia for enucleation and exenteration procedures were achieved with multiple local nerve blocks with mepivacaine hydrochloride (Intra-Epicaine, Dechra, Northwich, UK). Retrobulbar block, auriculopalpebral block, supraorbital nerve block, lacrimal nerve block, zygomatic nerve block and intratrochlear nerve blocks were performed in a routine manner. Topical anaesthesia was provided by 0.5% proxymetacaine hydrochloride (Bausch + Lomb; Kingston, UK) applied five minutes prior to surgery. If further desensitisation of the dorsomedial upper eyelid was required, local infiltration of mepivacaine hydrochloride (Intra-Epicaine, Dechra, Northwich, UK) was performed with a 25G 5/8” needle. Surgical protocolsBiopsy Incisional biopsy was performed, if required, before radical surgical procedures such as exenteration. An excisional biopsy was performed if the lesions appeared discrete and if excision was judged to be complete. Exenteration Standard exenteration included removal of the periosteum via a periosteal strip using periosteal elevators; the surgical site was closed with three layers of absorbable sutures. Intraorbital prosthetic implants were not used. Keratectomy Standard keratectomy and/or conjunctivectomy were performed in selected cases. The perimeter of the keratectomy site was demarcated with a 300 μm restricted depth blade (Sharpoint™ Wheel Knife, SJ Hales, Warwickshire, UK) to a depth of approximately 20%–40%, followed by lamellar dissection with a #64 Beaver blade, (SJ Hales, Warwickshire, UK). The surgical bed was not grafted. Cryotherapy Cryotherapy was performed either at the time of surgery or in the early post-operative period following the histopathological diagnosis. A compressed nitrous oxide cryotherapy unit (Cryomatic MKII Cryo Console, Keeler, Windsor, UK) was used. A closed probe was applied directly onto the surgical site, and a double freeze-thaw technique was used to achieve theoretical temperatures between -20℃ and -40℃ (Dugan et al., 1991). Post-operative regimen Post-operative medication comprised oral trimethoprim and sulfadiazine 30 mg/kg q12 hours (Trimediazine oral powder, Vetoquinol, Towcester, UK) and phenylbutazone 2.2 mg/kg q12 hours PO (Equipalazone, Dechra, Northwich, UK), both for five days. All horses had tetanus immunoprophylaxis. Topical medications varied by case. Ethical approvalEthical approval was not required for this study. ResultsClinical presentationThere were five geldings and five mares; the ages ranged from 10 to 21 years, with a mean of 16.2 years (median 18 years) (Table 1). The duration of clinical signs prior to presentation ranged from 2 to 8 weeks, with a mean of 6.7 weeks (median 4 weeks). The left eye was affected in four cases and the right eye in three cases. Serosanguinous ocular discharge was the most common clinical sign, noted in six cases. Three out of seven cases arose from the conjunctiva of the TEL, and four out of seven cases arose from the bulbar conjunctiva. Three were from the lateral bulbar conjunctiva, and 1 was from the medial bulbar conjunctiva. From the four cases arising from the bulbar conjunctiva two cases had corneal involvement. A difference in appearance was seen depending on location. The masses within the TEL all appeared contiguous with the conjunctiva and smoothly surfaced. The clinical appearance of the conjunctival masses on the TEL of cases 1, 3 and 7 are shown in Figure 1. The masses on the bulbar conjunctiva appear to be exophytic, lobulated and discrete. The clinical appearance of the bulbar conjunctival masses of cases 2, 5 and 6 are shown in Figure 2. ManagementAll cases were treated with surgical excision: incisional biopsy (n=2), excisional biopsy (n=3), and no biopsy prior to exenteration (n=2). Five exenterations were performed, 3 following a prior biopsy and 2 without biopsy (Table 2). Excisional biopsy included combined conjunctivectomy/keratectomy, (n=2) and TEL excision (n=1). Six cases had clear margins with the initial surgery. Unclear margins were not achieved in a case (case 4 with combined lamellar keratectomy-conjunctivectomy). Despite subsequent exenteration 4 weeks later, metastatic spread to the ipsilateral parotid salivary gland was confirmed histopathologically. Adjunctive cryotherapy was performed in two cases that had combined conjunctivectomy and keratectomy; case 5 had cryotherapy at the time of surgery, and case 6 had cryotherapy performed 2 months post-operatively; neither of these cases showed recurrence. Investigation into metastasis was performed in three cases, and no abnormalities indicative of metastasis were reported. Case 4 with metastatic spread did not have further imaging performed. Table 1. Signalment, clinical signs, and description of the masses.
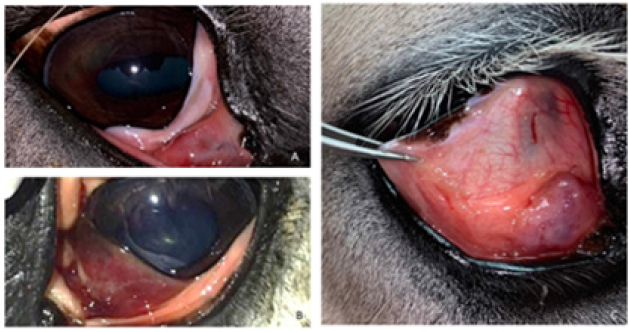
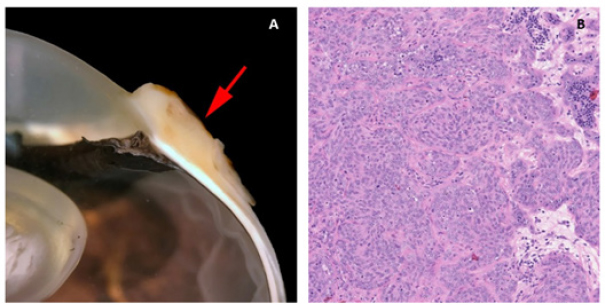
Fig. 1. Third eyelid haemangiosarcoma/angiosarcoma gross presentation. (A) Case 3 conjunctival angiosarcoma, (B) Case 1 conjunctival haemangiosarcoma, (C) Case 7 conjunctival haemangiosarcoma. Hyperaemic appearance and contiguous nature of the tumour are evident in all three cases.
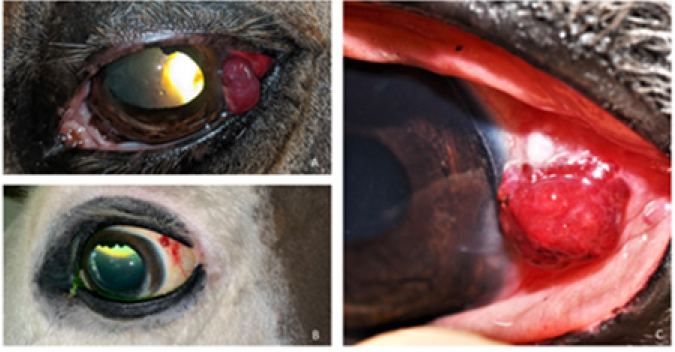
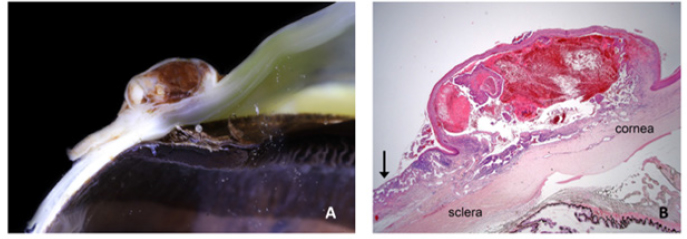
Fig. 2. Bulbar conjunctival haemangiosarcoma gross presentation. (A) Case 2 conjunctival and corneolimbal haemangiosarcoma, (B) Case 6 bulbar conjunctival haemangiosarcoma, (C) Case 5 bulbar conjunctival haemangiosarcoma. Note the exophytic, discrete and lobulated appearance of the tumour. Table 2. Location, surgical procedure performed, and outcome.
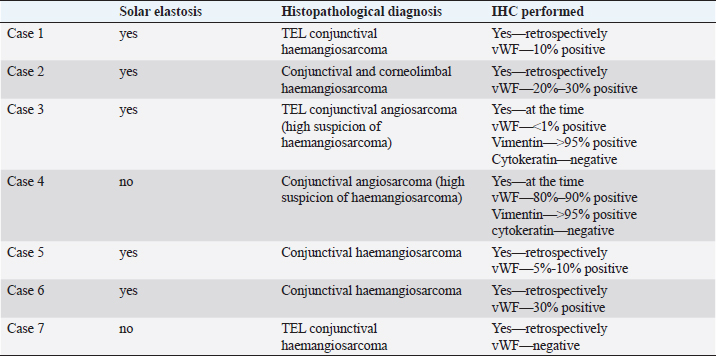
HistopathologyFive eyes from five horses were diagnosed with conjunctival haemangiosarcoma, and two eyes from two horses were diagnosed with conjunctival angiosarcoma with a high suspicion of haemangiosarcoma (Table 3). In the five horses diagnosed with conjunctival haemangiosarcoma, the histopathology findings confirmed the presence of a clearly vasoformative neoplasm in each case with the neoplastic endothelial cells either lining blood-filled vascular channels and/or forming solid nests (Fig. 3). Multiple lymphonodular aggregates or lymphoid follicles were commonly interspersed throughout the neoplastic infiltrate. Table 3. Histopathological findings and IHC results.
Fig. 3. H&E photomicrograph (400× magnification) of case 1 conjunctival haemangiosarcoma from the third eyelid of a horse. Irregular blood-filled vascular channels (star) formed by the tumour and lined by pleomorphic neoplastic endothelial cells (arrows).
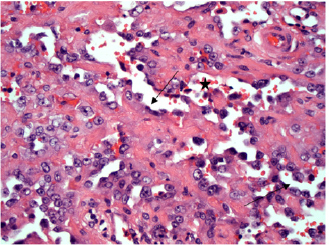
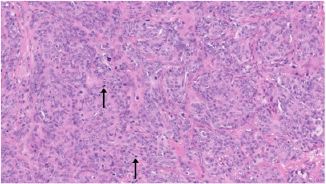
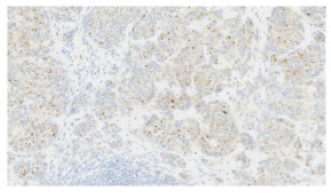
Fig. 4. H&E photomicrograph (20× magnification) of case 4, an angiosarcoma from the bulbar conjunctiva of a pony. Solid nests of neoplastic epithelioid cells devoid of vascular channel formation. Frequent mitoses are evident (arrows). Moderate to marked cellular pleomorphism and nuclear atypia were seen in all cases. Mitoses per 10HPF (2.73 mm2), varied from 3 to 34 per HPF, with case 4 displaying the highest number of mitoses and the only case with metastatic spread (Fig. 4). Solar elastosis was seen in five cases (Fig. 5). In the two horses diagnosed with conjunctival angiosarcoma, the histopathological findings reflected malignant conjunctival neoplasms that were poorly differentiated with regards to lineage. Both of these neoplasms had a solid epithelioid growth pattern and an absence of vascular channel formation, but vascular lineage was suspected given the location and H&E morphology of the tumour, accompanying perivascular lymphonodular aggregates and absence of overlying surface epithelial pathology with regards to dysplastic or neoplastic changes to indicate carcinoma (Fig. 6). IHC was performed in these two cases in an attempt to rule out the differential diagnosis of carcinoma and confirm vascular lineage as a requirement for further tumour identification (Fig. 7). IHC was performed retrospectively in all cases to investigate for concordance and to assess the value of immunohistochemical staining. Von Willebrand Factor (vWF, a vascular endothelial marker) was applied in all cases; vimentin (generalised mesenchymal marker) and cytokeratin (epithelial marker) were applied in the two poorly differentiated tumours at the time of diagnosis.
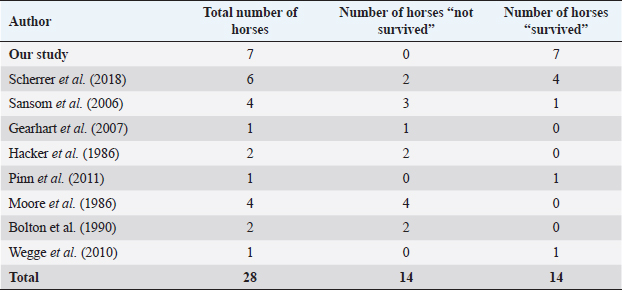
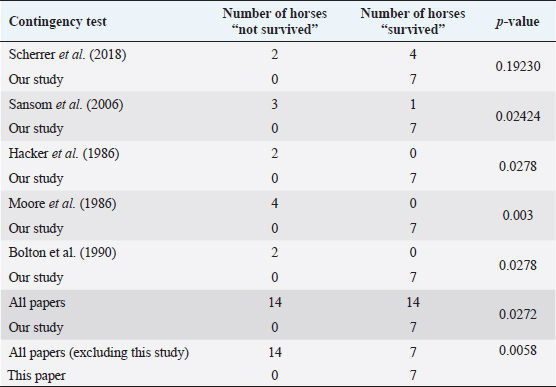
Fig. 5. H&E photomicrograph (100× magnification) of case 2 bulbar conjunctival haemangiosarcoma from a horse characterised by irregularly branching, blood-filled vascular channels. There is evidence of solar elastosis in the conjunctival stroma (arrow). Follow-up and outcomeAll horses were in good health at the last follow-up, which ranged from 2 months up to 5 years, with a mean of 2.9 years (median 2.5 years). Statistical Analysis Due to categorical data and small sample sizes, a Fisher’s exact test was performed with significance set at 95% confidence level (p-value <0.05). Contingency tables were created by comparing pairwise the survival cases number from this work against the survival cases of each study where conjunctival haemangiosarcoma was diagnosed (Table 4) (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018). Studies by Wegge and Pinn were excluded from individual calculations due to the sample size consisting of just one case. These were included when comparing our findings to the broader literature (Table 5). DiscussionSurvivalThis study reports a series of five cases of equine ocular haemangiosarcoma and two cases of equine ocular angiosarcoma (with a high suspicion of haemangiosarcoma). All cases were treated surgically, with two cases receiving adjunctive cryotherapy, and had a 100% survival rate at a mean of 2.9 years (34.8 months). This is in contrast to previous studies that demonstrated a high rate of euthanasia rate (73.6%) (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Scherrer et al., 2018). AgeConjunctival haemangiosarcoma appears to be a disease of older horses with an age range in the literature seen from 8 to 25 years old and the average age is 15.2 years (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Arenas-Gamboa and Mansell, 2011; Pinn et al., 2011; Scherrer et al., 2018). In this study, five out of seven horses were categorised as geriatric (aged 15 years or over) (McGowan, 2011). The age range of the horses in the current study was 10–21 years and an average age of 16.2 years. This is consistent with the previous literature (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018). The findings of this study, along with the literature, support that conjunctival haemangiosarcoma is a disease of older horses.
Fig. 6. (A) Case 4 gross pathology photomicrograph of a solid, poorly differentiated angiosarcoma, note the cream-coloured cut surface. (B) Histopathology photomicrograph, case 4, note the highly cellular and solid presentation with a lack of vasculature and red blood cells.
Fig. 7. vWF stained photomicrograph (×20 magnification) of case 4 angiosarcoma from the bulbar conjunctiva of a pony. The majority of neoplastic cells are immunopositive for vWF (IHC, 3,3′-Diaminobenzidine DAB chromogen). Confirming an endothelial cell origin to the tumour. Clinical signsThe most common clinical sign of equine conjunctival haemangiosarcoma in this case series was a serosanguinous ocular discharge, which was present in six of the seven horses (85.7%). This is in accordance with previous reports (75%) (Sansom et al., 2006; Scherrer et al., 2018). A serosanguinous ocular discharge is not seen with haemangioma, SCC or other tumour types at this location, and so its presence, particularly when of significant duration and refractive to first-line treatment, should raise the clinical index of suspicion for haemangiosarcoma and prompt further investigation (Giuliano, 2010; Malalana, 2022). PredispositionAll seven horses had a pale or dilute coat colour, which has been shown to be a predisposing factor in other studies (Sansom et al., 2006; Gearhart et al., 2007; Pinn et al., 2011; Scherrer et al., 2018). Previous literature also highlighted increased ultraviolet light exposure and being kept outside as potential risk factors (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart; et al., 2007; Wegge et al., 2010; Pinn et al., 2011). Six of the seven horses had histopathological features of solar elastosis. In a canine population, 39 of 46 dogs with cutaneous haemangiosarcoma had solar elastosis in the skin adjacent to the tumour (Hargis et al., 1978). The presence of solar elastosis is a risk factor for SCC in horse (Campbell et al., 1987) but has not been reported for cases of haemangiosarcomas. Tumour location is often thought to be associated with areas of greatest ultraviolet (UV) light exposure (e.g., the leading edge of TEL and temporal bulbar conjunctiva), suggesting a role for UV light; however, the exact pathogenesis warrants further research. Location and prognosisThe biological behaviour and prognosis associated with haemangiosarcomas are based on its location and the species affected. (Sorenmo et al., 2000; Schultheiss, 2004). Non-visceral ocular and peri-ocular locations are relatively uncommon locations for this tumour type in all species, with dogs being most frequently affected, followed by cats and then horses (Richardson and Deykin, 2021). A retrospective study investigating canine and feline non-visceral haemangiosarcomas identified excision with clear margins as a successful treatment with favourable outcomes in these species (Schultheiss, 2004). Previous studies have postulated that haemangiosarcomas do not display the same behaviour in the ocular and peri-ocular locations as in the cutaneous, visceral or disseminated forms (Scherrer et al., 2018). The available results from this study and others suggest that a positive outcome in horses is likely because of the possibility of obtaining clear surgical margins (Scherrer et al., 2018).
Fig. 8. (A) Case 2 gross pathology photomicrograph revealing the lobulated presentation and red colour to the cut surface. (B) Case 2 low-power histopathology photomicrograph revealing the well-differentiated haemangiosarcoma with blood-filled vessels, note the invasive nature of the tumour tissue (black arrow). Table 4. Comparison of survival of horses in this study versus survival of horses in other studies in the literature.
Table 5. Contingency table assessing pairwise survival outcomes from this study to each individual study in the literature and calculated p-values.
SurgeryLesions were excised with clear margins in six of the seven horses (85.7%). There was a recurrence in the one horse that did not have clear margins at the initial surgery. Although clear margins were subsequently obtained with exenteration 4 weeks later, metastatic spread to the ipsilateral parotid salivary gland was later confirmed. This supports the findings in a recent case series of ocular and peri-ocular haemangiosarcoma—complete excision at the initial surgery resulted in no recurrence in four of six horses (Scherrer et al., 2018). The two cases with post-excisional recurrence had histologic evidence of incomplete excision (Scherrer et al., 2018). The treatment options include surgical excision (conjunctivectomy, keratectomy, nictitating membrane removal, enucleation, and exenteration) with or without adjunctive therapies (cryotherapy or radiation therapy), or euthanasia (Hacker et al., 1986; Moore et al., 1986; Bolton et al., 1990; Sansom et al., 2006; Gearhart et al., 2007; Scherrer et al., 2018). There are only three reports of a successful outcome for equine ocular and periocular hemangiosarcoma (Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018). Survival was greater than 18 months in those three cases, two involving the TEL and one conjunctival mass (Wegge et al., 2010; Pinn et al., 2011; Scherrer et al., 2018). These cases, combined with those from our study, reveal that 8 out of 11 horses had a successful outcome when complete excision with clear margins was performed at the initial surgery. Metastatic disease in the ipsilateral parotid salivary gland was identified in one horse (case 4) (Kan et al., 2011). The clinical signs of facial nerve damage seen in this case were attributed to the parotid gland biopsy due to the course of the facial nerve over the parotid gland and their spontaneous resolution. (Boorman et al., 2020; Zetterström et al., 2022). The parotid gland swelling could be attributed to neoplastic infiltration and inflammation. The spontaneous resolution of the swelling without surgical excision, chemotherapy or radiotherapy may have been due to the non-steroidal anti-inflammatory treatment given after the surgical biopsy. Alternatively, or as well as this, an innate immune response (such as that mediated by natural killer cells) could have destroyed any small population of neoplastic cells present. Statistical significanceThrough the analysis of individual study results, we gained valuable insights into potential associations between survival and specific factors examined in this study, including age, dilute coat colour, solar elastosis, and serosanguinous ocular discharge. Comparing these findings with the available literature, we observed that the null hypothesis was not discarded only in the comparison between samples from this study (Scherrer et al., 2018). Remarkably, the seven survival cases from our work exhibited p-values below 0.05 in all other individual comparisons, strongly rejecting the null hypothesis. Furthermore, when compared with the total number of survival cases found in the literature, this rejection becomes even more pronounced, suggesting that the presence of seven survival cases is not merely a chance occurrence but likely due to an underlying contributing factor in this study. Given the limited number of samples available, it is challenging to determine the specific contributing factors to the survivability of our cases. The most suitable approach for addressing this question would involve Bayesian modelling. However, conducting such an analysis requires careful consideration of prior distributions and a more in-depth investigation of the relevant factors. Regrettably, this level of complexity is beyond the scope of the present work. Nevertheless, it opens the possibility for future research to build upon our findings and explore the factors influencing survivability in a more comprehensive and sophisticated Bayesian framework. HistopathologyAngiosarcoma is an umbrella term for tumours that are classified as malignant masses that originate within the vascular system (Fosmire et al., 2004) and can be further classified into haemangiosarcoma (tumours originating from the vasculature of blood vessels) or lymphangiosarcoma (tumours originating from the vasculature of the lymphatic system) (Halsey et al., 2016). Microscopically, the cell morphology of both tumour types is similar (Kim et al., 2015), and if the endothelial cell origin/lineage is unable to be categorised to the lymphatic system or the vascular system, then immunohistochemical markers can be employed (Fosmire et al., 2004). A diagnosis of haemangiosarcoma is made by the presence of erythrocytes within vascular channels formed by the tumour; lymphangiosarcoma is diagnosed by the absence of erythrocytes within the vascular channels (Swayne et al., 1989; Diessler et al., 2003; Halsey et al., 2016). The cases included in this study were all confirmed as haemangiosarcomas histopathologically or were deemed angiosarcoma with a high suspicion of haemangiosarcoma. Definitive diagnosis, therefore, always requires histopathological analysis of a biopsy sample (incisional or excisional), and so it is an important consideration for clinicians who are considering surgical intervention for ocular or periocular lesions. In Figure 8, a conjunctival haemangiosarcoma displays the more “classical” gross appearance of haemangiosarcoma; an exophytic mass with red appearance to both the cut surface and on histopathology. Blood-filled channels lined with neoplastic cells provide the tumour with a red appearance. Although clinically appearing as a discrete mass, the invasive nature of this tumour type can be seen with neoplastic cells infiltrating the cornea outside the mass. Contrast this to Figure (6), a poorly differentiated, solid angiosarcoma. Whilst still marginally exophytic, the gross appearance was cream/pale coloured, and histopathology revealed a lack of blood vessels. The tumour was solid in appearance with poorly differentiated cells making classification difficult. This highlights the importance of haemangiosarcoma as a differential for conjunctival masses in a horse, even if their appearance is not grossly red. IHC is not always required for a diagnosis, and in this case series, it was only indicated in two of the seven horses. The remaining five cases had histopathologic findings typical of haemangiosarcoma, and examination of H&E sections alone was sufficient for the diagnosis. IHC is typically performed on a poorly differentiated tumour to help further categorise it when defining morphological features of cell lineage are absent. Vimentin, cytokeratin and vWF immunohistochemical markers were performed in the two cases (Cases 3 and 4) where a solid conjunctival epithelioid neoplasm was diagnosed: vascular lineage was suspected, but IHC was performed in an attempt to confirm this and rule out the less likely differential diagnosis of carcinoma. Vimentin is a structural protein, the presence of which confirms a mesenchymal origin for a given tumour (Ifedioranma et al., 2020) but is otherwise not specific. Cytokeratin is a protein that provides mechanical support to epithelial cells and, therefore, confirms an epithelial cell origin for tumours. Both cases (Case 3 and 4) in this case series were immunonegative for cytokeratin and, therefore, did not support the differential diagnosis of carcinoma. vWF (also known as factor VIII-related antigen) is a vascular endothelial marker, confirming the origin of tissue to endothelial cells of the vascular system, e.g., an angiosarcoma (Smith et al., 1989). In one of the cases (Case 4), the majority of neoplastic cells were strongly immunopositive for vWF, and in the second case (Case 3), only rare neoplastic cells were immunopositive for vWF. The diagnosis of conjunctival angiosarcoma was made in both cases based on the location of the tumour, the H&E findings and the combination of immunochemistry results as outlined above. It is not uncommon for IHC to show a variability in the strength of immunostaining and/or the distribution pattern of immunostaining including the percentage of immunopositive neoplastic cells, particularly in the case of poorly differentiated malignancies. It is important to realise that although an immunopositive result can be helpful, an immunonegative result does not necessarily exclude the cell lineage being tested for depending on the level of sensitivity of the antibody for that particular neoplastic cell lineage in that particular species. Interestingly, vWF IHC was retrospectively performed in all remaining five cases of haemangiosarcoma and revealed a wide variation in immunostaining strength and pattern. Findings were measured against a consistent positive internal control (endothelial cells lining normal blood vessels). Up to a maximum of 30% neoplastic cells were immunopositive for vWF), in five cases (Cases 1, 2, 3, 5, and 6), 80%–90% neoplastic cells were immunopositive in one case (Case 4), and neoplastic endothelial cells were immunonegative in one case (Case 7) despite a clear histopathological diagnosis of haemangiosarcoma. This suggests that this particular antibody with the specific methodology and antibody dilution used in this case series of horses was not highly sensitive for labelling neoplastic endothelial cells and that vascular lineage could not be reliably excluded in a poorly differentiated neoplasm suspected to be an angiosarcoma if the neoplastic cells were immunonegative for vWF. This study highlighted the fact that their use does not always support the diagnosis fully and should only be utilised in cases of poorly differentiated tumours where a definitive diagnosis cannot be achieved with histopathological analysis alone. They can help to provide supplementary supporting information in an equivocal case but should not be relied on solely for the diagnosis of a haemangiosarcoma. There are no immunohistochemical stains routinely available that differentiate haemangiosarcoma from lymphangiosarcoma definitively. Two novel lymphatic endothelial cell-specific (LECs) markers, LYVE-1 and PROX-1, have been identified (Halsey et al., 2016) and are, in specific laboratories, available for the further differentiation of the two tumour types. These markers have yet to be validated in horses. Study limitationsThe retrospective nature of the study has limitations, including consistency with the diagnostic approach, management, and medical therapy. The number of cases is low, which reflects the condition being uncommon; case 7 was included despite only two months follow-up due to the uncommon presentation of this disease. Incisional biopsies were not performed prior to exenteration in two cases because of financial constraints; the authors recommend that a biopsy (incisional or excisional) should always be performed prior to enucleation or exenteration. ConclusionEquine ocular and periocular haemangiosarcomas are infrequently seen tumours; they appear to occur in older horses with pale coat colour. A persisting serosanguinous ocular discharge is a common clinical sign. A successful outcome can be achieved with early surgical intervention and complete excision at the initial surgery. The histopathological confirmation of clear surgical margins is important for the prognosis. Ultraviolet light may play a significant role in the aetiopathogenesis of ocular haemangiosarcoma. AcknowledgmentsThe authors would like to thank Gemma Turner for her contributions and support throughout the manuscript preparation process. Special thanks to Chez De Oliveira, Vim Kumaratunga, Tim Knott, and Josie Parker for the provision of clinical case notes and clinical photographs. Authors contributionsAndrea Kashani-Carver: Main author and contributor. Performed clinical examinations of some cases, performed owner phone calls, and drafted the full manuscript, excluding the statistical analysis section. Conor O’Halloran: Helped to edit and review the manuscript. Emma Scurrell: Prepared and interpreted all samples, reviewed all histopathology, generated images for the manuscript, and provided critical review. Heidi Featherstone: Helped to critically review and edit multiple manuscript drafts and provide supervision. Felipe Ferreira de Freitas: Data scientist. The drafted manuscript section on statistical analysis and provided tables. Rob Lowe: Supervisor of main author and paper. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Data availabilityThe authors confirm that the data supporting the findings of this study are available within the article. ReferencesArenas-Gamboa, A. and Mansell J. 2011. Epithelioid haemangiosarcoma in the ocular tissue of horses. J. Comp. Pathol. 144(4), 328–333. Bolton, J., Lees, M., Robinson, W., Thomas, J. and Klein, K. 1990. Ocular neoplasms of vascular origin in the horse. Equine Vet. J. 22(S10), 73–75. Boorman, S., Scherrer, , Stefanovski, D. and Johnson, . 2020. Facial nerve paralysis in 64 equids: clinical variables, diagnosis, and outcome. J. Vet. Int. Med. 34(3), 1308–1320. Campbell, G.A., Gross, T.L. and Adams R. 1987. Solar elastosis with squamous cell carcinoma in two horses. Vet. Pathol. 24(5), 463–464. Crawley, G.R., Bryan, G.M. and Gogolewski, R.P. 1987. Ocular hemangioma in a horse. Equine Pract. 9, 11. Diessler, M.E., Castellano, M.C., Massone, A.R., Portiansky, E.L., Allende, M.G., Idiart, J.R. and Gimeno E.J. 2003. Cutaneous lymphangiosarcoma in a young dog: clinical, anatomopathological and lectinhistochemical description. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 50(9), 452–456. Dugan, S.J., Curtis, C.R., Roberts, S.M. and Severin, G.A. 1991. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 198(2), 251–256. Fosmire, S.P., Dickerson, E.B., Scott, A.M., Bianco, S.R., Pettengill, M.J., Meylemans, H., Padilla, M., Frazer-Abel, A.A., Akhtar, N., Getzy, D.M., Wojcieszyn, J., Breen, M., Helfand, S.C. and Modiano, J.F. 2004. Canine malignant hemangiosarcoma as a model of primitive angiogenic endothelium. Lab. Invest. 84(5), 562–572. Gearhart, P.M., Steficek B.A. and Peteresen‐Jones S.M. 2007. Hemangiosarcoma and squamous cell carcinoma in the third eyelid of a horse. Vet. Ophthalmol. 10(2), 121–126. Giuliano, E.A. 2010. Equine periocular neoplasia: Current concepts in aetiopathogenesis and emerging treatment modalities. Equine Vet. J. 42(S37), 9–18. Hacker, D.V., Moore P.F. and Buyukmihci N.C. 1986. Ocular angiosarcoma in four horses. J. Am. Vet. Med. Assoc. 189(2), 200–203. Halsey, C.H.C., Worley, D.R., Curran, K., Charles J.B. and Ehrhart E.J. 2016. The use of novel lymphatic endothelial cell-specific immunohistochemical markers to differentiate cutaneous angiosarcomas in dogs. Vet. Comp. Oncol. 14(3), 236–244. Hargis, A.M., Lee A.C. and Thomassen, R.W. 1978. Tumor and tumor-like lesions of perilimbal conjunctiva in laboratory dogs. J. Am. Vet. Med. Assoc. 173(9), 1185–1190. Honavar, S.G. and Manjandavida F.P. 2015. Tumors of the ocular surface: a review. Indian J. Ophthalmol. 63(3), 187–203. Ifedioranma, O., Madukwe, J., Onyemelukwe, A. and Ngokere, A. 2020. Vimentin and cytokeratin immunostaining: the role in basic diagnosis and prognosis of sarcomas. J. Drug Deliv. Therap. 10, 175–178. Kan, L.-W.M.D., Leu, Y.-S.M.D., Tzen, C.-Y.M.D. and Wu, C.-H.M.D. 2011. Recurrent sebaceous gland carcinoma of eyelid previously diagnosed as basal cell carcinoma: case report. Am. J. Otolaryngol. 32(6), 620–623. Kim, J.-H., Graef, A.J., Dickerson, E.B. and Modiano, J.F. 2015. Pathobiology of hemangiosarcoma in dogs: research advances and future perspectives. Vet. Sci. 2(4), 388–405. Knottenbelt, D., Patterson-Kane, J. and Snalune, K. 2015. Vascular neoplasms, 1st ed. Clinical Equine Oncology, Amsterdam: Elsevier, pp: 332–341. Lavach, J. and Severin, G. 1977. Neoplasia of the equine eye, adnexa, and orbit: a review of 68 cases. J. Am. Vet. Med. Assoc. 170(2), 202–203. Liapis, I.K. and Genovese, L. 2004. Hemangiosarcoma of the third eyelid in a dog. Vet. Ophthalmol. 7(4), 279–282. Lopez, M., Talavera, C., Rest, J.R. and Taylor, D. 1996. Haemangiosarcoma of the conjunctiva of a giant panda. Vet. Rec. 138(1), 24–24. Malalana, F. 2022. Corneal neoplasia in horses. Equine Vet. Edu. 34(11), 570–572. McGowan, C. 2011. Welfare of aged horses. Animals (Basel) 1(4), 366–376. Moore, P.F., Hacker, D. and Buyukmihci, N. 1986. Ocular angiosarcoma in the horse: morphological and immunohistochemical studies. Vet. Pathol. 23(3), 240–244. Multari, D., Vascellari, M. and Mutinelli, F. 2002. Hemangiosarcoma of the third eyelid in a cat. Vet. Ophthalmol. 5(4), 273–276. Peiffer, R.L., Duncan, J. and Terrell, T. 1978. Hemangiona of nictitating-membrane in a dog. J. Am. Vet. Med. Assoc. 172(7), 832–833. Peiffer, R.L. and Terrell, T. 1978. Episcleral hemangioma in a dog. J. Am. Vet. Med. Assoc. 173(10), 1338–1340. Pinn, T.L., Cushing, T., Valentino L.M. and Koch S.A. 2011. Corneal invasion by hemangiosarcoma in a horse. Vet. Ophthalmol. 14(3), 200–204. Pirie, C.G. and Dubielzig, R.R. 2006. Feline conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of eight cases (1993–2004). Vet. Ophthalmol. 9(4), 227–231. Pirie, C.G., Knollinger, A.M., Thomas, C.B. and Dubielzig, R.R. 2006. Canine conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of 108 cases (1989–2004). Vet. Ophthalmol. 9(4), 215–226. Richardson, S. and Deykin A.R. 2021. Surgical treatment of conjunctival hemangioma and hemangiosarcoma: a retrospective study of 52 dogs. Vet. Ophthalmol. 24(5), 432–441. Ruggles, A.J., Irby, N.L., Saik, J.E. and Orsini, P.G. 1992. Ocular lymphangiosarcoma in a cow. J. Am. Vet. Med. Assoc. 200(12), 1987–1988. Sansom, J., Donaldson, D., Smith, K., Blunden, A., Petite, A. and Seeliger, M. 2006. Haemangiosarcoma involving the third eyelid in the horse: a case series. Equine Vet. J. 38(3), 277–282. Scherrer, N.M., Lassaline, M. and Engiles, J. 2018. Ocular and periocular hemangiosarcoma in six horses. Vet. Ophthalmol. 21(4), 432–437. Schultheiss, P.C. 2004. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. Invest. 16(6), 522–526. Smith, R.E., Janjan, N., Kretzschmar, S. and Hackbarth, D. 1989. The effect of radiation therapy on von Willebrand factor in patients with angiosarcoma. Radiother. Oncol. 16(4), 297–304. Sorenmo, K., Duda, L., Barber, L., Cronin, K., Sammarco, C., Usborne, A., Goldschmidt, M. and Shofer, F. 2000. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J. Vet. Int. Med. 14(4), 395–398. Sundberg, J., Burnstein, T., Page, E., Kirkham, W. and Robinson, F. 1977. Neoplasms of equidae. J. Am. Vet. Med. Assoc. 170(2), 150–152. Sutton, R.H. and McLennan, M.W. 1982. Hemangiosarcoma in a cow. Vet. Pathol. 19(4), 456–458. Swayne, D.E., Mahaffey, E.A. and Haynes, S.G. 1989. Lymphangiosarcoma and haemangiosarcoma in a cat. J. Comp. Pathol. 100(1), 91–96. Vestre, W., Turner, T. and Carlto, W. 1982. Conjunctival hemangioma in a horse. J. Am. Vet. Med. Assoc. 180(12), 1481–1482. Warren, A. and Summers, B. 2007. Epithelioid variant of hemangioma and hemangiosarcoma in the dog, horse, and cow. Vet. Pathol. 44(1), 15–24. Wegge, B., Gasthuys, F., Chiers, K. and Ducatelle, R. 2010. Haemangiosarcoma of the third eyelid in a horse. J. Comp. Pathol. 143(4), 337–337. Zetterström, S.M., Matz, B.M., Neto, R., Lindley, S.E.S., Cole, R.C., Wilhite, R. and Boone, L.H. 2022. Partial parotid sialoadenectomy in a horse with parotid ductal carcinoma: surgical description and case report. Vet. Surg. 51(2), 296–302. | ||
| How to Cite this Article |
| Pubmed Style Kashani-Carver A, Ohalloran C, Scurrell E, Featherstone H, de-Freitas FF, Lowe R. Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Vet J. 2023; 13(10): 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 Web Style Kashani-Carver A, Ohalloran C, Scurrell E, Featherstone H, de-Freitas FF, Lowe R. Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. https://www.openveterinaryjournal.com/?mno=162055 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i10.17 AMA (American Medical Association) Style Kashani-Carver A, Ohalloran C, Scurrell E, Featherstone H, de-Freitas FF, Lowe R. Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Vet J. 2023; 13(10): 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 Vancouver/ICMJE Style Kashani-Carver A, Ohalloran C, Scurrell E, Featherstone H, de-Freitas FF, Lowe R. Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Vet J. (2023), [cited July 27, 2024]; 13(10): 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 Harvard Style Kashani-Carver, A., Ohalloran, . C., Scurrell, . E., Featherstone, . H., de-Freitas, . F. F. & Lowe, . R. (2023) Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Vet J, 13 (10), 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 Turabian Style Kashani-Carver, Andrea, Conor Ohalloran, Emma Scurrell, Heidi Featherstone, Felipe Ferreira de-Freitas, and Robert Lowe. 2023. Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Veterinary Journal, 13 (10), 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 Chicago Style Kashani-Carver, Andrea, Conor Ohalloran, Emma Scurrell, Heidi Featherstone, Felipe Ferreira de-Freitas, and Robert Lowe. "Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom." Open Veterinary Journal 13 (2023), 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 MLA (The Modern Language Association) Style Kashani-Carver, Andrea, Conor Ohalloran, Emma Scurrell, Heidi Featherstone, Felipe Ferreira de-Freitas, and Robert Lowe. "Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom." Open Veterinary Journal 13.10 (2023), 1366-1378. Print. doi:10.5455/OVJ.2023.v13.i10.17 APA (American Psychological Association) Style Kashani-Carver, A., Ohalloran, . C., Scurrell, . E., Featherstone, . H., de-Freitas, . F. F. & Lowe, . R. (2023) Equine conjunctival haemangiosarcoma: Clinical presentation, management, and outcome of seven cases in the United Kingdom. Open Veterinary Journal, 13 (10), 1366-1378. doi:10.5455/OVJ.2023.v13.i10.17 |