| Research Article | ||
Open Vet J. 2023; 13(10): 1346-1351 Open Veterinary Journal, (2023), Vol. 13(10): 1346–1351 Original Research Subjective and objective observation of Tilapia skin as auto skin graft dressing in catsErwin Erwin1,*, Etriwati Etriwati2, Sugito Sugito1, and Hadi Mulki Satria31Laboratory of Veterinary Clinics and Surgery, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Student in Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Erwin Erwin. Laboratory of Veterinary Clinics and Surgery, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: erwin2102 [at] usk.ac.id Submitted: 22/06/2023 Accepted: 23/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
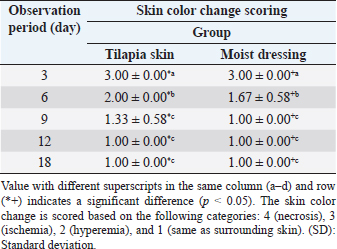
AbstractBackground: The recovery of auto skin graft is a dynamic and complex process that requires a suitable environment for vascularization as nutrition delivery to cells and donor skin reception. Aims: This research aimed to determine the effect of Tilapia skin dressing on the recovery of auto skin graft treatment on domestic cats through subjective and objective observation. Methods: Six male Indonesian local cats aged 1–2 years old weighing 3–4 kg were separated into two groups. The surgical procedure was performed in a sterile and aseptic environment. The first surgery created wound defects on the forelimb area 2 × 2 cm in size to whole groups. The wounds were left for 4 days and then treated with the following treatments; Group I (G-I) was treated with Tilapia skin dressing, and Group II (G-II) was treated with moist dressing Sofra-tulle®. The dressing of the two groups was replaced every 3 days and evaluated subjectively and objectively. Results: Subjective observation showed that skin was reddish (day 3), the bleeding test showed bleeding immediately occurred after incision, and pain response was observed on day 6 post-surgery on both treatment groups showed significantly differences with 95% confidence level (p < 0.05). Objective observation in the form of NaCl 0.9% absorption and medicine effect on auto skin graft did not show a significant difference between the two treatment groups (p > 0.05). Conclusion: Auto skin graft treatment by moist dressing showed better healing, but Tilapia skin dressing can be an alternative choice during auto skin graft treatment. Keywords: Tilapia skin dressing, Subjective, Objective, Graft, Cat. IntroductionWounds on the skin can be caused by injuries, burns, bites, and chemical irritants following tumor removal. Treating skin wounds usually involves bringing together the two edges of the wound. In cases of larger wounds with skin loose around, a skin flaps procedure is employed. However, when skin availability is limited, a skin graft is used as an alternative. A skin graft is the act of moving a part of or whole skin from one place to another to live, which requires neovascularization to ensure the livelihood of the skin. Autograft is a transplantation that moves tissue or organs from one part of the body to another part of the body in the same individual (Erwin et al., 2021). In general, wound healing may be complicated by graft rejections, graft-transmitted diseases, infections, pain, and substantial socioeconomic and treatment-related costs (Ibrahim et al., 2023). The use of moist dressing promotes skin graft recovery (Erwin et al., 2016). Several types of wound dressing are available in the market today; among them are silver-containing hydrophilic fiber dressing, antibacterial dressing, and wet dressing (Lei et al., 2019). The dressing is material used topically on the wound and is classified as traditional, biological, and synthetic dressing (Kamoun et al., 2017). The use of synthetic dressing incurs high costs for skin grafts. Tilapia skin is a biological dressing to treat burn wounds and contains microbes that are not infectious or pathogenic. Tilapia skin is an innovation, easy to apply, and low cost. Tilapia skin forms re-epithelization during 12–17 days of treatment, without dressing change and without side effects during recovery (Junior et al., 2019). The abundant collagen-rich Tilapia skin offers a moist wound environment that promotes tissue granulation and epithelization, facilitating more efficient wound healing (Fiakos et al., 2020). Tilapia skin contains type-1 collagen with a chain pattern identified as two α1 chains and one α2 chain (Song et al., 2021). Collagen is a structural protein that has been commercially used in the cosmetic and medical fields (Nasri, 2019). We often use these moist dressings in wound care, but the healing process of the skin graft method is still far away, requiring extensive work, and remains a challenge for the vet to apply appropriate dressings. Thus, the dressing of high collagen speeds up skin graft healing and provides a moist environment for wounds in order to support granulation and epithelization of tissue, thus permitting more effective wound healing (Erwin et al., 2021). This research observed the recovery of auto skin graft in domestic cats using Tilapia skin dressing with subjective and objective observation. Materials and MethodsResearch procedureThe cleaned Tilapia fish skin is stored in an isothermal box and separated from the muscles, then cut into sizes of 10 × 5 cm. The cut skin was washed with 0.9% saline solution and sterilized with 2% chlorhexidine gluconate (for 60 minutes). The Tilapia fish skin was then washed with sterile 0.9% saline solution and stored in a covered container with 70% glycerol and 25% saline. After 1 hour, the Tilapia fish skin is washed with sterile 0.9% saline solution and stored in a sterile container with 100% glycerol for 5 minutes. Subsequently, the fish skin is placed in a water bath for 3 hours and then centrifuged at a temperature of 37°C with a speed of 15 rpm. (Costa et al., 2019). This research is a laboratory experimental research using six male domestic cats aged 1.5–2 years old weighing 3–4 kg. The cats were adapted to individual cages for 1 month and fed 3 times daily with water ad libitum. As acclimatization, the cats were given amoxicillin and clavulanic acid antibiotics [62.5 mg/kg body weight (BW)] and metronidazole (17 mg/kg BW), praziquantel and pyrantel embonate (5 mg/kg BW), as well as vitamin supplements. After acclimatization, if a cat is clinically unwell, it will be replaced with another cat. The cats were divided into two treatment groups consisting of three cats. The cats were fasted for 8 hours before surgery. Premedication injection contained atropine sulfate (Atropine®, Ethica, Indonesia) 0,04 mg/kg BW subcutaneously. Fifteen minutes after, a general anesthetic containing ketamin 10% (Ketamil®, Troy Laboratories PTY Limited, Australia) 10 mg/kg BW and tranquilizer xylazine 2% (Xyla®, Interchemie, Holland) 1 mg/kg BW were administered via intramuscular injection. The surgical procedure was performed in lateral recumbency in a sterile and aseptic procedure. The first surgery was preceded by shaving hair and disinfecting the lateral area of the forelimb with povidone iodine. It was then followed by making an incision wound defect 2 × 2 cm. The wound was wrapped with sterile gauze, added povidone iodine, and then left for 4 days. The second surgery was done by harvesting skin from the thoracic area and applying it on the base of the recipient bed, which previously had been cleaned of subcutaneous tissue. Finally, the donor skin and recipient skin were stitched by a simple interrupted suture with polypropylene 3.0 United States Pharmacopeia (USP) thread. The skin graft area was with Tilapia skin dressing (G-I), and the moist dressing group was dressed with Sofra-tulle® (Pantheon UK Limited, Swindon, UK, for Sanovi-Aventis, Thailand) (G-II). Dressings were replaced 4 times on days 3, 6, 9, and 12, according to each group. Wound treatment post-surgery included antibiotics Amoxicillin and Clavulanic Acid 10 mg/kg BW (Claneksi®, Sanbe Farma, Indonesia) as well as Carprofen 2.2 mg/kg BW (Rymadyl®, Pfizer/Zoetis, USA) for 7 days two times a day. The auto skin graft healing assessment was performed subjectively and objectively (Erwin et al., 2016). Subjective parametersThey are as follows: a. The skin color of auto skin graft (donor site) and the surrounding skin post-surgery on days 3, 6, 9, 12, and 18. Assessment for skin color change categories were: 4 (black/necrosis), 3 (ischemia), 2 (hyperemia), and 1 (the same color as the surrounding skin). b. Pain response on auto skin graft on days 3, 6, 9, 12, and 18. Pain response assessment was performed by giving pressure on the donor skin area and categorized into: 3 (no response/necrosis), 2 (pain/inflammation), and 1 (no pain/healed). c. Bleeding test by incision (1 mm) on the donor skin area. Bleeding test assessment observes the blood quality and is categorized into: 2 (bleeding takes time after incision) and 1 (bleeding immediately occurs after incision). Objective parametersThey are as follows: a. Injection of 0.2 ml NaCl 0.9% subcutaneously around the skin donor site on day 18 post-2nd surgery was performed, and the time required to absorb was then observed. b. Injection 0.2 ml adrenalin subcutaneously on donor site on day 18 post-2nd surgery was performed, and sympathetic nerve reaction as the effect of sympathomimetic drug administration was then observed. Statistical analysisData from subjective observation was analyzed by ANOVA multivariate and post-hoc Duncan Test. Data subjective also analyzed the correlation between period observation with color auto skin graft and pain response. The objective observation was analyzed by T-test with the help of SPSS software for Windows 24. Ethical approvalThis research has been approved by the Veterinary Ethics Committee of the Faculty of Veterinary Medicine, Universitas Syiah Kuala, with certificate number: 98/KEPH/V/2021. ResultsColor changes on auto skin graftThe observation of auto skin graft color changes on days 3, 6, 9, 12, and 18 showed significant differences between the treatment group and observation period (p < 0,05), which are presented in Table 1 and Figure 1. On average, the auto skin graft color on day 3 for both treatment groups showed hyperemia with an average score of 3.00 ± 0.00. The color of the auto skin graft on days 9, 12, and 18 for moist dressing (G-II) started to look similar with surrounding skin (1.00 ± 0.00), which was significantly different (p < 0,05) with day 3 and day 6 post-treatment. Skin graft with Tilapia skin dressing showed the same color as surrounding skin on day 12 with an average score of 1.00 ± 0.00, which was not significantly different with day 9 and day 6 post-surgery, but significantly different with day 3 post-surgery. The color change of auto skin graft into the same color as surrounding skin is faster in moist dressing (G-II) compared to Tilapia skin dressing (G-I). There was a strong negative correlation between the observation period with color skin graft on G-I (r=−0.849, p < 0.001) and G-II (r=−0.750, p < 0.001). The application of Tilapia skin as skin graft dressing showed slower color change compared to moist dressing. Sterilizing Tilapia skin with chlorhexidine for an extended period of time causes a decrease in the integrity, organization, and collagen intensity within the Tilapia skin. This reduction has an impact on the granulation and neovascularization processes in the healing of auto-skin grafts, resulting in a higher score for graft color in Tilapia skin dressing (G-I). Table 1. Average (±SD) score for skin color change in auto skin graft on days 3, 6, 9, 12, and 18 after treatment with Tilapia skin and moist dressing.
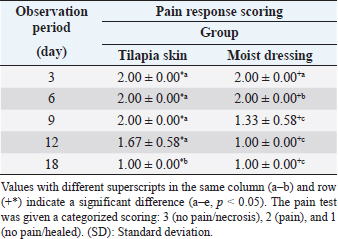
Pain responseAuto skin graft area showed good response in both treatment groups with significant difference between groups and observation period (p < 0.05) (Table 2). Pain response/inflammation resolved (healed) faster in moist dressing (G-II) compared to Tilapia skin dressing (G-I). On days 3 and 6, auto skin graft treated by Tilapia skin dressing (G-I) still showed pain response/inflammation with a score of 2.00 ± 0.00 and 2.00 ± 0.00, respectively, giving no significant difference (p > 0.05). Pain response in Tilapia skin dressing (G-I) had gone/recovered on day 18, which is significantly different (p < 0.05) with days 3, 6, 9, and 12.
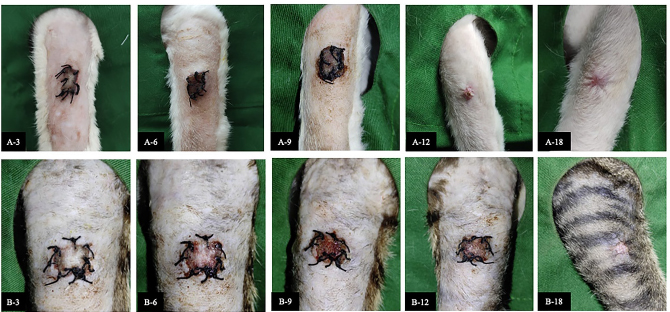
Fig. 1. The recovery of auto skin graft for each observation period. Group I auto skin graft treated by Tilapia skin dressing on day 3 post-surgery (A-3), day 6 post-surgery (A-6), day 9 post-surgery (A-9), day 12 post-surgery (A-12) and day 18 post-surgery (A-18). Group II auto skin graft treated by moist dressing on day 3 post-surgery (B-3), day 6 post-surgery (B-6), day 9 post-surgery (B-9), day 12 post-surgery (B-12) and day 18 post-surgery (B-18). Table 2. Average value (±SD) of pain response for auto skin graft on days 3, 6, 9, 12, and 18 after being treated by Tilapia skin and mist dressing.
Skin graft with moist dressing (G-II), pain response was gone/recovered faster on day 9 with a score of 1.33 ± 0.58 which is significantly different (p < 0.05) from day 6 (2.00 ± 0.00) and day 3 (2.00 ± 0.00) post-surgery. There was a strong negative correlation between the observation period with pain response skin graft on G-I (r=−0.806, p < 0.001) and G-II (r=−0.808, p < 0.001). Pain response was resolved/healed fasted in moist dressing (G-II) compared to Tilapia skin dressing (G-I). Pain response is one of the symptoms of inflammation. The decrease in collagen function of Tilapia skin dressing due to immersion in sterilizing chlorhexidine leads to a reduction in cell proliferation capability during the healing of auto skin grafts in cats. Bleeding test, absorption test, and drug effectA bleeding test on skin graft was performed on day 18 post-surgery. Blood was bright red and came out immediately after incision on both treatment groups with a scoring value of (1.00 ± 0.00) and did not show a significant difference (p > 0.05). After the skin graft tissue has integrated perfectly, necrotic tissue will be replaced by collagen. A large amount of collagen replacing necrotic tissue will cause the skin to lose its elasticity, and blood will take time to flow out after incision. The amount of collagen formed also influences absorption time and the onset of the drug effect. The 0.2 ml NaCl 0,9% absorption time for skin graft using Tilapia skin dressing (G-I) was (53.3 ± 5.50) seconds, longer compared to moist dressing (G-II) which was (50.0 ± 5.00 seconds). However, according to the independent T-test, no significant difference was found between the two groups (p > 0.05). The time required until pupil dilatation did not show a significant difference between the two treatment groups (p > 0.05). The average onset for pupil dilatation was (66.6 ± 4.04) seconds for Tilapia skin dressing (GI) and (66.0 ± 2.64) seconds for moist dressing (G-II) after adrenaline injection. Moist wound dressing provides adequate moisture and has a positive impact on the healing of auto-skin grafts. Prolonged stages of sterilizing Tilapia skin dressing in chlorhexidine result in a decreased collagen function within the Tilapia skin for the healing of auto-skin grafts. Wound healing is also hindered by several local factors, such as excessive exudation and crust, dehydration, necrotic tissue, and repeated trauma. In the two treatment groups, no fluid was found on the auto skin graft area. DiscussionCorrect wound treatment management can improve the recovery process (Kamoun et al., 2017). Technique choice also influences the success of skin grafts. Skin graft recovery consists of the inhibition phase, revascularization phase, and maturation phase (Junior et al., 2019). Wound treatment in a moist environment is better than dry. The optimum moisture for wound recovery is in a gelatinous environment with moisture ranging between damp and dry. Over moisture on the wound may cause the graft to be detached from the wound bed, and maceration could possibly occur in the wound area (Alam and Jeffery, 2019). Auto skin graft color changing into hyperemia is an inflammation/hemostatic process. This phase comes with artery vasodilatation followed by an increase in blood supply, thus making the skin turn red and swelling around the skin grafting wound area. Framycetin sulfate (moist dressing) has the advantage of facilitating a moist environment in the wound area because it contains antibiotics (Erwin et al., 2016). However, many researches showed moist wound healing as one of the factors that positively influence wound healing (Svenjo et al., 2000). Tilapia skin dressing has emerged as a potentially cost-effective wound treatment method with improved wound healing outcomes (Luze et al., 2022). Tilapia skin provides a large amount of collagen type 1 that is similar to human skin morphology with high endurance (Junior et al., 2019). Collagen has an important function in wound healing because it has the ability in hemostasis, interact with thrombocytes, interact with fibronectin, increase fluid exudation, increase cellular component, increase growth factor, and accelerate fibroplasia process, and occasionally epidermis proliferation (Werner and Grose, 2003). Collagen dressings are widely used due to their beneficial properties, including low antigenicity, enhanced inflammation, and hemostasis, and accelerated fibroplasia and epithelization (Luze et al., 2022). Collagen dressings derived from cattle and pig skin or chicken waste are inappropriate due to the risk of disease transmission or religious and cultural reasons (Alam and Jeffery, 2019; Ii et al., 2021). The inflammation process causes pain in the wound area due to the release of chemical mediators. The difference in score for this pain response is influenced by the period of the inflammation process for each treatment group. Tilapia skin is a good source of collagen, with antibacterial and antioxidant properties due to collagen peptides and omega-3 polyunsaturated fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (Ibrahim et al., 2023). The inflammation process caused pain around the auto skin graft tissue area. If the inflammation process did not occur, the wound area would remain a source of pain, and wound healing would be hindered. Tilapia skin acts by reducing inflammatory responses and advancing proinflammatory cytokines that promote wound healing (Luze et al., 2022). Previous evidence states that omega-3 polyunsaturated fatty acids, EPA, and docosahexaenoic acid, which are abundant in fish skin, reduce inflammatory responses and advance proinflammatory cytokines that promote wound healing (Yang et al., 2016). Acellular fish skin (AFS) containing these omega-3 polyunsaturated fatty acids can, therefore, facilitate the transition out of the inflammatory phase of the wound healing process (Michael et al., 2019). The quality of blood was determined by good proliferation between wound bed and skin graft donor. This showed that the auto skin graft area has a good proliferation process. The proliferation process is indicated by angiogenesis (neovascularization), which is the formation of a large amount of capillary blood vessels that give adequate nutrition for the cell regeneration process (Erwin et al., 2021). Through the bleeding test, the skin graft treated with moist dressing and Tilapia skin dressing was a success, and no vein congestion was found. If dark blood comes out after the incision on the skin graft area, this indicates vein congestion (Eric, 2006). Skin graft recovery with a lot of connective tissue and few neovascularization will cause blood to remain under the skin for a long time (Erwin et al., 2016). The rate of absorption and distribution in subcutaneous administration depends on vascularization supply in respective tissues. In the capillary section, absorption is eased by endothelial porous with approximately 3 μm radius. If the compound has reached the blood vessel, the test of the distribution is done by blood circulation. Distribution of respective drug compounds is dependent on the size of the molecules, plasma protein and tissue protein bond, solubility, and chemical properties (Adams and Ramsey, 2005; Bachmann et al., 2008). Tilapia skin is a readily available, quality, safe, inexpensive material that is easy to apply (Costa et al., 2019). Nile Tilapia skin also has a high level of biocompatibility in nature as the collagen extract is a biocompatible type I collagen with potential as a biomedical material for use in clinical regenerative medicine (Ibrahim et al., 2020). Collagen in normal skin is a combination of tropocollagen molecules into fibril. Fibrils then undergo cross-linking into bundles (Junior et al., 2019). Collagen synthesis is required in large amounts as an effort to rebuild the extracellular matrix (ECM) damaged during tissue damage (Ackermann, 2012). Normal tissue does not need ECM reparation since no damage is found. Collagen fiber in Tilapia skin can improve the wound-healing process in rats by improving adhesion, proliferation, and cell differentiation. Tilapia skin collagen induces epidermal growth factor and the expression of fibroblast growth factor. This increases the proliferation and differentiation of fibroblast and keratinocyte, which plays a role in wound healing. However, in this research, the treatment of auto skin grafts using moist dressing resulted in a faster recovery compared to Tilapia skin dressing (p < 0.05). Prolonged sterilization of Tilapia skin dressing using chlorhexidine led to collagen damage. Since irradiation can lead to collagen matrix cross-linking or the breakdown of peptide bonds inside the collagen molecules, sterilizing skin grafts with irradiation damages the fundamental components of the skin matrix (Ibrahim et al., 2020). Tilapia skin can form reepithelization within 12–17 days of treatment without dressing replacement and does not cause side effects during recovery (Junior et al., 2019). ConclusionThis research has managed to perform subjective and objective observation on the recovery of auto skin graft, which utilized Tilapia skin dressing and moist dressing. The recovery of auto skin graft treated by moist dressing showed better recovery, but Tilapia skin dressing can be an alternative to use in skin graft treatment. Tilapia skin dressing is widely accessible, good quality, safe, easy to obtain, and reasonably priced. Tilapia skin dressing holds promising potential for auto skin graft treatments by replacing chemical compounds used for sterilization during its production. AcknowledgmentsThe authors are highly thankful to the Rector of Universitas Syiah Kuala for the Candidate Professor Research Grant in 2023 and to the Dean of the Faculty of Veterinary Medicine, Universitas Syiah Kuala. The authors are also thankful to the Laboratory of Veterinary Surgery, Veterinary Radiology and Veterinary Pathology, Faculty of Veterinary Medicine of the Universitas Syiah Kuala for providing the necessary facilities for this study. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study was funded by The Universitas Syiah Kuala through Candidate Professor Research Grant No. No. 96/UN11.2.1/PT.01.03/ PNBP/2023. Authors’ contributionsThe authors will declare the contribution of each author. Erwin Erwin, Etriwati Etriwati, and Sugito Sugito conceived and designed, developed methodology, and implemented the study of anesthesia and surgery. Erwin Erwin and Hadi Mulki Satria executed the postsurgical treatment, analysis, and interpretation of Tilapia skin dressing for skin graft. All the authors reviewed and provided critical comments on the manuscript and read and approved the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAckermann, M.R. 2012. Inflammation and healing. In Pathology basis of veterinary disease. Eds., Zachary, J.F. and McGavin, M.D. St. Louis, MO: Elsevier Mosby, pp: 135–146. Adams, D.C. and Ramsey, M.L. 2005. Grafts in dermatologic surgery. Dermatol. Surg. 31(8 Pt 2), 1055–1067. Alam, K. and Jeffery, S.L.A. 2019. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: a case series. Mil. Med. 184(Suppl 1), 16–20. Bachmann, B.O., Bock, F., Weigand, S.J., Maruyama, K., Dana, M.R., Kruse, F.E., Leutjen-Drecoll, E. and Curseifen, C. 2008. Promotion of graft survival by vascular endothelial growth factor-A neutralization after high risk corneal transplantation. Arch. Ophthalmol. 126(1), 71–77. Costa, B.A., Lima Júnior, E.M., de Moraes Filho, M.O., Fechine, F.V., de Moraes, M.E.A., Silva Júnior, F.R., do Nascimento Soares, M.F.A. and Rocha, M.B.S. 2019. Use of Tilapia skin as a xenograft for pediatric burn treatment: a case report. J. Burn. Care. Res. 40(5), 714–717. Eric, R.P. 2006. Head and facial wounds in dog and cat. Vet. Clin. North. Am. Small. Anim. Pract. 36(4), 793–817. Erwin, E., Etriwati, E., Zamzami, R.S. and Hosea, C.T.P. 2021. Moist wound dressing and its application in distant skin flap in cats. Vet. World. 14(3), 734–738. Erwin, E., Gunanti, G., Handharyani, E. and Noviana, D. 2016. Subjective and objective observation of skin graft recovery on Indonesian local cat with different periods of transplantation time. Vet. World. 9(5), 481–486. Fiakos, G., Kuang, Z. and Lo, E. 2020. Improved skin regeneration with acellular fish skin grafts. Eng. Regen. 1, 95–101. Ibrahim, M., Ayyoubi, H.S., Alkhairi, L.A., Tabbaa, H., Elkins, I. and Narvil, R. 2023. Fish skin grafts versus alternative wound dressings in wound care: a systematic review of the literature. Cureus 15(3), e36348. Ibrahim, A., Hassan, D., Kelany, N., Kotb, S. and Solimman, M. 2020. Validation of three different sterilization methods of Tilapia skin dressing: impact on microbiological enumeration and collagen content. Front. Vet. Sci. 7, 597751. Ii, R.S., Saathoff, E.C., Larson, D.A., Wall, J.T., Wienandt, N.A., Magnusson, S., Kjartansson, H., Natesan, S. and Christy, R.J. 2021. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int. J. Mol. Sci. 22(4), 1590. Junior, E.M.L., Filho, M.O.D.M., Costa, B.A., Fechine, F.V., Moraes, M.E.A.D., Junior, F.R.S., Soares, M.F.A.D.N., Rocha, M.B.S. and Leontsinis, C.M.P. 2019. Innovative treatment using Tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J. Surg. Case. Rep. 6, 1–4. Kamoun, E.A., Kenawy, E.R. and Chen, X. 2017. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 8(3), 217–233. Lei, J., Sun, L., Li, P., Zhu, C., Lin, Z., Mackey, V., Coy, D.H. and He, Q. 2019. The wound dressings and their applications in wound healing and management. Health. Sci. J. 13(4), 662. Luze, H., Nischwitz, S.P., Smolle, C., Zrim, R. and Kamolz, L.P. 2022. The use of acellular fish skin grafts in burn wound management-a systematic review. Medicina (Kaunas) 58(7), 912. Michael, S., Winters, C. and Khan, M. 2019. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds 3(10), 262–268. Nasri, M. 2019. Bioactive peptides from fish collagen byproducts. In Hyproducts from agriculture and fisheries. Eds., Benjamin, K.S., Alberta, N.A.A. and Toldra, F. Hoboken, NJ: Wiley Online Library, pp: 309–333. Song, Z., Liu, H., Chen, L., Chen, L., Zhou, C., Hong, P. and Deng. C. 2021. Characterization and comparison of collagen extracted from the skin of the Nile Tilapia by fermentation and chemical pretreatment. Food. Chem. 15(340), 128139. Svenjo, T., Pomahac, B., Yao, F. and Slama, J. 2000. Accelerated healing of full-thickness skin wounds in a wet environment. Plast. Reconstr. Surg. 106(3), 602–612. Werner, S. and Grose, R. 2003. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83(3), 835–870. Yang, C.K., Polanco, T.O. and Lantis, J.C 2nd. 2016. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds 28(4), 112–118. | ||
| How to Cite this Article |
| Pubmed Style Erwin E, Etriwati E, Sugito S, Satria HM. Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Vet J. 2023; 13(10): 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 Web Style Erwin E, Etriwati E, Sugito S, Satria HM. Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. https://www.openveterinaryjournal.com/?mno=158486 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i10.14 AMA (American Medical Association) Style Erwin E, Etriwati E, Sugito S, Satria HM. Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Vet J. 2023; 13(10): 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 Vancouver/ICMJE Style Erwin E, Etriwati E, Sugito S, Satria HM. Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Vet J. (2023), [cited July 01, 2025]; 13(10): 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 Harvard Style Erwin, E., Etriwati, . E., Sugito, . S. & Satria, . H. M. (2023) Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Vet J, 13 (10), 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 Turabian Style Erwin, Erwin, Etriwati Etriwati, Sugito Sugito, and Hadi Mulki Satria. 2023. Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Veterinary Journal, 13 (10), 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 Chicago Style Erwin, Erwin, Etriwati Etriwati, Sugito Sugito, and Hadi Mulki Satria. "Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats." Open Veterinary Journal 13 (2023), 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 MLA (The Modern Language Association) Style Erwin, Erwin, Etriwati Etriwati, Sugito Sugito, and Hadi Mulki Satria. "Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats." Open Veterinary Journal 13.10 (2023), 1346-1351. Print. doi:10.5455/OVJ.2023.v13.i10.14 APA (American Psychological Association) Style Erwin, E., Etriwati, . E., Sugito, . S. & Satria, . H. M. (2023) Subjective and objective observation of Tilapia skin as auto skin graft dressing in cats. Open Veterinary Journal, 13 (10), 1346-1351. doi:10.5455/OVJ.2023.v13.i10.14 |