| Research Article | ||
Open Vet J. 2023; 13(8): 983-990 Open Veterinary Journal, (2023), Vol. 13(8): 983-990 Original Research Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar ratsNurina Titisari1,2, Ahmad Fauzi3, Intan Shameha Abdul Razak1, Nurdiana Samsulrizal4 and Hafandi Ahmad1,5*1Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia 2Department of Veterinary Physiology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 3Department of Veterinary Clinical Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 4Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, Malaysia 5Institute of Tropical Agriculture and Food Safety, Universiti Putra Malaysia, Serdang, Malaysia *Corresponding Author: Hafandi Ahmad. Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia. Email: hafandi [at] upm.edu.my Submitted: 03/04/2023 Accepted: 23/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
AbstractBackground: Fish oil, which is regarded as the primary source of omega-3 fatty acids, has been long studied for its potential as an antidiabetic therapy. However, its protective ability against insulin resistance and pancreatic islet alteration remains unclear and controversial. Aim: To investigate the beneficial effects of fish oil consumption on the progression of insulin resistance and pancreatic islet dysfunction in a rat model of diabetes. Methods: Diabetic rats model (n=30) were divided into five groups and received; 1) NS injection + NS oral (normal control); 2) NS injection + 3 g/kg fish oil (fish oil control); 3) streptozotocin (STZ) injection + NS oral [diabetes control (DC)]; 4) STZ injection + 1 g/kg fish oil (DFO1); and 5) STZ injection + 3 g/kg fish oil (DFO3). Fasting blood insulin was analyzed by commercial rat insulin enzyme-linked immunosorbent assay; meanwhile, the determination of insulin sensitivity was calculated by homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of beta-cell function. A histological study was conducted on pancreas tissue using H and E staining. Results: Fish oil supplementation reduced hyperglycemia and ameliorated HOMA-IR in STZ-induced animal models indicating that fish oil supplementation improved insulin sensitivity. Furthermore, animals treated with fish oil at a dose of 3 g/kg (DFO3) showed an enhancement in pancreatic islets, which was displayed by less abnormal structures than DC animals. This could imply that the administration of fish oil, especially rich in bioactive omega-3 fatty acids effectively inhibits insulin resistance and restore islet of Langerhans alteration in rats injected with STZ. Conclusion: Thus, the current study suggested that fish oil supplementation could support the treatment of diabetes but should not be considered as an alternative therapy. Keywords: Omega-3 fatty acids, Diabetes mellitus, Insulin, Islet of Langerhans, Wistar rats. IntroductionDiabetes mellitus (DM) treatment still lacks an ideal drug choice to combat the disease and its associated complications (Inzucchi and McGuire, 2008; Nasri and Rafieian-Kopaei, 2014; Harrison, 2021; Fralick et al., 2022). Insulin remains the primary therapy for DM, especially for type 1 DM (Malik and Taplin, 2014; Mesa, 2015). In the case of type 2 DM, lifestyle changes are required, as well as taking diabetes medication. Aside from being costly, non-insulin medication can also lead to other complications (Corathers et al., 2013). Nevertheless, the use of insulin hormone therapy is not also the ultimate solution. Health risks from having excess insulin in the blood are the development of hypertension, weight gain, and endothelial dysfunction (Kolb et al., 2020). Additionally, insulin therapy must be considered, particularly in patients with congenital diseases such as heart failure (Smooke et al., 2005). Therefore, it is essential to propose alternative strategies for preserving and regenerating beta-cells and preventing or delaying diabetes from its early stages (Meier, 2008; Reimann et al., 2009). The study of environmental factors contributing to the development of type 1 DM has focused on viruses and factors that might affect the gut immune system, including hygiene and diet (Warshauer et al., 2020). Diet has a substantial effect that may slow the progression of diabetes and diabetes-related complications (Asif, 2014; Khazrai et al., 2014). The consumption of products rich in omega-3 fatty acids, such as plant oil, fish, and seafood, is important for diabetes management (Mali et al., 2016). Many bioactive agents in marine resources, such as polyphenols, peptides, pigments, phlorotannins, and sterols, could be used to prevent and manage diabetes and its complications (Barde et al., 2015). The benefits of omega-3 fatty acids supplementation in diabetes can be defined by at least two mechanisms, including anti-inflammatory activity and suppression of autoimmunity (Purdel et al., 2021). Omega-3 fatty acids have been shown in numerous studies to have a beneficial impact on DM (Norris et al., 2007; Li et al., 2014; Iwase et al., 2015; Ricordi et al., 2019). In a study, fish oil from hybrid catfish was given orally to diabetic rats for 12 weeks could inhibit insulin resistance and pancreatic cell damage (Keapai et al., 2016). Another study found a protective effect against progressive beta-cell damage when 8% of fish oil was added to a high fructose diet (Soltan, 2012). However, other researchers argue the health advantages and assert a lack of effects or even could increase the risk of diabetes (Poudyal et al., 2011; Chauhan et al., 2017; Brown et al., 2019; Hu et al., 2022). Therefore, a better understanding of the potential role of omega-3 fatty acids in the development and progression of diabetes sheds light on the importance of consuming omega-3 fatty acids derived from fish oil to prevent the disorder’s development. Materials and MethodsChemicalsStreptozotocin (STZ) (Santa Cruz Biotechnology, CA, USA) was purchased from Gamma Scientific Biolab Company. The STZ solution is prepared by dissolving in sodium citrate buffer 100 mM (pH 4.5). Experimental animalsEight-week-old male Wistar rats (n =30) weights between 250 and 280 g were used in these experiments. The Wistar rats were used in this study due to their multi-purpose characteristics in physiological and toxicological studies (Onyibe et al., 2021; Edo, 2022; Edo et al., 2023). The animals were acclimatized for a week and maintained in a temperature-controlled room at 24°C ± 2°C with a relative humidity of 60% ± 10%. The animals were housed as three rats per cage and fed with a regular chow diet and plain water ad libitum until the end of the study. Diabetes inductionAfter 1 week of acclimatization, diabetes induction was conducted through multiple intraperitoneal injections of 0.5 ml STZ (45 mg/kg) for 3 days to create a type 1 DM animal model. Before injection, animals were fasted for 8 hours to maximize the STZ effect. After 1 week, the blood glucose levels were estimated from the animal tail with a digital blood glucose meter (Gluco-Dr). Animals with diabetes are characterized by fasting blood glucose (FBG) exceeding 11 mMol/l (Zin et al., 2019; Onyibe et al., 2021). Fish oil administrationThe animal was treated with menhaden fish oil (F8020) (Sigma-Aldrich company), which contains 8%–15% docosahexaenoic acid and 10%–15% eicosapentaenoic acid. Fish oil was administered orally using oral gavage once a day, between 8 and 9 am, in a volume of 0.5 ml per rat. Experimental designThe experimental animals were randomly allocated into five groups (n=6) (Table 1). The treatment groups consist of normal control (NC), fish oil control (FC), diabetes control (DC), diabetic treated with fish oil dose 1 g/kg (DFO1), and diabetic treated with fish oil dose 3 g/kg (DFO3). Body weight (BW) was measured at the beginning and the end of the experiment. On day 7 after STZ injection, animals with FBG below 11 mMol/l were excluded from the study. Following that, depending on the group, the animals were either given normal saline or fish oil orally for the next 3 weeks. At the end of the experiment, all animals were sacrificed by an overdose of anesthesia (e.g., ketamine and xylazine) for analysis of the second FBG and pancreas tissue. Determination of insulin sensitivityIndirect indexes for the assessment of insulin sensitivity were calculated using fasting serum insulin and blood glucose at the end of the study. Serum insulin levels were determined using a double-antibody enzyme-linked immunosorbent assay (ELISA). Insulin ELISA kit (Rat insulin ELISA kit, BT lab) procedures followed the manufacturer’s instructions. The homeostatic model assessment of insulin resistance (HOMA-IR) and the homeostatic model assessment of beta-cell function (HOMA B) index were calculated using the equation as described below (Wallace et al., 2004):
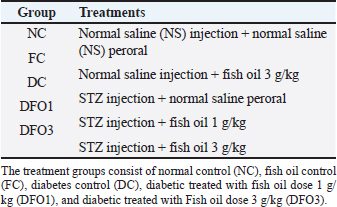
Histological analysisFor histological analysis, the pancreas tissue of each group was fixed in phosphate buffer formalin (10%) overnight. The samples were dehydrated in graded alcohol series and cleared with xylol. Later on, dehydrated samples were embedded in paraffin and cut into sections (4 μm). The pancreas sections were stained with hematoxylin and eosin (H and E). Images were obtained with an optilab camera microscope (software image raster) using 40× objective lens magnification. Statistical analysisStatistical analysis was conducted using the standard software Statistical Package for the Social Sciences version 20.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism, version 6.0 (GraphPad Sofware, Inc., La Jolla, CA). All data were analyzed using SPSS 20.0 software, and the data are reported as mean ± SEM (n=6/group). Statistical analysis was performed by analysis of variance for blood glucose, BW, and insulin sensitivity, followed by the Tukey post hoc test to elucidate significant means. The p value (p < 0.05) was considered a statistical significance between groups. Ethical approvalAll animal experimental care and procedures were approved by the Institutional for Animal Care and Use Committee (IACUC), Universiti Putra Malaysia, Selangor, Malaysia (UPM/IACUC/AUP-R017/2022). ResultsThe general examination in this study concluded that the diabetic classic signs were found in diabetic animals (DC, DFO1, and DFO3) which are polydipsia, polyphagia, and polyuria. Water intake of diabetic animals was higher (average 100–120 ml/rat) than control groups (NC, FC) after 7 days of STZ injection (average 50–70 ml/rat). Food intake also increased in the last 2 weeks of the experiment on diabetic animals (average 30–32 g/rat) than control animals (average 23–25 g/rat). Animal observation also showed alterations in physical appearances and physiology between the control group and the diabetic group. Acute weight loss, decreased activity, rough coat, and diarrhea were observed in the diabetic group throughout the study. Table 1. Experimental design of the treatment groups.
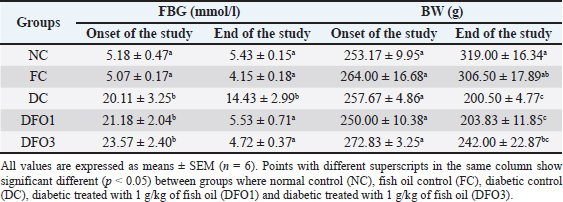
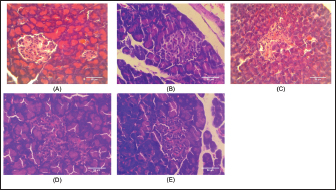
BW and FBG levels in rats with STZ-induced diabetes before and after fish oil administration were shown in Table 2. As expected, the diabetic rats (DC, DFO1, and DFO3) exhibited increased blood glucose and decreased BW after 1 week of STZ injections. In the initial study, the FBG of diabetic groups was significantly different from NC and FC groups (p < 0.05), however, BW differences were not statistically significant. After 3 weeks of fish oil supplementation in diabetic animals (DFO1 and DFO3), blood glucose had returned to normal but could not prevent BW loss. This result showed a positive effect of fish oil supplementation rich in omega-3 fatty acids toward hyperglycemia inhibition in STZ-induced rats. The NC group clearly showed significantly higher insulin levels (p < 0.05) compared to the diabetic groups (STZ-treated animals) (Fig. 1A). Furthermore, the HOMA-IR index, which implied body insulin resistance, was remarkably increased only in the DC group (Fig. 1B). The HOMA-B scores on DFO1 and DFO3 were significant difference (p < 0.05) from the NC (Fig. 1C). This means that fish oil supplementation for 3 weeks has not been able to restore beta-cells function even though the glucose level is not different from the NC group (Table 2). As shown in Figure 2A, B, and E, Langerhans’s islet appeared normal and paler than the surrounding exocrine cells. On the other hand, the islets of Langerhans of group DC and DFO1 depicted distorted pancreatic cell islets (Fig. 2C and D). Sections of the diabetic pancreas undergo shrinkage or atrophy with degeneration and necrosis of islets components cells and the disappearance of cell borders in the pancreatic islets. Nonetheless, the pathological changes in diabetic rats which received 3 g/kg/day of fish oil were less severe than those in the diabetic control group. Meanwhile, fish oil supplementation of 3 g/kg/day showed beneficial histological changes as it remarkably reduced pathological changes. Table 2. Effect of fish oil supplementation on BW and FBG after 3 weeks of treatment.
Fig. 1. Control negative group (NC) had higher insulin levels (A), the diabetic control group (DC) was insulin resistant (B), and beta-cell dysfunction (C). All values are expressed as means ± SEM (n=6). *p < 0.05 versus NC; **p < 0.01 versus NC.
Fig. 2. Histology of treated animal pancreas tissue was as follows: (A) NC rat with a normal architecture of pancreatic islets and acinar cell; (B) FC rat (received fish oil with 3 g/kg) showed the same structure with NC rat; (C) diabetic control rat (received multiple doses of 45 mg/kg of STZ) showed distorted pancreatic islet and necrotic cells; (D) 1 g/kg of fish oil treatment on STZ-treated rat depicted islet Langerhans deformed structure but not as severe as the diabetic control group; and (E) STZ-treated rat received 3 g/kg of fish oil treatment showed slightly pathological changes on the islet of Langerhans and appear almost identical as normal group and FC group (40× magnification, H and E staining, scale bar: 50 µm). DiscussionOur results showed fish oil supplementation could not compromise typical symptoms of diabetes disorder in rats injected with STZ, which are conditions of excessive thirst, excessive urine, increased food consumption, and weight loss (Gyamfi et al., 2019). These symptoms arise due to insulin deficiency, which causes the body to exchange proteins and fatty acids for energy sources, resulting in weight loss (Ghule et al., 2010). Furthermore, high blood glucose due to insulin insufficiency initiates the kidneys to produce more urine to eliminate the glucose from the body. This loss of fluids then causes a syndrome of polydipsia, inducing the individual to increase water intake (Bakris et al., 2009; Gerich, 2010; Uduak Akpan et al., 2012). Even so, in this study, the fish oil-treated STZ-induced animals showed abnormalities characteristic of diabetes even though the blood glucose reached the normal level. We suspected STZ injection causing complications in other organs besides the pancreas, such as the kidneys and liver. As it has been established that STZ binding to the glucose transporter 2 (GLUT2) in the plasma membrane then produces pathological effects on the development of diabetes in the animal model (Lenzen, 2008; Šoltésová and Herichová, 2011). Whereas other cells besides the pancreas could express GLUT2 transporters, such as renal tubular cells and hepatocytes, which then impact the organ structure and function (Eleazu et al., 2013). Moreover, the complication is not scientifically proven due to limitations in this study, but some investigators reported that fish oil could not completely inhibit pathological changes in the kidneys (Ghadge et al., 2016; Parveen et al., 2019) or liver (Jangale et al., 2013) of rats injected with STZ. FBG levels in the diabetic control rats increased at the beginning, indicating that the STZ injection method in this study was damaging pancreatic beta-cells. Surprisingly, all diabetic rats had lower blood glucose levels by the end, yet DC rats were still considered hyperglycemia which is defined as being above 11 mMol/l (Dong et al., 2014; DiMeglio et al., 2018; Samsulrizal et al., 2021). Meanwhile, the fish oil treatment group in this study was within normal limits (4–6 mMol/l) (Wang et al., 2010). In line with previous observations, FBG was reduced after 4 weeks of fish oil supplementation in STZ-induced rats (Parveen et al., 2019). These FBG results were then calculated with fasting serum insulin levels to obtain HOMA-IR and HOMA-B. It is well-known that HOMA is a non-invasive method of measuring insulin resistance and pancreatic beta-cell function with a formula derived from examining plasma glucose and insulin levels (Bellia et al., 2018). As depicted in Figure 1, serum insulin levels, HOMA-B, and HOMA-IR ratio were alleviated markedly in the DC rats compared to the NC and FC rats. The DC group had the highest HOMA-IR ratio, revealing that animals encountered insulin resistance. Considering that HOMA-IR has been proven as a reliable indicator of insulin resistance in Wistar rats (Antunes et al., 2016). In this study, STZ-treated rats with fish oil supplementation significantly lowered insulin resistance, as seen from the lower HOMA-IR ratio. However, both fish oil treatments failed to improve the HOMA-B scores, which were thought to be related to pancreatic beta-cell damage. It is well known that a higher HOMA-B value indicates lower beta-cell pancreas dysfunction (Wallace et al., 2004; Festa et al., 2008). The HOMA-B ratio is closely associated with lower insulin production in DFO1 and DFO3 rats. This result implies that insulin secretion was not completely recovered after 3 weeks of oral fish oil administration. In contrast, scientific studies reported that omega-3 fatty acids may improve insulin secretion (Baynes et al., 2018; Zou et al., 2023). In the cell culture study, fish oil can prevent pancreatic cell death and enhance insulin secretion (Wei et al., 2010; Das et al., 2022). Meanwhile, in in vivo animal model, the study was limited, and the data was inconsistent. Omega-3 fatty acids-rich safflower oil has been shown to protect Fat-1 transgenic mice from insulin deficiency, high blood sugar, and beta-cell apoptosis (Bellenger et al., 2011). On the other hand, long-term fish oil dietary supplementation on healthy boars had no impact on plasma glucose and insulin sensitivity (Castellano et al., 2010). Data from humans also revealed conflicting findings (Mori et al., 2000; Woodman et al., 2002; Hendrich, 2010), indicating that the species under study, the duration of the treatment, and the capacity to control the amount of fatty acids absorbed can all have an impact on the outcomes (Wang and Chan, 2015). Histology of pancreas tissue displays two morphological and functionally distinct components by H and E staining. The exocrine pancreas (80%–85% of the pancreas) consists of acinar cells and ductal cells, and the endocrine pancreas (less than 5% of the total pancreas) consists of the islet of Langerhans (Eurell and Frappier, 2013; Zhou and Melton, 2018). The islets of Langerhans contain five types of cells: alpha cells (α-cells), beta-cells (β-cells), delta cells (δ-cells), pancreatic polypeptide cells (PP cells), and upsilon cells (ɛ-cells) (Longnecker, 2014; El Sayed and Mukherjee, 2017). Meanwhile, to distinguish beta-cells from other cells in the islet of Langerhans requires special staining such as Gomori’s aldehyde fuchsin or Mallory-Azan stain (Baskin, 2015; Hull and Baskin, 2015). As a result, the present study could only display the degeneration of pancreatic islet. The islets of Langerhans were almost entirely lost in STZ-treated rats (Fig. 2C). On the other hand, the DFO3 displays a nearly normal pancreatic islet structure compared with DFO1. This study’s result supports the theory of the protective effect of omega-3 fatty acids against pancreas abnormality changes in STZ-treated rats. In fact, our research finding was in line with Bellenger et al. (2011) and Soltan (2012) who reported that an omega-3 fatty acids enrichment diet prevents necrotic changes and atrophy of the pancreatic islet. Indeed, omega-3 fatty acids provoke beta-cell regeneration and protect the pancreas from apoptosis (Habib, 2013; Li et al., 2014). According to pancreatic islet observations, 3 weeks of fish oil supplementation in STZ-induced rats appears to be able to preserve islet structural integrity, particularly with 3 g/kg fish oil. However, the fact that insulin levels were lower in STZ-treated rats implies that beta-cells damage remains to occur, as indicated by the lower HOMA-B ratio. On the other hand, fish oil supplementation clearly showed sharply declined blood glucose levels as well as an improvement of the HOMA-IR ratio. These findings suggest that the anti hyperglycemic ability of fish oil may be due to improved insulin sensitivity, as supported by the HOMA-IR ratio. Thus, fish oil treatment likely reduced the hyperglycemia in diabetic animal models, and consequently will protect pancreatic islets from alteration. ConclusionIn conclusion, these studies indicated that fish oil supplementation decreased blood glucose, prevented insulin resistance, and improved pancreatic islets structure in STZ-induced diabetic rats. However, it was unable to reverse the BW loss and metabolic changes caused by STZ induction in Wistar rats. Based on the limitation of this study, we suggested further investigations regarding the long-term effect of omega-3 fatty acids in the sub-acute and high-dose toxicity studies. AcknowledgmentsThe authors would like to thank the research officers at the Physiology Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia, and Universitas Brawijaya for supporting this study. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Author contributionNurina Titisari, Hafandi Ahmad: Conceptualization, Methodology, Analysis, Writing-Original draft. Ahmad Fauzi: Analysis. Intan Shameha Abdul Razak, Nurdiana Samsulrizal: Supervision. All Authors read and approved the final manuscript. FundingThis work was supported by Universiti Putra Malaysia through Putra Grant Scheme (IPS) (9722900). Data availabilityData are available from the corresponding author, Associate Professor Dr. Hafandi Ahmad for researchers who meet the criteria for access to confidential data. ReferencesAntunes, L.C., Elkfury, J.L., Jornada, M.N., Foletto, K.C. and Bertoluci, M.C. 2016. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch. Endocrinol. Metab. 60(2), 138–142. Asif, M. 2014. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Health Promot. 3(1), 1. Bakris, G.L., Fonseca, V.A., Sharma, K. and Wright, E.M. 2009. Renal sodium–glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 75(12), 1272–1277. Barde, S.R., Sakhare, R.S., Kanthale, S.B., Chandak, P.G. and Jamkhande, P.G. 2015. Marine bioactive agents: a short review on new marine antidiabetic compounds. Asian Pac. J. Trop. Dis. 5(1), S209–S213. Baskin, D.G. 2015. A historical perspective on the identification of cell types in pancreatic islets of Langerhans by staining and histochemical techniques. J. Histochem. Cytochem. 63(8), 543. Baynes, H.W., Mideksa, S. and Ambachew, S. 2018. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 7(2), 81–87. Bellenger, J., Bellenger, S., Bataille, A., Massey, K.A., Nicolaou, A., Rialland, M., Tessier, C., Kang, J.X. and Narce, M. 2011. High pancreatic n-3 fatty acids prevent sTZ-induced diabetes in fat-1 mice: inflammatory pathway inhibition. Diabetes 60(4), 1090. Bellia, C., Zaninotto, M., Cosma, C., Agnello, L., Lo Sasso, B., Altavilla, P., Bivona, G., Pizzolanti, G., Bernardini, S., Plebani, M. and Ciaccio, M. 2018. Glycated albumin is correlated to insulin resistance and -cell secretory function in subjects at risk of developing diabetes. Biochim. Clin. 42(3), 234–239. Brown, T. J., Brainard, J., Song, F., Wang, X., Abdelhamid, A. and Hooper, L. 2019. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 366, l4697. Castellano, C.A., Audet, I., Laforest, J.P., Chouinard, Y. and Matte, J.J. 2010. Fish oil diets do not improve insulin sensitivity and secretion in healthy adult male pigs. Br. J. Nutr. 103(2), 189–196. Chauhan, S., Kodali, H., Noor, J., Ramteke, K. and Gawai, V. 2017. Role of omega-3 fatty acids on lipid profile in diabetic dyslipidaemia: Single blind, randomised clinical trial. J. Clin. Diagn. Res. 11(3), OC13–OC16. Corathers, S.D., Peavie, S. and Salehi, M. 2013. Complications of diabetes therapy. Endocrinol. Metab. Clin. North Am. 42(4), 947. Das, M., Banerjee, A. and Roy, R. 2022. A novel in vitro approach to test the effectiveness of fish oil in ameliorating type 1 diabetes. Mol. Cell. Biochem. 477(8), 2121–2132. DiMeglio, L.A., Evans-Molina, C. and Oram, R.A. 2018. Type 1 diabetes. Lancet. 391(10138), 2449–2462. Dong, Y., Jing, T., Meng, Q., Liu, C., Hu, S., Ma, Y., Liu, Y., Lu, J., Cheng, Y., Wang, D. and Teng, L. 2014. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed. Res. Int. 2014, 160980. Edo, G.I. 2022. Effects of paraquat dichloride on adult male wistar rat. an approach in the toxicity of body weights and hematological tissues. J. Anal. Pharm. Res. 11(1), 1–7. Edo, G.I., Ugbune, U., Onoharigho, F.O., Ezekiel, G.O. and Agbo, J.J. 2023. Antioxidant activities of Reissantia indica willd. (mopane paddle-pod) and nephroprotective effect on paracetamol-induced nephrotoxicity in male Wistar rats. Nutrire 48(1), 1–12. Eleazu, C.O., Eleazu, K.C., Chukwuma, S. and Essien, U.N. 2013. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 12(1), 1–7. El Sayed, S.A. and Mukherjee, S. 2017. Physiology, pancreas. Treasure Island, FL: StatPearls Publishing. Eurell, J.A. and Frappier, B.L. 2013. Dellmann’s textbook of veterinary histology, 6th ed. Ames, IA: Blackwell Pub. Festa, A., Williams, K., Hanley, A.J.G. and Haffner, S.M. 2008. Β-Cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes 57(6), 1638–1644. Fralick, M., Jenkins, A.J., Khunti, K., Mbanya, J.C., Mohan, V. and Schmidt, M.I. 2022. Global accessibility of therapeutics for diabetes mellitus. Nat. Rev. Endocrinol. 18(4), 199–204. Gerich, J.E. 2010. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet. Med. 27(2), 136–142. Ghadge, A., Harsulkar, A., Karandikar, M., Pandit, V. and Kuvalekar, A. 2016. Comparative anti-inflammatory and lipidnormalizing effects of metformin and omega-3 fatty acids through modulation of transcription factors in diabetic rats. Genes Nutr. 11(1), 1–12. Ghule, S., Prakash, T., Kotresha, D., Karki, R., Surendra, V. and Goli, D. 2010. Anti-diabetic activity of Celosia argentea root in streptozotocin-induced diabetic rats. Int. J. Green Pharm. 4(3), 206–211. Gyamfi, D., Awuah, E.O. and Owusu, S. 2019. Molecular aspects and biochemical regulation of diabetes mellitus. In Molecular nutrition: carbohydrates. Ed., Patel, V.B. London, UK: Academic Press an Imprint of Elsevier, pp: 35–57. Habib, E.K. 2013. Possible role of omega-3 on the pancreas of streptozotocin-induced diabetes in adult albino rats: histological and immunohistochemical study. Egypt. J. Histol. 36(3), 579–591. Harrison, L.C. 2021. The dark side of insulin: a primary autoantigen and instrument of self-destruction in type 1 diabetes. Mol. Metab. 52, 101288. Hendrich, S. 2010. (n-3) Fatty acids: clinical trials in people with type 2 diabetes. Adv. Nutr. 1(1), 3–7. Hu, M., Fang, Z., Zhang, T. and Chen, Y. 2022. Polyunsaturated fatty acid intake and incidence of type 2 diabetes in adults: a dose response meta-analysis of cohort studies. Diabetol. Metab. Syndr. 14(1), 1–23. Hull, R.L. and Baskin, D.G. 2015. Histochemical insights into pancreatic islet biology. J. Histochem. Cytochem. 63(8), 541. Inzucchi, S.E. and McGuire, D.K. 2008. New drugs for the treatment of diabetes. Circulation. 117(4), 574–584. Iwase, Y., Kamei, N. and Takeda-Morishita, M. 2015. Antidiabetic effects of omega-3 polyunsaturated fatty acids: from mechanism to therapeutic possibilities. Pharmacol. Pharm. 6(3), 190–200. Jangale, N.M., Devarshi, P.P., Dubal, A.A., Ghule, A.E., Koppikar, S.J., Bodhankar, S.L., Chougale, A.D., Kulkarni, M.J. and Harsulkar, A.M. 2013. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chem. 141(1), 187–195. Keapai, W., Apichai, S., Amornlerdpison, D. and Lailerd, N. 2016. Evaluation of fish oil-rich in MUFAs for anti-diabetic and antiinflammation potential in experimental type 2 diabetic rats. Korean J. Physiol. Pharmacol. 20(6), 581–593. Khazrai, Y.M., Defeudis, G. and Pozzilli, P. 2014. Effect of diet on type 2 diabetes mellitus: a review. Diabetes Metab. Res. Rev. 30(S1), 24–33. Kolb, H., Kempf, K., Röhling, M. and Martin, S. 2020. Insulin: too much of a good thing is bad. BMC Med. 18(1), 224. Lenzen, S. 2008. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51(2), 216–226. Li, P., Zhang, L., Tian, X. and Xing, J. 2014. Docosahexaenoic acid has an anti-diabetic effect in streptozotocin-induced diabetic mice. Int. J. Clin. Exp. Med. 7(9), 3021–3029. Longnecker, D.S. 2014. Anatomy and histology of the pancreas (Version 1.0). Pancreapedia: the exocrine pancreas knowledge base. American Pacreatic Association. Mali, A.V., Bhise, S.S. and Katyare, S.S. 2016. Omega-3 fatty acids and diabetic complications. In Omega-3 fatty acids. Eds., Hegde, M., Zanwar, A. and Adekar, S. Cham, Switzerland: Springer, pp: 221–227. Malik, F.S. and Taplin, C.E. 2014. Insulin therapy in children and adolescents with type 1 diabetes. Pediatr. Drugs 16(2), 141–150. Meier, J.J. 2008. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia 51(5), 703–713. Mesa, J. 2015. New insulin types in type 1 diabetes mellitus. Med. Clin. (Barc) 145(2), 70–75. Mori, T.A., Burke, V., Puddey, I.B., Watts, G.F., O’Neal, D.N., Best, J.D. and Beilin, L.J. 2000. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hypedipidemic men. Am. J. Clin. Nutr. 71(5), 1085–1094. Nasri, H. and Rafieian-Kopaei, M. 2014. Metformin: current knowledge. J. Res. Med. Sci. 19(7), 658. Norris, J.M., Yin, X., Lamb, M.M., Barriga, K., Seifert, J., Hoffman, M., Orton, H.D., Barón, A.E., Clare-Salzler, M., Chase, H.P., Szabo, N.J., Erlich, H., Eisenbarth, G.S. and Rewers, M. 2007. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. J. Am. Med. Assoc. 298(12), 1420–1428. Onyibe, P.N., Edo, G.I., Nwosu, L.C. and Ozgor, E. 2021. Effects of vernonia amygdalina fractionate on glutathione reductase and glutathione-S-transferase on alloxan induced diabetes Wistar rat. Biocatal. Agric. Biotechnol. 36, 102118. Parveen, K., Siddiqui, W.A., Arif, J.M., Kuddus, M., Shahid, S.M.A. and Kausar, M.A. 2019. Evaluation of vegetables and fish oils for the attenuation of diabetes complications. Cell. Mol. Biol. 65(7), 38–45. Poudyal, H., Panchal, S. K., Diwan, V. and Brown, L. 2011. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog. Lipid Res. 50(4), 372–387. Purdel, C., Ungurianu, A. and Margina, D. 2021. Metabolic and metabolomic insights regarding the omega-3 PUFAs intake in type 1 diabetes mellitus. Front. Mol. Biosci. 8, 1–15. Reimann, M., Bonifacio, E., Solimena, M., Schwarz, P.E.H., Ludwig, B., Hanefeld, M. and Bornstein, S.R. 2009. An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol. Ther. 121(3), 317–331. Ricordi, C., Clare-Salzler, M., Infante, M., Baggerly, C., Aliano, J., McDonnell, S. and Chritton, S. 2019. Vitamin D and omega 3 field study on progression of type 1 diabetes. CellR4 Repair Replace Regen. Reprogram. 7, e2737. Samsulrizal, N., Goh, Y.M., Ahmad, H., Md Dom, S., Azmi, N.S., NoorMohamad Zin, N.S. and Ebrahimi, M. 2021. Ficus deltoidea promotes bone formation in streptozotocin-induced diabetic rats. Pharm. Biol. 59(1), 66–73. Smooke, S., Horwich, T.B. and Fonarow, G.C. 2005. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am. Heart J. 149(1), 168–174. Soltan, S. 2012. The effects of varieties sources of omega-3 fatty acids on diabetes in rats. Food Nutr. Sci. 3(10), 1404–1412. Šoltésová, D. and Herichová, I. 2011. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetol. Metab. Endokrinol. Vyziva. 14(3), 130–138. Uduak Akpan, O., Owo, D., Udokang, N., Udobang, J. and Ekpenyong, C. 2012. Oral administration of aqueous leaf extract of Ocimum gratissimum ameliorates polyphagia, polydipsia and weight loss in streptozotocin-induced diabetic rats. Am. J. Med. Med. Sci. 2(3), 45–49. Wallace, T.M., Levy, J.C. and Matthews, D.R. 2004. Use and abuse of HOMA modeling. Diabetes Care. 27(6), 1487–1495. Wang, X. and Chan, C.B. 2015. N-3 Polyunsaturated fatty acids and insulin secretion. J. Endocrinol. 224(3), R97–R106. Wang, Z., Yang, Y., Xiang, X., Zhu, Y., Men, J. and He, M. 2010. Estimation of the normal range of blood glucose in rats. J. Hyg. Res. 39(2), 133–137. Warshauer, J.T., Bluestone, J.A. and Anderson, M.S. 2020. New frontiers in the treatment of type 1 diabetes. Cell Metab. 31(1), 46–61. Wei, D., Li, J., Shen, M., Jia, W., Chen, N., Chen, T., Su, D., Tian, H., Zheng, S., Dai, Y. and Zhao, A. 2010. Cellular production of n-3 PUFAs and reduction of n-6 -to-n-3 ratios in the pancreatic β-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 59(2), 471–478. Woodman, R.J., Mori, T.A., Burke, V., Puddey, I.B., Watts, G.F. and Beilin, L.J. 2002. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am. J. Clin. Nutr. 76(5), 1007–1015. Zhou, Q. and Melton, D.A. 2018. Pancreas regeneration. Nature 557(7705), 351–358. Zin, N.S.N.M., Hashim, N., Samsulrizal, N. and Azmi, N.S. 2019. The protective effect of Azadirachta excelsa leaves extract and quercetin treatment on the learning and memory impairments in relation with insulin and amylin levels in the brain of streptozotocin-induced diabetic rats. J. King Saud Univ. Sci. 31(3), 299–307. Zou, H.Y., Zhang, H.J., Zhao, Y.C., Li, X.Y., Wang, Y.M., Zhang, T.T. and Xue, C.H. 2023. N-3 PUFA deficiency aggravates streptozotocin-induced pancreatic injury in mice but dietary supplementation with DHA/EPA protects the pancreas by suppressing inflammation, oxidative stress and apoptosis. Mar Drugs. 21(1), 39. | ||
| How to Cite this Article |
| Pubmed Style Titisari N, Fauzi A, Abdul-razak IS, Samsulrizal N, Ahmad H. Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Vet J. 2023; 13(8): 983-990. doi:10.5455/OVJ.2023.v13.i8.4 Web Style Titisari N, Fauzi A, Abdul-razak IS, Samsulrizal N, Ahmad H. Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. https://www.openveterinaryjournal.com/?mno=148332 [Access: July 11, 2025]. doi:10.5455/OVJ.2023.v13.i8.4 AMA (American Medical Association) Style Titisari N, Fauzi A, Abdul-razak IS, Samsulrizal N, Ahmad H. Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Vet J. 2023; 13(8): 983-990. doi:10.5455/OVJ.2023.v13.i8.4 Vancouver/ICMJE Style Titisari N, Fauzi A, Abdul-razak IS, Samsulrizal N, Ahmad H. Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Vet J. (2023), [cited July 11, 2025]; 13(8): 983-990. doi:10.5455/OVJ.2023.v13.i8.4 Harvard Style Titisari, N., Fauzi, . A., Abdul-razak, . I. S., Samsulrizal, . N. & Ahmad, . H. (2023) Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Vet J, 13 (8), 983-990. doi:10.5455/OVJ.2023.v13.i8.4 Turabian Style Titisari, Nurina, Ahmad Fauzi, Intan Shameha Abdul-razak, Nurdiana Samsulrizal, and Hafandi Ahmad. 2023. Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Veterinary Journal, 13 (8), 983-990. doi:10.5455/OVJ.2023.v13.i8.4 Chicago Style Titisari, Nurina, Ahmad Fauzi, Intan Shameha Abdul-razak, Nurdiana Samsulrizal, and Hafandi Ahmad. "Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats." Open Veterinary Journal 13 (2023), 983-990. doi:10.5455/OVJ.2023.v13.i8.4 MLA (The Modern Language Association) Style Titisari, Nurina, Ahmad Fauzi, Intan Shameha Abdul-razak, Nurdiana Samsulrizal, and Hafandi Ahmad. "Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats." Open Veterinary Journal 13.8 (2023), 983-990. Print. doi:10.5455/OVJ.2023.v13.i8.4 APA (American Psychological Association) Style Titisari, N., Fauzi, . A., Abdul-razak, . I. S., Samsulrizal, . N. & Ahmad, . H. (2023) Protective potential of fish oil supplementation against insulin resistance and pancreatic islet damage in STZ-induced Wistar rats. Open Veterinary Journal, 13 (8), 983-990. doi:10.5455/OVJ.2023.v13.i8.4 |