| Case Report | ||
Open Vet J. 2023; 13(8): 1037-1043 Open Veterinary Journal, (2023), Vol. 13(8): 1037-1043 Case Report Standing sedation management of a domesticated reindeer for third eyelid removalSara J. Lawrence-Mills1*, Maria-Christine Fischer1, Sophie Talbot2, Jenny Reed2 and Carolina Palacios Jimenez11Queen Mother Hospital for Animals, The Royal Veterinary College, Hatfield, UK 2Royal Veterinary College, Equine Hospital and Specialists, Hatfield, UK *Corresponding Author: Sara J. Lawrence-Mills. Queen Mother Hospital for Animals, The Royal Veterinary College, Hatfield, UK. Email: smills21 [at] rvc.ac.uk Submitted: 02/04/2023 Accepted: 17/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
AbstractBackground: Reindeer are becoming popular animals within petting farms. Few case reports describe the sedation of domesticated reindeer, but none describe the use of ocular local anesthetic blocks in this species. Case Description: A 9-year-old, female, Svalbard reindeer (Rangifer tarandus platyrhynchus) presenting for removal of a squamous cell carcinoma involving the third eyelid. Standing sedation was performed using initial boluses of medetomidine and butorphanol via intramuscular injection before catheter placement and maintenance with a variable rate infusion of medetomidine. Supraorbital, auriculopalpebral, infratrochlear blocks and local infiltration of the base of the third eyelid were performed using mepivacaine. Following the surgical removal of the third eyelid, atipamazole was administered intramuscularly to antagonize the effects of medetomidine. The patient recovered without complications. Conclusion: Medetomidine-butorphanol in combination with local anesthetic blocks provided a sufficient plane of sedation and analgesia for extra ocular surgery in a domesticated reindeer. Keywords: Svalbard reindeer, Anesthesia, Local anesthetic blocks for ophthalmic surgery, Ocular squamous cell carcinoma. IntroductionReindeer are becoming very popular in the UK, typically hand reared at farms, and commonly used in meet and greet events. Due to this increase in numbers, veterinarians must know about the husbandry and anesthesia of this species. There are very few case reports detailing the anesthetic management of domestic reindeer in the literature (Lehnus, 2018). Primarily reports for this species are focused on the capture of non-domesticated wild or captive animals (Ryeng et al., 2001a; Evans et al., 2013). As such, anesthetic protocols focus on immobilization (Ryeng et al., 2001a). The most commonly referenced pharmacological combination, intramuscular xylazine-ketamine, is associated with significant anesthetic complications (Monticelli et al., 2017). To date, there are no reports describing the use of variable rate infusion to sedate domesticated reindeer or the use of local anesthetic blocks to facilitate ophthalmic surgery in this species. Case DetailsA 9-year-old, 71 kg, female Svalbard reindeer (Rangifer tarandus platyrhynchus) presented to a referral veterinary hospital for the reappearance of a left ocular mass on her third eyelid. Five years prior the reindeer had undergone eyelid mass removal for a suspected squamous cell carcinoma of her right eye at the same institution. She was systemically well with a 9-month-old calf at the foot. On conscious ophthalmic examination, the margin of the third eyelid was nonpigmented and displayed a poorly defined mass lesion with an irregular surface involving the leading edge of the nictitating membrane (Fig. 1). The owner elected to remove the third eyelid without further diagnostic tests. The reindeer was starved for 20 hours, water was removed 2 hours before the procedure. Her calf remained with her throughout the hospitalization and surgical procedure. Before surgery, her antlers were wrapped to maintain the safety of the anaesthetic and surgical team. The morning of the surgery, the reindeer was moved to an equine recovery box as this provided soft padded flooring in the likely occurrence that she became recumbent. The relevant equipment and anesthetic machine were prepared to enable easy intubation and conversion to general anesthesia if required. Premedication consisted of 30 ug/kg medetomidine and 0.05 mg/kg butorphanol via intramuscular injection. The onset of action was 10 minutes, at this time the reindeer became ataxic with low head carriage. After clipping and prepping the area a 20-gauge cephalic catheter was placed and a further 5 ug/kg medetomidine was administered intravenously. The reindeer became unsteady on her feet and was assisted into sternal recumbency. Her head remained elevated by the use of soft padded blocks (Fig. 2). Peri-operative intravenous fluids at 5 ml/kg/hour of compound sodium lactate were commenced. Monitoring consisted of electrocardiogram (ECG), non-invasive blood pressure, and capnography via placement of an extension line into the right nostril. Mask oxygen was supplied at 6 l/minute. Sedation depth was monitored throughout by assessment of palpebral reflex, ear flick, eye position, and response to auditory stimulation. Thirty minutes after the initial intramuscular injection the reindeer regained a strong palpebral reflex and started moving her legs in response to auditory stimulation. At this time an infusion of 10 ug/kg/hour of medetomidine was commenced. The infusion was reduced to 5 ug/kg/hour and subsequently stopped forty minutes later due to progressive bradycardia. Sedation depth remained adequate and no further pharmacological agents were required.
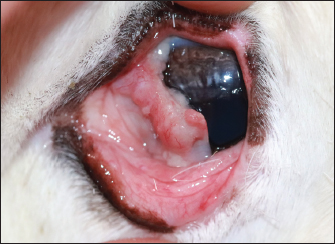
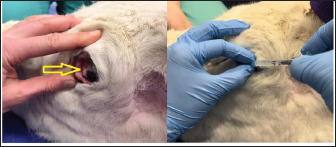
Fig. 1. Demonstration of the mass present on the reindeer’s third eyelid.
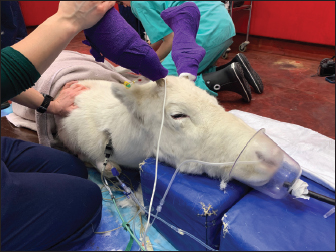
Fig. 2. Elevation of the patient’s head on soft padded blocks, in addition to mask oxygen supplementation and monitoring with ECG, capnography, and pulse oximetry. The cephalic intravenous catheter can be seen in the front right forelimb with a three-way tap connecting the medetomidine infusion and intravenous fluid therapy.
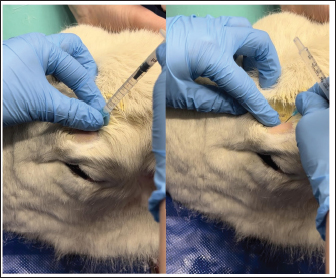
Fig. 3. Administration of the supraorbital nerve block showing needle placement and injection.
Fig. 4. Injection of the auriculopalpebral nerve block.
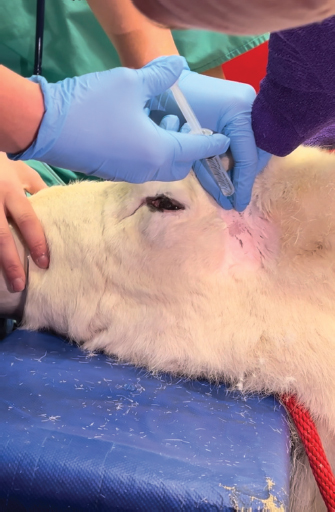
Fig. 5. Infiltration of the infratrochlear nerve block, the yellow arrow pointing to the palpable infratrochlear notch. Local anesthetic blocks were administered for the provision of analgesia and to facilitate third eyelid removal by blocking the palpebral reflex. Mepivicaine at a dose of 2 mg/kg was chosen. The supraorbital nerve block was performed by palpating the frontal nerve as it travelled through the supraorbital foramen located at the dorsolateral orbital rim, 1 ml of mepivacaine 2% was injected (Fig. 3). The auriculopalpebral nerve block was performed by palpating the nerve as it traversed horizontally over the dorsal most ridge of the zygomatic process of the temporal bone, 2 ml of mepivacaine 2% were injected just dorsal to the zygomatic arch and rostral to the base of the ear (Fig. 4). The infratrochlear nerve block was performed by palpating the infratrochlear notch at the medial canthus, 1 ml of mepivacaine 2% was then injected aiming medially beneath the orbital rim (Fig. 5). Local infiltration of the base of the third eyelid in the region that was not macroscopically affected by the neoplastic change was performed with 2 ml of mepivacaine 2%. A 2 ml splash block of the ocular surface was performed using lidocaine 2% and epinephrine 1:200,000. Lubricant using carmellose sodium (Celluvisc 1%) was applied to both eyes every 30 minutes until the palpebral reflex was regained. Physiological parameters remained within acceptable limits. Mild bradycardia at 32 beats per minute (bpm) was documented before the cessation of medetomidine infusion, pre-anaesthetic heart rate was 60 bpm. Mean arterial non-invasive blood pressure remained between 90 and 105 mmHg. Respiratory rate was stable between 12 and 19 breaths per minute maintaining an end tidal carbon dioxide between 40 and 50 mmHg. Pulse oximetry was >98% throughout the procedure. The temperature remained stable between 38.2 and 38.4 degrees Celsius. The surgical procedure consisted of aseptic preparation of the periocular region using 1:10 diluted iodine and flushing of the conjunctival sack using 1:50 diluted iodine over 3 minutes followed by a 10 ml saline flush. The patient was subsequently draped and a lidocaine/adrenaline splash block of 2 ml was applied to the ocular surface. The base of the third eyelid was clamped and sharply excised anterior to the haemostats. Following release minimal haemorrhage and mild orbital fat prolapse in the region of the medial canthus occurred. The orbital fat was repositioned and the entire surgical wound was closed with a continuous inverting suture pattern using 6-0 Vicryl. Adjunctive cryotherapy was performed using the Cryo-Alfa Super handheld cryosurgery unit. N2O gas cryospray was applied to the surgical incision site and the surrounding conjunctiva in two cycles of freezing and thawing. During this surgical step, the cornea was protected with lubricating carmellose sodium eye drops (Celluvisc 1%) and a surgical drape. Histological examination of the mass confirmed a squamous cell carcinoma with no evidence of vascular invasion. The neoplasm was fully excised with the narrowest histological margin measuring 4 mm. Upon completion of the procedure, medetomidine was antagonized with atipamezole, and a total of 70 ug/kg was given. This was administered in two boluses, 35 ug/kg intramuscularly and then 15 minutes later 35 ug/kg intravenously. After 30 minutes the reindeer was standing although she remained mildly ataxic and dull for a further 2 hours. She was perceived to be quiet for 12 hours after surgery. She was discharged the next day with ongoing oral meloxicam (Metacam®) 0.5 mg/kg q 48 hours, topical gentamicin sulphate 3.0 mg/ml eye drops (Tiacil®) q 8 hours, and bromfenac sodium sesquihydrate 0.9 mg/ml eye drops (Yellox®) q 8 hours. At 24 hours follow up there was mild to moderate blepharospasm and mild mucoid ocular discharge. The surgical wound looked unremarkable, no suture material was visible and there was no evidence of corneal ulceration. The conjunctiva surrounding the surgical site was markedly hyperaemic and chemotic. DiscussionFew studies describe the anesthetic management of reindeer. The combination of medetomidine and ketamine has been investigated for the immobilization of captive reindeer (Rangifer tarandus tarandus) (Ryeng et al., 2001a). They concluded a 100 ug/kg medetomidine in combination with 0.5 mg/kg ketamine via intramuscular injection was sufficient for induction of anesthesia. This dose of medetomidine is significantly higher than was required for the reindeer in our case report. This likely reflects the different pharmacological requirements for different subspecies of reindeer, in addition to the significant effect of domestication. Moreover, reindeer are reported to have seasonal variation in their resting metabolic rate; some papers reference a reduction of up to 30% in winter months (Nilssen et al., 1984). This may contribute to reduced drug elimination. However, pharmacokinetic studies are yet to prove this theory (Ranheim et al., 1997). Our case represents a very well-domesticated animal accustomed to frequent handling. The importance of environmental surroundings is evidenced by significantly higher drug doses being required when darting the same animals in an outdoor compared to an indoor environment (Ryeng et al., 2001a). Further, it is described that when the animals were in a paddock they were more easily disturbed and stressed than when already restrained. The same paper discusses the significantly increased levels of stress and excitement demonstrated in free-ranging reindeer when approached by humans, compared to captive animals. In addition to the effect these catecholamines have on the efficacy of anesthetic drugs, an investigation into impala capture identified the greatest influence on cardiovascular parameters was the duration of the exposure to stress, not the pharmacological agents used (Meyer et al., 2008a). This highlights the importance of considering an animal’s temperament and likely stress levels when selecting an anesthetic protocol. Importantly, cortisol and catecholamine levels have been shown to influence the incidence of capture hyperthermia (Meyer et al., 2008b). A case report detailing the use of medetomidine-ketamine in semi-domesticated Eurasia Tundra reindeer identified high levels of morbidity and mortality associated with high ambient temperatures (Tryland et al., 2021). These case reports demonstrate the importance of regular temperature measurement in these species. The risk of capture hyperthermia and/or myopathy was likely lower in the present case due to the reindeer being used for frequent handling. The most commonly documented anesthetic complications in reindeer appear to be hypoventilation and subsequent hypoxia. A study investigating medetomidine-ketamine in 12 healthy captive reindeer found an initial period of apnoea followed by deep respirations increasing in frequency during the recovery period (Ryeng et al., 2001b). A similar study in wild Norweigen reindeer demonstrated all seven to be hypoxaemic, determined by arterial blood gas sampling, before oxygen supplementation (Evans et al., 2013). A more recent case series detailing the anesthetic complications associated with xylazine +/− ketamine in four reindeer identified severe hypercapnia in one case and hypoxaemia in another (Monticelli et al., 2017). In one case of hypercapnia, the reindeer was intubated, and intermittent positive pressure ventilation was initiated, this improved the respiratory acidosis as documented on arterial blood gas analysis. This demonstrates the benefit of having the equipment ready to enable intubation if required. In all cases, the respiratory depression and hypoxaemia were attributed to alpha-2-agonist administration (Celly et al., 1997). The pulse oximetry and capnography values remained within normal physiological limits in the present case. This may reflect the lower doses of medetomidine administered and the early supplementation with oxygen. Interestingly, the primary physiological abnormality identified here was bradycardia, a decrease from a pre-anaesthetic heart rate of 62 to 32 bpm. While this was not associated with hypotension, without any arterial blood gas measurements the overall impact on tissue perfusion is difficult to ascertain. Further, pulse oximetry devices have not been validated for use in reindeer, thus monitoring with unvalidated equipment must be interpreted with caution. The present case also documented a prolonged recovery; the reindeer remained dull for 2 hours after the procedure and quiet for 12 hours post-operatively. This is likely the influence of an insufficient atipamazole dose. This was chosen due to the significant amount of time elapsed between the initial dose in combination with the returning reflexes already noted in the patient. It may also reflect the longer elimination half-life of medetomidine compared to atipamazole. One pharmacokinetic study documented reindeer becoming re-sedated 0.5—1 hours after reversal with atipamezole (Ranheim et al., 1997). If the reindeer was being released, or in an environment where close monitoring was not possible, a quicker recovery would have been desirable. The blepharospasm documented 12 hours after the procedure likely indicated ongoing pain, this may have contributed to the reindeer’s prolonged recovery. Post-operative analgesia consisted primarily of meloxicam dosed every 48 hours. This interval was informed by a recent pharmacokinetic study in semi-domesticated reindeer which concluded a single 0.5 mg/kg dose intravenously or orally maintained therapeutic concentrations for up to 3 days (Nurmi et al., 2022). However, studies are yet to investigate the analgesic effect of meloxicam over this time period in reindeer. Plasma profiles in sheep administered meloxicam subcutaneously and intramuscularly demonstrated 1 mg/kg to have anti-inflammatory and analgesic effects from 6 to 48 hours, determined by a reduction in lameness scoring (Woodland et al., 2019). However, it may be that more regular meloxicam dosing would have been beneficial in this case. Variable rate infusions of alpha-2 agonists are commonly implemented in other species. Studies in horses have found continuous infusion provides steady plasma concentrations which are directly proportional to adequate sedation (Dunlop et al., 1991). Continuous rate infusions (CRI) have been investigated in other ruminant species. One study utilized a detomidine CRI at 60 ug/kg/hour in eight adult sheep, they found this produced a satisfactory level of sedation with no significant changes in mean arterial blood pressure or cardiac output (de Moura et al., 2017). In this study one animal became hypoxaemic (PaO2 66.9 mmHg), with a low PaO2 for the duration of the procedure, however, all eight sheep recovered without complication. Another experimental study investigated the effects of dexmedetomidine infusion at 1 ug/kg/hour for 3 hours in pregnant ewes (Uemura et al., 2012). This infusion produced overt sedation. Repeated maternal arterial blood sampling evidenced no significant respiratory depression and no effect on foetal cerebral oxygenation. Medetomidine specifically has been implemented as a CRI in impala (Buck et al., 2017). This prospective study in ten adult female impala found constant rate infusions of 5 ug/kg/hour medetomidine and 1.5 mg/kg/hour ketamine in combination with a variable rate infusion of propofol starting at 0.2 mg/kg/minute successfully immobilized impala with rapid and calm recovery in all animals. They also concluded oxygen supplementation was required with hypoxaemia and hypercapnia commonly identified. The variable rate infusion utilized in this study started at 10 ug/kg/hour medetomidine as a sole agent. This produced profound sedation and in retrospect, a lower starting dose of 5 ug/kg/hour may have provided sufficient sedation without profound bradycardia. One other case report details the use of the opioid butorphanol at a similar dose (0.04 mg/kg of 0.05 mg/kg) in a reindeer (Lehnus, 2018). The combination of detomidine-butorphanol has been found as a safe reliable combination for standing sedation in a range of ungulate species (Bouts et al., 2017). Butorphanol has been shown an effective analgesic in sheep (Waterman et al., 1991), cows (Lauder et al., 2019), and llamas (Carroll et al., 2001). The implementation of analgesic medication is challenging in ruminants due to the lack of licensed agents. Despite this, the prevention of pain is vital to maintain good animal welfare. Furthermore, butorphanol and meloxicam have licensed formulations for horses (Veterinary Medicine Directorate, 2001 and 2007) and thus may be implemented via the cascade. In the present case report the use of local anesthetic blocks permitted the procedure to be performed under sedation rather than general anesthesia. This has the benefit of maintaining swallowing and gag reflexes thus decreasing the risk of regurgitation and subsequent aspiration pneumonia. Regurgitation is a common risk in ruminant species, while it has not been reported in reindeer, it has been noted as a complication in moose (Kreeger, 2000) and brown brocket deer (Munerato et al., 2013). To the author’s knowledge, this is the first case report to detail the use of local anesthetic blocks for ocular surgery in reindeer. Local anesthetic blocks are commonly utilized in other species to enable the reduction of systemic anesthetic agents thus minimizing cardiorespiratory depression and opioid requirements (de Henriksen and Brooks, 2014). The ocular nerve blocks used are well-described and commonly implemented in horses for ophthalmic surgery (Labelle and Clark-Price, 2013). The auriculopalpebral nerve provides motor innervation to the orbicularis oculi muscle that closes the eyelids; its blockade facilitates surgery by preventing blinking. The sensory input to the eyelids is supplied by cranial nerve V, the trigeminal nerve. This was blocked by anaesthetising the frontal nerve at the supraorbital foramen and the infratrochlear nerve at the medial canthus. The present case report demonstrates the translatability of equine anatomical landmarks to reindeer. Indeed, cadaveric studies investigating antler nervous supply support a similar anatomy in deer and equids (Woodbury and Haigh, 1996). The local anaesthetic mepivacaine was chosen for its intermediary duration of action; to provide analgesia for the entirety of the procedure whilst quickly enabling the recovery of the palpebral reflex (Harcourt et al., 2021). This local anaesthetic has the added benefit of being licensed in horses (Veterinary Medicine Directorate, 2017). ConclusionThis case report demonstrates the use of low doses of medetomidine in combination with butorphanol to achieve reliable sedation in a domesticated reindeer. It is the first report to document the successful implementation of ophthalmic blocks in this species to facilitate third eyelid removal. AcknowledgmentsThe authors would like to thank their colleagues at the Royal Veterinary College Equine Hospital. Conflict of interestThe authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article. FundingThere was no funding source for this study. Authors contributionsAll authors contributed to the clinical management of this case. The first draft of the manuscript was prepared by Sara Lawrence-Mills. All authors commented on the manuscript and the final version is a collaborative effort. Data availabilityAll relevant data is presented directly in the article. ReferencesBouts, T., Dodds, J., Berry, K., Arif, A., Taylor, P., Routh, A. and Gasthuys, F. 2017. Detomidine and butorphanol for standing sedation in a range of zoo-kept ungulate species. J. Zoo. Wildl. Med. 48(3), 616–626. Buck, R.K., Meyer, L.R.C., Stegmann, G.F., Kästner, S.B.R., Kummrow, M., Gerlach, C., Fosgate, G.T. and Zeiler, G.E. 2017. Propofol–medetomidine–ketamine total intravenous anaesthesia in thiafentanil–medetomidine-immobilized impala (Aepyceros melampus). Vet. Anaesth. Analg. 44(1), 138–143. Carroll, G.L., Boothe, D.M., Hartsfield, S.M., Martinez, E.A., Spann, A.C. and Hernandez, A. 2001. Pharmacokinetics and pharmacodynamics of butorphanol in llamas after intravenous and intramuscular administration. J. Am. Vet. Med. Assoc. 219(9), 1263–1268. Celly, C.S., McDonell, W.N., Young, S.S. and Black, W.D. 1997. The comparative hypoxaemic effect of four α2 adrenoceptor agonists (xylazine, romifidine, detomidine and medetomidine) in sheep. J. Vet. Pharmacol. Ther. 20(6), 464–471. de Henriksen, M.L. and Brooks, D.E. 2014. Standing ophthalmic surgeries in horses. Vet. Clin. North. Am. Equine. Pract. 30(1), 91–110. de Moura, R.S., Bittar, I.P., da Silva, L.H., Villela, A.C.V., dos Santos Júnior, M.B., Borges, N.C. and Franco, L.G. 2017. Sedative and cardiorespiratory effects of detomidine constant rate infusion in sheep. Lab. Anim. 52(1), 51–58. Dunlop, C.I., Daunt, D.A., Wagner, A.E., Shafer, S.L., Chapman, P.L. and Maze, M. 1991. Comparison of cardiopulmonary responses to detomidine administered as an intravenous steady-state infusion vs intravenous bolus in standing horses. Vet. Anaesth. Analg. 18, 117–120. Evans, A.L., Lian, M., das Neves, C.G., Øs, O., Andersen, R., Aanes, R., Strand, O., Tryland, M. and Arnemo, J.M. 2013. Physiologic evaluation of medetomidine-ketamine anesthesia in free-ranging Svalbard (Rangifer tarandus platyrhynchus) and wild Norwegian reindeer (Rangifer tarandus tarandus). J. Wildl. Dis. 49(4), 1037–1041. Harcourt, M., Smith, R. and Hosgood, G. 2021. Duration of skin desensitisation following palmar digital nerve blocks with lidocaine, bupivacaine, mepivacaine and prilocaine. Aust. Vet. J. 99(12), 541–546. Kreeger, J.T. 2000. Xylazine-induced aspiration pneumonia in shira’s moose. Wildl. Soc. Bull. 28(3), 751–753. Labelle, A.L. and Clark-Price, S.C. 2013. Anesthesia for ophthalmic procedures in the standing horse. Vet. Clin. North. Am. Equine. Pract. 29(1), 179–191. Lauder, J.K., Marti, S., Schwartzkopf-Genswein, K.S., Jelinski, M.D. and Janzen, E.D. 2019. Measuring behavioural and physiological responses to pain mitigation for ovariectomy in Bos taurus yearling beef heifers. J. Anim. Sci. 98(1), skz386. Lehnus, K. 2018. Endotracheal tube obstruction with a blood clot following aspiration of rumen contents in a reindeer. Vet. Rec. Case. Rep. 6(3), e000641. Meyer, L.C.R., Fick, L., Matthee, A., Mitchell, D. and Fuller, A. 2008b. Hyperthermia in captured impala (Aepyceros melampus): a fright not flight response. J. Wildl. Dis. 44(2), 404–416. Meyer, L.C.R., Hetem, R.S., Fick, L.G., Matthee, A., Mitchell, D. and Fuller, A. 2008a. Thermal, cardiorespiratory and cortisol responses of impala (Aepyceros melampus) to chemical immobilisation with 4 different drug combinations. J. S. Afr. Vet. Assoc. 79(3), 121–129. Monticelli, P., McSloy, A., Morath, U. and Adami, C. 2017. Challenging anaesthetic management of captive reindeer (Rangifer tarandus): report of 4 cases. Schweiz. Arch. 159(12), 657–662. Munerato, M.S., Duarte, J.M.B., Pereira, G.T. and Marques, J.A. 2013. Physiologic effects of three different protocols of isoflurane anaesthesia in captive brown brocket deer (Mazama Gouazoubira). J. Zoo. Wildl. Med. 44(4), 889–898. Nilssen, K.J., Sundsfjord, J.A. and Blix, A.S. 1984. Regulation of metabolic rate in Svalbard and Norwegian reindeer. Am. J. Physiol. Regul. Integr. Comp. Biol. 247(5), 837–841. Nurmi, H., Laaksonen, S., Raekallio, M. and Hanninen, L. 2022. Wintertime pharmacokinetics of intravenously and orally administered meloxicam in semi-domesticated reindeer (Rangifer tarandus tarandus). Vet. Anaesth. Analg. 49(4), 423–428. Ranheim, B., Horsberg, T.E., Nymoen, U., SØli, N.E., Tyler, N.J.C. and Arnemo, J.M. 1997. Reversal of medetomidine-induced sedation in reindeer (Rangifer tarandus tarandus) with atipamezole increases the medetomidine concentration in plasma. J. Vet. Pharmacol. Ther. 20(5), 350–354. Ryeng, K.A., Arnemo, J.M. and Larsen, S. 2001a. Determination of optimal immobilizing doses of a medetomidine hydrochloride and ketamine hydrochloride combination in captive reindeer. Am. J. Vet. Res. 62(1), 119–126. Ryeng, K.A., Larsen, S., Ranheim, B., Albertsen, G. and Arnemo, J.M. 2001b. Clinical evaluation of established optimal immobilizing doses of medetomidine-ketamine in captive reindeer (Rangifer tarandus tarandus). Am. J. Vet. Res. 62(3), 406–413. Tryland, M., Josefsen, T.D., Romano, J.S., Marcin, N., Mørk, T. and Arnemo, J.M. 2021. Case report: subclinical verminous pneumonia and high ambient temperatures had severe impact on the anesthesia of semi-domesticated Eurasian Tundra Reindeer (Rangifer tarandus tarandus) with medetomidine–ketamine. Front. Vet. Sci. 8, 606323. Uemura, K., Shimazutsu, K., McClaine, R.J., McClaine, D.J., Manson, R.J., White, W.D., Benni, P.B. and Reynolds, J.D. 2012. Maternal and preterm fetal sheep responses to dexmedetomidine. Int. J. Obstet. Anesth. 21(4), 339–347. Veterinary Medicine Directorate. 2001. Product details: metacam 20 mg/kg solution for injection for cattle, pigs and horses. Available via https://www.vmd.defra.gov.uk/ProductInformationDatabase/product/A005552 (Accessed 25 February 2023). Veterinary Medicine Directorate. 2007. Product details: dolorex 10 mg/ml solution for injection for horse, dog and cat. Available via https://www.vmd.defra.gov.uk/ProductInformationDatabase/product/A006939 (Accessed 25 February 2023). Veterinary Medicine Directorate. 2017. Product details: mepivacaine richter 20 mg/kg solution for injection for horses. Available via https://www.vmd.defra.gov.uk/ProductInformationDatabase/product/A009757 (Accessed 25 February 2023). Waterman, A.E., Livingston, A. and Amin, A. 1991. Analgesic activity and respiratory effects of butorphanol in sheep. Res. Vet. Sci. 51(1), 19–23. Woodbury, M.R. and Haigh, J.C. 1996. Innervation and anesthesia of the antler pedicle in wapiti and fallow deer. Can. Vet. J. 37(8), 486–489. Woodland, A.N., Van der Saag, D., Kimble, B., White, P.J., Govendir, M. and Lomax, S. 2019. Plasma pharmacokinetic profile and efficacy of meloxicam administered subcutaneously and intramuscularly to sheep. PLoS One 14(4), e0215842. | ||
| How to Cite this Article |
| Pubmed Style Lawrence-mills S, Fischer M, Talbot S, Reed J, Jimenez CP. Standing sedation management of a domesticated reindeer for third eyelid removal. Open Vet J. 2023; 13(8): 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 Web Style Lawrence-mills S, Fischer M, Talbot S, Reed J, Jimenez CP. Standing sedation management of a domesticated reindeer for third eyelid removal. https://www.openveterinaryjournal.com/?mno=148236 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i8.11 AMA (American Medical Association) Style Lawrence-mills S, Fischer M, Talbot S, Reed J, Jimenez CP. Standing sedation management of a domesticated reindeer for third eyelid removal. Open Vet J. 2023; 13(8): 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 Vancouver/ICMJE Style Lawrence-mills S, Fischer M, Talbot S, Reed J, Jimenez CP. Standing sedation management of a domesticated reindeer for third eyelid removal. Open Vet J. (2023), [cited July 01, 2025]; 13(8): 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 Harvard Style Lawrence-mills, S., Fischer, . M., Talbot, . S., Reed, . J. & Jimenez, . C. P. (2023) Standing sedation management of a domesticated reindeer for third eyelid removal. Open Vet J, 13 (8), 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 Turabian Style Lawrence-mills, Sara, Maria-christine Fischer, Sophie Talbot, Jenny Reed, and Carolina Palacios Jimenez. 2023. Standing sedation management of a domesticated reindeer for third eyelid removal. Open Veterinary Journal, 13 (8), 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 Chicago Style Lawrence-mills, Sara, Maria-christine Fischer, Sophie Talbot, Jenny Reed, and Carolina Palacios Jimenez. "Standing sedation management of a domesticated reindeer for third eyelid removal." Open Veterinary Journal 13 (2023), 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 MLA (The Modern Language Association) Style Lawrence-mills, Sara, Maria-christine Fischer, Sophie Talbot, Jenny Reed, and Carolina Palacios Jimenez. "Standing sedation management of a domesticated reindeer for third eyelid removal." Open Veterinary Journal 13.8 (2023), 1037-1043. Print. doi:10.5455/OVJ.2023.v13.i8.11 APA (American Psychological Association) Style Lawrence-mills, S., Fischer, . M., Talbot, . S., Reed, . J. & Jimenez, . C. P. (2023) Standing sedation management of a domesticated reindeer for third eyelid removal. Open Veterinary Journal, 13 (8), 1037-1043. doi:10.5455/OVJ.2023.v13.i8.11 |