| Research Article | ||
Open Vet J. 2023; 13(7): 942-947 Open Veterinary Journal, (2023), Vol. 13(7): 942-947 Original Research Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot studyTomohiro Yonezawa*, Cris Niño Bon B. Marasigan, Yuki Matsumiya, Shingo Maeda, Tomoki Motegi and Yasuyuki MomoiLaboratory of Veterinary Clinical Pathobiology, Department of Veterinary Medical Sciences, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan *Corresponding Author: Tomohiro Yonezawa. Laboratory of Veterinary Clinical Pathobiology, Department of Veterinary Medical Sciences, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan. Email: tomohiro.yonezawa [at] gmail.com Submitted: 12/04/2023 Accepted: 19/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
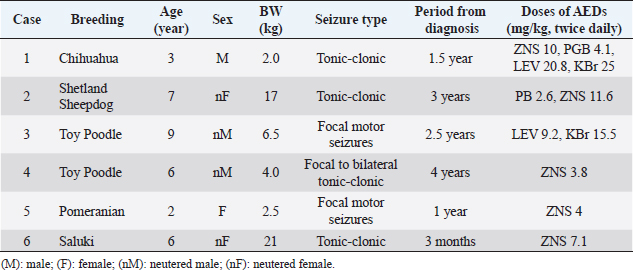
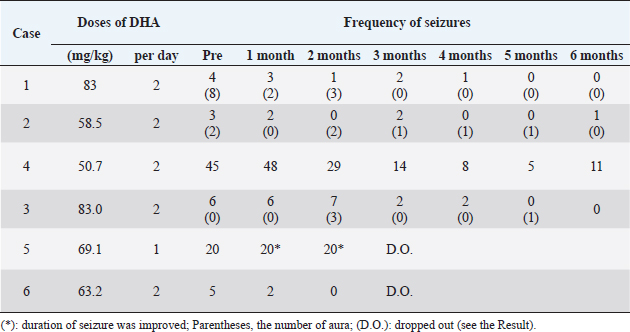
AbstractBackground: The anti-epileptic effects of docosahexaenoic acid (DHA) in dogs and humans remain controversial. The dosage and efficacy of DHA were various in the previous reports. Aim: The effects of high-dose DHA supplementation as add-on therapy for idiopathic epilepsy in dogs were evaluated. Methods: An open-label clinical trial was designed in this pilot study. Six dogs (median age: 6 years) with idiopathic epilepsy were included. All the patients were diagnosed with idiopathic epilepsy using magnetic MRI and cerebrospinal fluid examination (median: 2.0 years before the trial). They had 5–45 seizures and/or auras (median: 9.0) in the month before starting DHA supplementation. DHA was adjunctively administered at doses of 69–166 mg/kg/day without changing other prescriptions. Results: Four of the six patients completed the 6-month observation period. All the patients showed a decrease in seizure frequency of 50% or more within 2–3 months after the start of the administration, and three patients decreased to a frequency of 0–1 per month after 5–6 months. No clear adverse events were observed in the general condition or blood test results in any patients. Conclusion: Although the sample size was small and the study was not a randomized controlled trial, the data suggest that add-on supplementation of DHA could be useful in reducing the frequency of seizures in canine idiopathic epilepsy. Keywords: Docosahexaenoic acid, Dogs, Drug-resistant epilepsy, Idiopathic epilepsy, Omega-3 fatty acids. IntroductionIn dogs, idiopathic epilepsy is a condition of repeated seizures in which there are no organic lesions in the brain and no clear origin of the seizures (Berendt et al., 2015). This form of epilepsy is one of the most common chronic neurological disorders observed in dogs. Although prevalence varies depending on the study, it has been reported to be from 0.62% to 1.84% (Kearsley-Fleet et al., 2013; Heske et al., 2014; Hamamoto et al., 2016). The International Veterinary Epilepsy Task Force published a consensus statement regarding the treatment of idiopathic epilepsy in dogs (Sofie et al., 2015). In many cases, the frequency of seizures can be sufficiently reduced by prescribing antiepileptic drugs (AEDs) according to guidelines. However, in less than 30% of these cases, the frequency and severity of seizures cannot be reduced even with multiple AEDs (Trepanier et al., 1998). Therefore, the development of medications that support the action of AEDs has garnered significant research attention with the goal of improving quality of life. Docosahexaenoic acid (DHA) is an omega-3 fatty acid abundantly found in blue fish and crustaceans. Although DHA intake is known to have a positive effect on brain function (Lauritzen et al., 2016), its association with neural diseases such as epilepsy remains controversial. In a 2018 systematic review, four of nine studies in human patients reported a significant positive association between omega-3 fatty acids and epileptic seizures; however, the authors could not conclude a beneficial effect because of the low statistical power (Pourmasoumi et al., 2018). Another clinical study of 99 patients reported that the administration of DHA at a dose of approximately 33.4–49.1 mg/kg/day reduced the frequency of seizures in patients with refractory epilepsy (Ibrahim et al., 2018). Two papers have been published on the administration of omega-3 fatty acids for idiopathic epilepsy in dogs; however, the AEDs of DHA have not been clearly indicated (Scorza et al., 2009; Matthews et al., 2012). In these studies, dogs were administered 2 g/head/day of whole omega 3 fatty acid and 25 mg/kg/day of DHA, respectively, which were lower doses than those reported in humans, and the ratio of DHA components to fatty acids was ambiguous in one study (Scorza et al., 2009). In addition, the NOAEL of DHA + EPA in healthy dogs has been reported to be sufficiently higher than these doses (Takeda Pharmaceutical Company, 2020), suggesting that DHA can be safely administered at higher doses than those reported in previous studies. Therefore, we conducted an open-label clinical trial to clarify the effects of supplemental treatment with a high dose of DHA in dogs with idiopathic epilepsy. Materials and MethodsInclusion criteriaAnimals that had been in regular follow up at the center were enrolled in the trial between 2019 and 2021 (Table 1). The inclusion criteria were as follows: 1- to 10 -year-old dogs; clinically diagnosed with idiopathic epilepsy; that had been on AEDs at the same dose(s) for at least 2 months; and had seizure and/or aura episodes more than twice per month. The diagnosis of idiopathic epilepsy (with a Tier II confidence level) was concluded in accordance with the guidelines of the International Veterinary Epilepsy Task Force (De Risio et al., 2015) as follows: absence of neurological abnormalities during the interictal period; unremarkable results of cranial MRI examination; and reactive seizures ruled out via medical records, physical examination, and blood examination. As part of the selection process, the trial was discussed with the owners, and consent was obtained. Six dogs were identified as fit and enrolled in the trial (Table 1). ProcedureDHA supplements (DHA70 BAPT, Bizen Chemical, Okayama, Japan) were prescribed at a dose of 50–100 mg/kg to be taken every 12 hours (100–200 mg/kg/day) for 6 months. The DHA capsules were oval in shape, measuring 13 mm long and 7.5 mm in diameter. During the trial period, the owners were advised to continue administering AEDs that had been prescribed prior to the study. Follow-up visits were performed each month to check for the following: seizure episodes, vital signs, physical assessment, and changes in patient behavior/activity. Clinical pathology, including routine complete blood count, alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), and creatinine (CRE) was assessed every 3 months for clinical assessment. Total cholesterol (TCHO) and triglyceride (TG) were obtained from one patient. Routine blood examinations were performed by a veterinarian using a ProCyte Dx hematology analyzer (IDEXX Laboratories, MA, USA) and DRI-CHEM 7000V (FUJIFILM VET Systems, Tokyo, Japan). As humane endpoints, any animal that showed negative reactions to supplementation was withdrawn. The dropout criteria were defined as follows: occurrence of status epilepticus and/or cluster seizures; critically poor general physical condition; loss of more than 5% of total body weight post-administration; the presence of remarkable clinical pathology results; non-compliance with DHA administration; and owner withdrawal. Statistical analysisAll statistical analyses were conducted using XLSTAT Life Science (version 2021.2.2.1141, Addinsoft, Paris, France) as an add-on application in Microsoft Excel (Microsoft Corporation, Redmond, Washington, DC). Friedman and Nemenyi post-hoc tests were used to compare the time periods. No missing data were imputed. The significance level was set at p-value <0.05. Ethical approvalThis open-label prospective canine clinical trial was conducted at the Veterinary Medical Center, University of Tokyo (Tokyo, Japan), following approval from the ethics and welfare committee of the center (#VMC2018-5). ResultsSix dogs were initially enrolled in this study, as shown in Table 1. Four patients had a history of generalized tonic-clonic seizures, while the other two had recurrent focal motor seizures in the forelimbs. All patients were diagnosed with idiopathic epilepsy using MRI and CSF tests following the guidelines under the Tier II confidence level for diagnosing idiopathic epilepsy in dogs. The median age of the animals was 6 years (range: 2–9 years), and the median period of diagnosis was 2.0 years before the start of the trial (range: 3 months to 4 years). Doses and responses to DHA supplementation are shown in Table 2. With a median dose of 121.8 mg/kg/day (range: 69.1–166 mg/kg/day), five patients received oral DHA capsules twice daily, whereas the remaining patient only received the capsule once daily due to a misunderstanding. One patient withdrew from the program due to inconsistent adherence to the trial protocol, and another withdrew due to changes in primary AED medication. Table 1. Patient characteristics and AED doses.
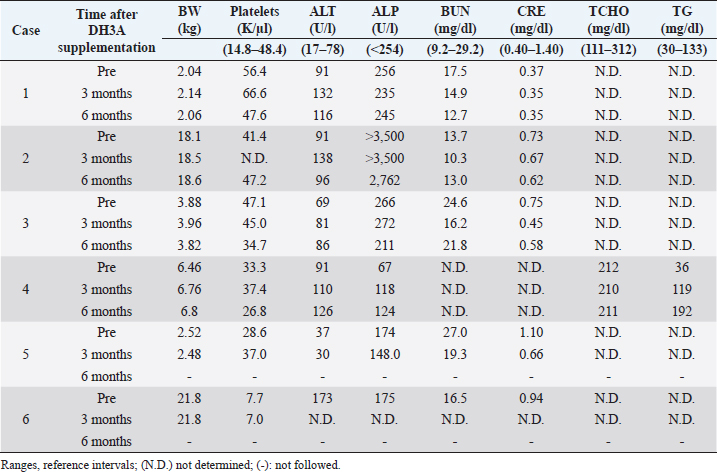
The assessment of seizure activity revealed that all remaining animals receiving DHA supplementation showed improvements in the frequency and severity of seizures and auras (Table 2). They had 5–45 seizures and/or auras (median: 9) within 1 month of starting DHA supplementation. They were treated at doses of 41.5 to 83 mg/kg once or twice daily in total 69.1 to 166 mg/kg/day of DHA (median, 109.2 mg/kg/day). Four of the six dogs completed the 6-month observed period without changes in medication. The seizure frequencies of all patients decreased by 50% or more by 2–3 months after the start of DHA supplementation, and those of three patients decreased to 0–1 per month at 5–6 months. Statistical analyses using the Friedman test ( p=0.00249) showed differences in seizure frequency among the groups at the time of the trial. Further analyses using the Nemenyi post-hoc test ( p=0.0338) revealed that the total frequency of seizures and auras was significantly different between the pre-trial period and at 5 months after the start of supplementation. The results of body weight and blood examination tests are shown in Table 3. Adverse events related to supplementation were not observed in any cases. No problems were identified during physical examinations or follow-up interviews. Two patients had to be withdrawn from the trial. Case 5 was a 2-year-old intact female Pomeranian dog with a history of focal motor seizures. She was administered oral DHA supplementation at an inappropriate dose of 69.1 mg/kg once daily instead of twice due to miscommunication. Owing to the size of the DHA capsule, the owner struggled to administer it. The veterinarian advised the owner to decapsulate it and mix the contents with the feed; however, it appeared that the patient missed the appropriate amount of DHA despite multiple attempts during the observation period. The owners were not able to record the precise number of seizures at the time of the trial but felt that the severity and frequency of seizures decreased. After consulting with a veterinarian, the patient withdrew from the program. Case 6, a 6-year-old neutered female Saluki dog with tonic-clonic seizures, was also excluded from the trial due to an alteration in AED medication. Prior to withdrawal, the seizure activity of the patient had apparently reduced at a dose of 63.2 mg/kg twice daily. DiscussionIn this study, an open-label clinical trial was conducted on six dogs with idiopathic epilepsy. Four of the six patients completed the 6-month observed period without changes in their primary AED regimens. All dogs showed a decrease in seizure frequency of 50% or more at 2–3 months after the start of administration, and three of the four dogs decreased to 0–1 per month at the end of the study period. These data suggest that DHA may be a useful supplement for treating canine idiopathic epilepsy. While statistical changes were observed, the sample size was small, and the power was quite low. A large-scale trial is needed to verify the effectiveness of DHA as a supplement for idiopathic epilepsy in dogs. Table 2. Effects of DHA supplementation on seizure frequency.
Table 3. Pre- and post-supplementation body weights and test results.
There have been few reports on DHA administration in dogs with idiopathic epilepsy showing a clear effect. In 2009, a 2-year-old Great Dane with idiopathic epilepsy was treated with 2 g/head/day of omega-3 fatty acids derived from fish oil (Scorza et al., 2009). The body weight of the dog was not reported. The seizure frequency decreased from 2 to <1 within 2–3 months. Although this result suggests that omega-3 fatty acids could be effective for idiopathic epilepsy in dogs, the dosage was unclear, and the proportion of DHA in the omega-3 fatty acids was not indicated. In another report by Matthews et al. (2012) 40 mg/kg EPA, 25 mg/kg DHA, and 2.2 mg/kg vitamin E were administered to 15 dogs with idiopathic epilepsy treated with AEDs for 12 weeks. While no adverse effects were observed, there was no clear decrease in seizure frequency or severity. In this study, all patients were administered DHA at doses of 69 to 166 mg/kg/day, which was more than two to six times higher than those used in previous studies, including in human reports (Ibrahim et al., 2018; Pourmasoumi et al., 2018). Moreover, the DHA/EPA ratio was higher than that previously reported. The high dose and purity of DHA could be one of the reasons for its high efficacy in this study. There are similarities in the pathogenesis and pathophysiology of canine and human epilepsy (Chandler, 2006), implying that research on canine patients could be translated to treatment in humans. The present data also indicate the scope for reconsidering effective dosages of DHA in human patients with epilepsy. The mechanism by which DHA reduces seizure frequency in epilepsy is not well understood. In reports using the epileptic mice model, DHA is suggested to increase serotonin secretion, promote serotonin receptors in postsynaptic membranes, and increase neurosteroid secretory activity (Patrick and Ames, 2015; Ishihara et al., 2017). In addition, omega-3 fatty acids, such as DHA, are abundant in nerve cells in the cerebral cortex and are known to increase the fluidity of cell membranes and cell plasticity (Lauritzen et al., 2016). DHA also reduces the inward calcium currents and prolongs the inactivated state of cells after action potentials (Xiao et al., 1997). Identifying the mechanism of action of DHA was beyond the scope of this study. However, our findings provide evidence regarding its positive effect and are expected to support future research analyzing the physiological effects of DHA in epilepsy. The efficacy of DHA tended to be observed approximately 1–3 months after the start of treatment, and statistically significant differences were observed 5 months after the start of treatment. This is consistent with the results of a previous study in patients with epilepsy (Ibrahim et al., 2018). In the body, DHA function is dependent on dietary supply (Gao et al., 2009). DHA is first trapped in low-density lipoprotein (LDL) pools, and the free form of DHA is systemically released after the LDL pool is saturated (Taha et al., 2010). It has been reported that the concentration of DHA in the CSF increases after consistent dietary intake (Levi et al., 2014). These distribution systems may take a long time to exert the antiepileptic effects of DHA. There are several known adverse effects of DHA in humans. The DHA/EPA preparation omega-3 fatty acid ethyl (Lotriga, Takeda Pharmaceutical Company, Tokyo, Japan) was approved in 2012 for hyperlipidemia treatment. According to the interview form, 948 adults were treated with 2 or 4 g of triga. Of these, 91 (9.6%) had adverse effects, mainly diarrhea (2.5%) (Takeda Pharmaceutical Company, 2020). As other serious side effects, the elevation of AST, ALT, AL-P, γ-GTP, LDH, bilirubin, etc. has been suggested (Takeda Pharmaceutical Company, 2020). In a clinical trial of DHA administration in pediatric epilepsy reported by Ibrahim et al. (2018) loss of appetite, fever, and rash were observed by treatment of not only EPA or DHA but also placebo. In addition, DHA and EPA are known to have anticoagulant effects (Swanson et al., 2012), suggesting that they should be used with caution in patients with diseases associated with a high risk of bleeding and in those taking anticoagulants. A repeated-dose toxicity study using healthy adult dogs was conducted using Lotriga’s interview form. According to one report, the NOAEL was >1,000 mg/kg/day for over 52 weeks (Takeda Pharmaceutical Company, 2020). As the DHA content of the drug is approximately 36.7% (Takeda Pharmaceutical Company, 2020), >367 mg/kg/day of DHA is estimated to be the NOAEL in dogs. Mild suppression of body weight gain was observed without changes in food consumption or coat contamination (Takeda Pharmaceutical Company, 2020). In another study, the breath smell worsened (Scorza et al., 2009). However, no side effects suggestive of severe abnormalities were observed in any of the dog. In this study, no adverse effects were observed during the observation period. Nevertheless, based on previous reports, it is better to continue paying close attention to gastrointestinal symptoms, liver disorders, coagulation systems, body weight, and coating conditions. This study had several limitations. This was a pilot study, and the reproducibility of the results is not guaranteed, although the findings were found to be statistically significant. Because the test was designed as an open-label trial rather than a blind test, it is subject to owner and observer biases. A control group was not included because of ethical considerations. The other prescriptions, except for DHA, did not change from 2 months before the start of the trial to the end; however, this does not completely account for the possibility that seizure symptoms were improved by drugs other than DHA during the observation period. ConclusionThis pilot study investigated the potential of high-dose DHA preparation as an ancillary treatment for canine idiopathic epilepsy. After several months of supplementation, the frequency of seizures and auras decreased without any apparent adverse effects. A large-scale study will likely prove its effectiveness as a supplement in idiopathic epilepsy. AcknowledgmentsDHA70 BAPT was a gift from Bizen Chemicals. We are grateful to Dr. Yoshihisa Misawa for coordinating the supply of formulations. Conflict of interestThe authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. FundingThe authors received no financial support for the research. Availability of dataThe datasets analyzed during the current study are available from the corresponding author upon reasonable request. Author contributionsConceptualization, TY; Methodology, TY, CNBBM, YM; Validation, CNBBM, YM; Statistical Analysis, TY, TM; Resources, SM, TM, YM; Data Curation, TY; Writing – Original Draft Preparation, CNBBM; Review and Editing, TY; Supervision, TY; Project Administration, TY. ReferencesBerendt, M., Farqhuar, R.G., Mandigers, P.J.J., Pakozdy, A., Bhatti, S.F.M., De Risio, L., Fischer, A., Long, S., Matiasek, K., Muñana, K., Patterson, E.E., Penderis, J., Platt, S., Podell, M., Potschka, H., Pumarola, M.B., Rusbridge, C., Stein, V.M., Tipold, A. and Volk, H.A. 2015. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC. Vet. Res. 11, 182–192. Chandler, K. 2006. Canine epilepsy: what can we learn from human seizure disorders? Vet. J. 172(2), 207–217. De Risio, L., Bhatti, S., Muñana, K., Penderis, J., Stein, V., Tipold, A., Berendt, M., Farqhuar, R., Fischer, A., Long, S., Mandigers, P.J., Matiasek, K., Packer, R.M., Pakozdy, A., Patterson, N., Platt, S., Podell, M., Potschka, H., Batlle, M.P., Rusbridge, C. and Volk, H.A. 2015. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC. Vet. Res. 11, 148. Gao, F., Kiesewetter, D., Chang, L., Ma, K., Bell, J.M., Rapoport, S.I. and Igarashi, M. 2009. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified alpha-linolenic acid in unanesthetized rats. J. Lipid. Res. 50(4), 749–758. Hamamoto, Y., Hasegawa, D., Mizoguchi, S., Yu, Y., Wada, M., Kuwabara, T., Fujiwara-Igarashi, A. and Fujita, M. 2016. Retrospective epidemiological study of canine epilepsy in Japan using the International veterinary epilepsy task force classification 2015 (2003-2013): etiological distribution, risk factors, survival time, and lifespan. BMC. Vet. Res. 12(1), 248. Heske, L., Nødtvedt, A., Jäderlund, K.H., Berendt, M. and Egenvall, A. 2014. A cohort study of epilepsy among 665,000 insured dogs: incidence, mortality and survival after diagnosis. Vet. J. 202(3), 471–476. Ibrahim, F.A.S., Ghebremeskel, K., Abdel-Rahman, M.E., Ahmed, A.A.M., Mohmed, I.M., Osman, G., Elseed, M., Hamed, A., Rabinowicz, A.L., Salih, M.A.M., Elbashir, M.I. and Daak, A.A. 2018. The differential effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on seizure frequency in patients with drug-resistant epilepsy—a randomized, double-blind, placebo-controlled trial. Epilepsy. Behav. 87, 32–38. Ishihara, Y., Itoh, K., Tanaka, M., Tsuji, M., Kawamoto, T., Kawato, S., Vogel, C.F.A. and Yamazaki, T. 2017. Potentiation of 17beta-estradiol synthesis in the brain and elongation of seizure latency through dietary supplementation with docosahexaenoic acid. Sci. Rep. 7(1), 6268. Kearsley-Fleet, L., O'Neill, D.G., Volk, H.A., Church, D.B. and Brodbelt, D.C. 2013. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec. 172(13), 338. Lauritzen, L., Brambilla, P., Mazzocchi, A., Harsløf, L.B.S., Ciappolino, V. and Agostoni, C. 2016. DHA effects in brain development and function. Nutrients 8(1), 6. Levi, Y.F., Vedin, I., Cederholm, T., Basun, H., Irving, G.F., Eriksdotter, M., Hjorth, E., Schultzberg, M., Vessby, B., Wahlund, L.O., Salem, Jr. N. and Palmbladr, J. 2014. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. J. Intern. Med. 275(4), 428–436. Matthews, H., Granger, N., Wood, J. and Skelly, B. 2012. Effects of essential fatty acid supplementation in dogs with idiopathic epilepsy: a clinical trial. Vet. J. 191(3), 396–398. Patrick, R.P. and Ames, B.N. 2015. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB. J. 29(6), 2207–2222. Pourmasoumi, M., Vosoughi, N., Derakhshandeh-Rishehri, S.M., Assarroudi, M. and Heidari-Beni, M. 2018. Association of omega-3 fatty acid and epileptic seizure in epileptic patients: a systematic review. Int. J. Prev. Med. 9, 36. Scorza, F.A., Cavalheiro, E.A., Arida, R.M., Terra, V.C., Scorza, C.A., Ribeiro, M.O. and Cysneiros, R.M. 2009. Positive impact of omega-3 fatty acid supplementation in a dog with drug-resistant epilepsy: a case study. Epilepsy. Behav. 15(4), 527–528. Sofie, F.M., De Risio, B.L., Muñana, K., Penderis, J., Stein, V.M., Tipold, A., Berendt, M., Farquhar, R.G., Fischer, A., Long, S., Löscher, W., Mandigers, P.J.J., Matiasek, K., Pakozdy, A., Patterson, E.E., Platt, S., Podell, M., Potschka, H., Rusbridge, C. and Volk, H.A. 2015. International veterinary epilepsy task force consensus proposal: medical treatment of canine epilepsy in Europe. BMC. Vet. Res. 11, 176. Swanson, D., Block, R. and Shaker, A. 2012. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 3(1), 1–7. Taha, A.Y., Burnham, W.M. and Auvin, S. 2010. Polyunsaturated fatty acids and epilepsy. Epilepsia 51(8), 1348–1358. Takeda Pharmaceutical Company. 2020. EPA/DHA pharmaceutical formulation; Lotriga granular capsule 2 g. Pharmaceutical interview form. Available via https://www.takedamed.com/mcm/medicine/download.jsp?id=152&type=INTERVIEW_FORM Trepanier, L.A., Schoick, A.V., Schwark, W.S. and Carrillo, J. 1998. Therapeutic serum drug concentrations in epileptic dogs treated with potassium bromide alone or in combination with other anticonvulsants: 122 cases (1992–1996). J. Am. Vet. Med. Assoc. 213(10), 1449–1453. Xiao, Y.F., Gomez, A.M., Morgan, J.P., Lederer, W.J. and Leaf, A. 1997. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U.S.A. 94(8), 4182–4187. | ||
| How to Cite this Article |
| Pubmed Style Yonezawa T, Marasigan CNBB, Matsumiya Y, Maeda S, Motegi T, Momoi Y. Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Vet J. 2023; 13(7): 942-947. doi:10.5455/OVJ.2023.v13.i7.14 Web Style Yonezawa T, Marasigan CNBB, Matsumiya Y, Maeda S, Motegi T, Momoi Y. Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. https://www.openveterinaryjournal.com/?mno=147025 [Access: July 11, 2025]. doi:10.5455/OVJ.2023.v13.i7.14 AMA (American Medical Association) Style Yonezawa T, Marasigan CNBB, Matsumiya Y, Maeda S, Motegi T, Momoi Y. Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Vet J. 2023; 13(7): 942-947. doi:10.5455/OVJ.2023.v13.i7.14 Vancouver/ICMJE Style Yonezawa T, Marasigan CNBB, Matsumiya Y, Maeda S, Motegi T, Momoi Y. Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Vet J. (2023), [cited July 11, 2025]; 13(7): 942-947. doi:10.5455/OVJ.2023.v13.i7.14 Harvard Style Yonezawa, T., Marasigan, . C. N. B. B., Matsumiya, . Y., Maeda, . S., Motegi, . T. & Momoi, . Y. (2023) Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Vet J, 13 (7), 942-947. doi:10.5455/OVJ.2023.v13.i7.14 Turabian Style Yonezawa, Tomohiro, Cris Niño Bon B. Marasigan, Yuki Matsumiya, Shingo Maeda, Tomoki Motegi, and Yasuyuki Momoi. 2023. Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Veterinary Journal, 13 (7), 942-947. doi:10.5455/OVJ.2023.v13.i7.14 Chicago Style Yonezawa, Tomohiro, Cris Niño Bon B. Marasigan, Yuki Matsumiya, Shingo Maeda, Tomoki Motegi, and Yasuyuki Momoi. "Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study." Open Veterinary Journal 13 (2023), 942-947. doi:10.5455/OVJ.2023.v13.i7.14 MLA (The Modern Language Association) Style Yonezawa, Tomohiro, Cris Niño Bon B. Marasigan, Yuki Matsumiya, Shingo Maeda, Tomoki Motegi, and Yasuyuki Momoi. "Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study." Open Veterinary Journal 13.7 (2023), 942-947. Print. doi:10.5455/OVJ.2023.v13.i7.14 APA (American Psychological Association) Style Yonezawa, T., Marasigan, . C. N. B. B., Matsumiya, . Y., Maeda, . S., Motegi, . T. & Momoi, . Y. (2023) Effects of high-dose docosahexaenoic acid supplementation as an add-on therapy for canine idiopathic epilepsy: A pilot study. Open Veterinary Journal, 13 (7), 942-947. doi:10.5455/OVJ.2023.v13.i7.14 |