| Research Article | ||
Open Vet J. 2023; 13(9): 1167-1174 Open Veterinary Journal, (2023), Vol. 13(9): 1167–1174 Original Research Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogsManuela Crasta1†, Kevin Arteaga1*†, Teresa Peña2,3 and Marta Leiva2,31AniCura Visionvet, Eye Clinic, San Giovanni in Persiceto, Bologna, Italy 2Departament de Medicina i Cirurgia Animals, Facultat de Veterinària, Universitat Autònoma de Barcelona, Bellaterra, Spain 3Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain †Both authors contributed equally to this work. *Corresponding Author: Kevin Arteaga. AniCura Visionvet, Eye Clinic, San Giovanni in Persiceto, Bologna, Italy. Email: kevinof91 [at] hotmail.com Submitted: 02/05/2023 Accepted: 25/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
AbstractBackground: Crystalline corneal dystrophy (CCD) is the most common type of corneal lipidic deposition in dogs. CCD is a primary metabolic disorder of the corneal fibroblast featuring an accumulation of extracellular and intracellular lipid deposits. Corneal lipid deposits create a corneal opacity and modify the interfibrillar collagen distance, inducing light scattering. Corneal vascularization is not usually associated with the disease, but, in case of chronicity, cell death may produce inflammation, and new corneal vessels are developed. To the best of the authors’ knowledge, this is the first report of a medical approach for CCD treatment in veterinary medicine. Aim: To evaluate the efficacy of topical 1% cyclosporine eyedrops (1% CsA) for the treatment of CCD in dogs. Methods: Medical records of dogs with CCD were retrospectively reviewed (2009–2020). Corneal opacification description (COD) [size (mm), depth, and opacification degree (0–3)] was evaluated at 0, 3, 6, 9, 12, and 15 months postinitial diagnosis. Dogs were classified into three groups: the control group (G0), the group receiving topical 1% CsA once per day (G1), and the group receiving topical 1% CsA twice daily (G2). Results: Ninety-two client-owned dogs (163 eyes) of different breeds, ages, and gender fulfilled the inclusion criteria. When compared to G0, where the eyes significantly increased COD (p < 0.001), G1 and G2 significantly decreased COD (p < 0.001). In fact, the probability of reducing COD was about three times higher in G2 than in G1, being nearly the same for the right [odds ratio (OR)=2.94; 95% confidence interval (95% CI)=0.55–15.78] and left eye (OR=2.92; 95% CI=0.49–17.26). In addition, for each additional month of treatment in G2, the probability of reducing COD increased significantly (OR=1.12; 95%CI=1.00–1.26 for the right eye and OR=1.16; 95%CI=1.02–1.32 for the left eye). Conclusion: Long-term treatment with topical 1% CsA eyedrops significantly improved CCD in dogs, being the probability of reducing COD higher when applying the treatment twice daily. Keywords: Cornea, Cyclosporine, Deposits, Dog, Dystrophy. IntroductionCrystalline corneal dystrophy (CCD) is the most common type of corneal lipidic deposition in dogs (Barsotti et al., 2008). CCD is a primary metabolic disorder of the corneal fibroblast featuring an accumulation of extracellular and intracellular lipid deposits (Barsotti et al., 2008). Some purebreds (e.g., Beagle, Siberian Husky, and Cavalier King Charles Spaniel) are prone to suffer from CCD, which tends to affect young dogs and both eyes more frequently (Spangler et al., 1982; Cooley and Dice, 1990; Crispin, 2002). CCD is characterized by metallic and refracting opacities in the central or paracentral cornea, with different shapes (i.e., oval, round, discoid, or ringlike lesion) (Whitley and Ralph, 2021). Corneal lipid deposits create a corneal opacity and modify the interfibrillar collagen distance, inducing light scattering. Corneal vascularization is not usually associated with the disease, but, in case of chronicity, cell death may produce inflammation, and new corneal vessels are developed (Crispin, 2002). Schnyder’s crystalline stromal dystrophy is a very similar condition described in humans. Histochemical and ultrastructural studies in both species have pointed to the presence of cholesterol, cholesterol ester, and phospholipids in the affected cornea as responsible for the structural disorganization of corneal collagen fibers. However, a definitive relationship between dyslipoproteinemia has not been found in dogs’ or humans’ disease (Winder, 1994; Crispin, 2002). In dogs with CCD, subtle defects in vision are difficult to assess and confirm. Nonetheless, scientific reports in humans have asserted that photopic vision impairment is caused by the glare from corneal crystalline deposits, with some patients requiring lamellar or penetrating keratoplasty (Jayne, 2009). Lamellar keratectomy in dogs with CCD is recommended only as a last-resort option if the opacity significantly obstructs vision since the corneal deposit is likely to recur with time. To date, no medical treatment has been shown to decrease or eliminate the opacity in CCD (Whitley and Ralph, 2021). Cyclosporine A (CsA) is a cyclic peptide commonly used to treat immune-mediated keratoconjunctivitis sicca in dogs due to its lacrimostimulant and lacrimomimetic properties, improving corneal transparency by reducing corneal vascularization and pigmentation. CsA also disrupts immune-mediated processes by preventing T-cell production. Besides its lipophilic nature, CsA enhances epithelium absorption into the corneal and conjunctival areas (Whitley and Ralph, 2021). In addition, CsA has been shown to stimulate mucin production, secreted by conjunctival goblet cells, on the ocular surface (Phillips et al., 2000; Moore et al., 2001). Importantly, the safety of topical CsA, in a solution of 80% pure vitamin E (DL-α-tocopherol acetate, racemic mixture) and 20% medium-chain fatty acids, has been established previously in dogs and cats (Arteaga et al., 2021). The purpose of the present study was to evaluate the efficacy of topical 1% cyclosporine eyedrops (1% CsA) for the treatment of CCD in dogs. Materials and MethodsMedical records of dogs diagnosed with CCD at two referral veterinary ophthalmology centers, Eye Clinic Anicura Visionvet (San Giovanni in Persiceto, Italy) and Veterinary Teaching Hospital of the Autonomous University of Barcelona (Bellaterra, Spain), from 2009 to 2020, were retrospectively reviewed. Full approval from the hospitals to use the medical record information was obtained. Only dogs diagnosed with CCD after a complete ophthalmic examination, with Schirmer tear test 1 (STT-1) values >15 mm/minute, no sign of ocular discomfort, a minimum follow-up of 3 months, and with digital photographic records, were included in the study. Exclusion criteria included concomitant systemic metabolic diseases (e.g., hyperadrenocorticism, diabetes mellitus, or hypothyroidism), hypertriglyceridemia, hypercholesterolemia, disruption of topical treatment before 3 months, and concomitant ocular surface diseases other than those related to breed’s anatomical conformation (e.g., brachycephalic dogs). Recorded medical data included date of presentation, breed, age, gender, the affected eye, main complaint, topical treatment, complications, and follow-up time. In addition, spectral domain-optical coherence tomography (SD-OCT) (Optovue, Inc; Freemont, CA) of the corneal dystrophic area was recorded, when available. Dogs were classified into three groups: G0, the control group with no treatment, G1 receiving topical 1% CsA eyedrops once daily, and G2 receiving topical 1% CsA twice daily. In all the cases, 1% CsA eyedrops were compounded in sterile conditions as a solution in 80% pure vitamin E (DL-α-tocopherol acetate, racemic mixture) and 20% medium-chain fatty acids (from coconut oil). In all the dogs, a comparative assessment of corneal opacification description (COD) by double-masked evaluation of the digital photographic records (Nikon D3500® and AF-S Micro Nikkor 60 mmf/2.8 G ED macro lens) was performed at the first visit and follow-ups. For all dogs, grade 2 was considered as the baseline grading detected at the first visit. At follow-up evaluations, grades 0, 1, and 3 were considered depending if the corneal deposit was no longer detectable, diminished, or increased, respectively (Fig. 1). For those dogs in which no changes in terms of opacification were observed, grade 2 was assigned. Statistical analysisThe statistical analysis was carried out using STATA Statistical Software (Release 14.2. College Station, TX: StataCorp LP) with no adjustment for multiplicity and a statistical significance set at <0.05. Categorical variables were described as absolute frequencies and percentages, continuous variables as mean and standard deviation (SD), when normally distributed, and media and ranges, when non-normally. Categorical variables were compared using Pearson chi-square and continuous variables using the Kruskal–Wallis test. Logistic regression analyses to estimate association [odds ratio (OR) and 95% confidence interval (95%CI)] between efficacy (considering dogs as healed/improved vs. stable/worsened, depending on the difference between the COD grade at baseline and at the last follow-up) and treatment group (G0 vs. G1 and G0 vs. G2), adjusting for gender, age, breed, and treatment follow-up. Analyses were run separately for each eye, considering the right eye [oculus dexter (OD)] and left eye [oculus sinister (OS)].
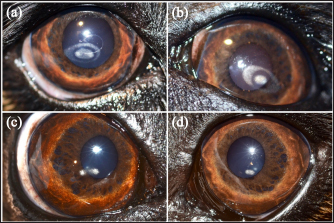
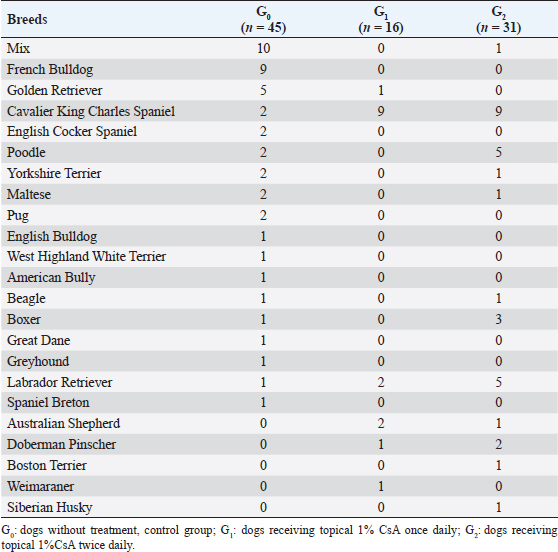
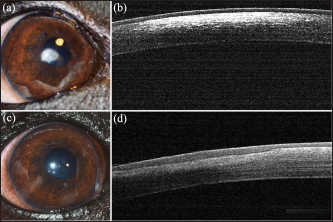
Fig. 1. Cavalier King Charles Spaniel with (a) CCD OD and (b) OS classified as grade 2 at first presentation, and subsequently classified as grade 1 after 12 months of topical 1% CsA treatment (c) OD and (d) OS. Ethical approvalNot needed as this was a retrospective study. ResultsNinety-two dogs (163 eyes) of 23 different breeds fulfilled the inclusion criteria (Table 1). Brachycephalic breeds were overrepresented (38/92; 42%). There were 50 females and 42 males of a median age of 6 years at presentation (range: 1–16 years) (Table 2). Seventy-one dogs (71/92; 77%) were bilaterally affected (142 eyes), and 21 unilaterally (21/92; 23%), being 12 ODs and 9 OSs affected. Table 1. Breed distribution according to their treatment group.
Table 2. Baseline characteristics of dogs according to their treatment group.
Table 3. Topical 1%CsA efficacy in oculus dexter and oculus sinister from first to last visit. The figure represents absolute numbers of eyes (and %) unless otherwise stated.
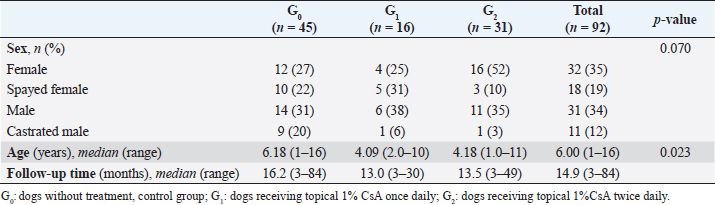
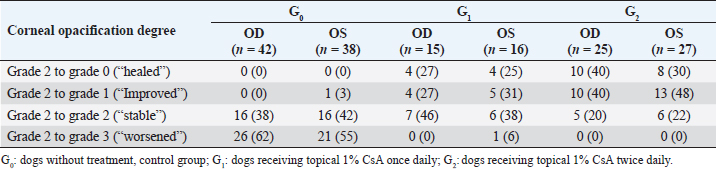
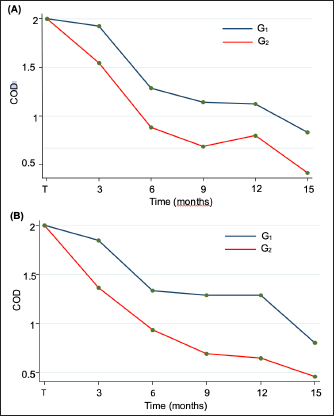
The main complaints reported were the presence of a white spot on the cornea (63/92 dogs; 69%), dull eyes (13/92 dogs; 14%), and increased ocular discharge (11/92 dogs; 12%). When classified among groups, there were 45 dogs (80 eyes) in G0, 16 dogs (31 eyes) in G1, and 31 (52 eyes) in G2 (Table 2). Gender (p=0.070) and bilaterality (p=0.087) were not statistically different among the three groups, age (p=0.023) was not (p=0.023). The median follow-up for the three groups was 14.9 months, ranging from 3–84 months. At the last follow-up, G1 and G2 had a statistically significant decrease in COD (p < 0.001) while G0 had a significant increase (p < 0.001) (Table 3). Only one eye from G0 went from grade 2 to grade 1. No statistically significant difference in COD between OD and OS at the last follow-up was found in any group (G0, p=0.513; G1, p=0.763; G2, p=0.730). Similarly, no significant difference was observed between brachycephalic and nonbrachycephalic breeds in any group (G0, p=0.636; G1, p=0.132; G2, p=0.248). When compared, the probability of reducing COD was about three times higher in G2 than in G1, being nearly the same for OD (OR=2.94; 95% CI=0.55–15.78) and OS (OR=2.92; 95% CI=0.49–17.26) (Fig. 2). Logistic regression analysis indicated that for each additional month of follow-up in G2, and therefore, treatment with 1% CsA eyedrops twice daily, the probability of reducing COD increased significantly (p=0.044 for OD and p=0.019 for OS) (OR=1.12; 95% CI=1.00–1.26 for OD and OR=1.16; 95% CI=1.02–1.32 for OS) (Fig. 3). SD-OCT was performed in 2 and 3 eyes (5/163; 3%) from 1 G1 and 2 G2 dogs, respectively. A hyperreflective area located in the anterior part of the axial stroma was found at the site where deposits were clinically detected in all eyes. Focal thickening of the epithelium was evident after 9 months of treatment with topical 1% CsA twice daily in the region where deposits were previously located (Fig. 4).
Fig. 2. Longitudinal analysis of efficacy in treated groups (G1 and G2) showing a COD reduction as the treatment time increased in (A) OD and (B) OS. COD: Corneal opacification degree; G1: Group of dogs treated with 1%CsA once daily; G2: Group of dogs treated with 1%CsA twice daily. Apart from 2 G2-dogs (3/163 eyes; 2%) which had excessive tears, no other side effects were noticed. The first dog (1 eye) presented with epiphora during the last examination at 6 months. The second one (2 eyes), with epiphora at 9 months of therapy, had no longer corneal deposits, and the tearing resolved after reducing frequency to once daily. DiscussionCCD is a common ophthalmic disease in dogs that, if left untreated, can cause amblyopia, discomfort, corneal erosion, and ulceration (Cooley et al., 1990). This study analyzes the clinical efficacy of topical 1% CsA eyedrops on the treatment of CCD in dogs. To the best of the authors’ knowledge, this is the first report of a medical approach for CCD treatment in veterinary medicine.
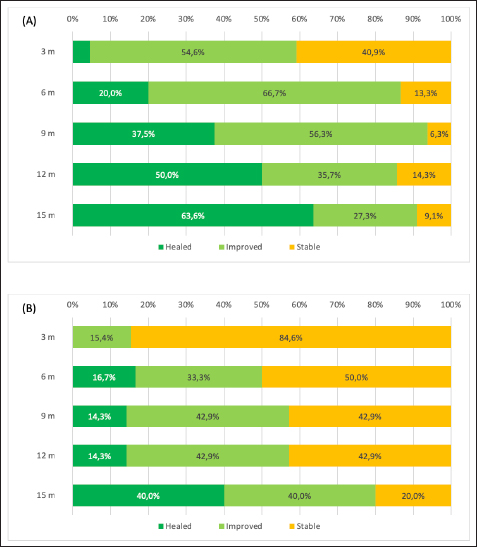
Fig. 3. Longitudinal analysis of G2 showing the percentages of total eyes classified by COD grade during the follow-up (months). Results are presented for (A) the right and (B) the left eye. The three COD grades were: grade 0 (deposit no longer detectable with slit-lamp biomicroscopy, “healed,” shown in dark green), grade 1 (reduction of opacity, “improved,” shown in light green), and grade 2 (baseline, “stable,” shown in yellow). Grade 3 (increased opacity, “worsened”) is not shown since no dogs presented this COD grade in G2. COD: Corneal opacification degree; G2: dogs receiving topical 1% CsA twice daily. Although CCD has been described in many different canine breeds, brachycephalic dogs were overrepresented in the present study, which is not surprising based on their anatomical conformation features such as short noses, shallow orbits, and projected eyeballs, predisposing these individuals to suffer from tear film instability and dry eye syndrome (DES) (Dinesh and Pookode, 2019). In addition, they may have low corneal sensitivity, incomplete and less frequent blinking, and a thin lipid layer tear film, leading to areas of chronic drying, especially the central part of the cornea (Carrington et al., 1989; Packer et al., 2015). Interestingly, DES has been associated with abnormal tear lipid deposition in mice (Chen et al., 2020). In general, CCD does not respond to medical treatment, and topical anti-inflammatory medications may exacerbate the lesion. Low-fat diets are anecdotally suggested to prevent the progression of the disease (Whitley and Ralph, 2021). The corneal lesions can be removed by keratectomy if the opacity is obstructing vision significantly. However, the opacities may recur after keratectomy; therefore, surgery is recommended for corneal dystrophy only as a last-resort measure in dogs with significant visual deficits. Due to the ophthalmic complications that could be derived from a nontreated CCD, finding a topical medication that reduces corneal opacity is mandatory.
Fig. 4. Representative digital photographs and SD-OCT of a Cavalier King Charles Spaniel dog from group G2. (a) Before treatment, a digital photograph of the right eye showed a white and oval refracting opacity in the paracentral cornea and (b) an SD-OCT of the same eye revealed a hyperreflective area located in the anterior part of the axial stroma. (c) After 9 months of treatment with 1% CsA twice daily, no deposits were detected and (d) focal thickening of the epithelium was evident in the region where deposits were previously located. SD-OCT: Spectral Domain-Optical Coherence Tomography. Topical CsA has been shown to have a stimulatory effect on both the number of conjunctival goblet cells and the amount of mucin production (Phillips et al., 2000; Moore et al., 2001). Increasing mucin production would likely increase the time of permanence of the lacrimal film, improving the tear film stability and thus protecting the central part of the cornea. Although the present study shows that the use of topical 1%CsA eyedrops decreases the deposition of lipids in the central cornea, the mechanism of action is not fully understood. Increasing mucin production may extend the durability of the lacrimal tear film and, hence, avoid the deposition of lipids in the central cornea. Unfortunately, break-up times were not evaluated in this study, thus, this theory could not be demonstrated yet; however, we are currently investigating the possible correlation between CCD and the qualitative alteration of the precorneal tear film interferometry. In CCD, crystal deposits tend to be distributed in the central and coolest part of the cornea, and a temperature-dependent enzyme defect has been hypothesized to be the cause (Fielder et al., 1981). It has been demonstrated that local temperature changes can affect the melting point and thermal transition phases of lipids (Crispin, 2002; Tkáčová et al., 2011). Previous studies have evaluated the temperature of the cornea in patients with DES and have revealed that the temperature of the central cornea was significantly lower (Mori et al., 1997; Craig et al., 2000; Kamao et al., 2011; Versura et al., 2015). However, except for those kept in warmed conditions, all types of tear substitutes decrease the corneal temperature right after their application, but the temperature will increase with eyelid closure during normal blinking (Fujishima et al., 1996; Fujishima et al., 1997). Further investigation is required to assess whether topical CsA use modifies corneal temperature in dogs with CCD and its association with lipid deposition. In the present study, COD reduction seems to be faster and more marked with two daily medications than with one, indicating a frequency-dependent response to topical 1%CsA. If CsA is used for this purpose, monitoring STT values is strongly recommended, as overproduction of tears could be seen. Topical CsA has been used in human ophthalmology for more than 40 years and evidence supports its safety and efficacy in treating various ocular surface disorders (McCarthy et al., 1992; Reinhard and Sundmache, 1999; Sundmacher et al., 2001; Bonfioli et al., 2005; Spadavecchia et al., 2006; Duszak 2007). Ophthalmic emulsions of CsA are prepared in several different oil-based vehicles with concentrations ranging from 0.05% to 2% (Acheampong et al., 1998). In previous studies, our research group has shown that a combination of CsA and vitamin E was a safe and effective treatment for keratoconjunctivitis sicca and chronic superficial keratitis in dogs (Arteaga et al., 2021). Ophthalmic α-tocopherol (TOC) is the most effective antioxidant of the vitamin E family and is receiving special attention to prevent and treat retinopathies, cataracts, and DES, among other ocular pathologies (Xin et al., 2016). In our study, 100% TOC was used as a new solvent of CsA. TOC has been reported to substantially reduce the concentration of free radicals in the tear film and to improve the protection of corneal and conjunctival cells, thus, reducing the risk of inflammation and restoring the ocular surface from damage exposure (Aydemir et al., 2004; Zapata et al., 2008; Ribeiro et al., 2013; Olchawa et al., 2015; Tredici et al., 2020). Furthermore, TOC decreases the rate of corneal autolysis and keratocyte apoptosis (Bilgihan et al., 2001). Therefore, as the stromal cell cycle is reduced, there is less deposition of material rich in cholesterol and phospholipids. Even if the use of topical CsA has been associated with some adverse effects in dogs, in our study, only two dogs showed excessive tear production that was reversed after reducing the treatment frequency from twice to once daily (Gregory et al., 1989; Kaswan et al., 1989; Radziejewski and Balicki, 2016). The limitations of this study are mostly due to its design. Dogs and treatment were not randomized and the ophthalmologist performing the examination and follow-up evaluation was not masked. Another group only treated with ophthalmic TOC could have increased the power of the study. In the author’s experience, most patients with CCD also have a lipid tear film deficiency when evaluated by ocular surface interferometry (unpublished results), therefore, only TOC is unlikely to reduce CCD. Cristal corneal deposits were not classified by size thus, we could not objectively evaluate whether the dimension of the deposit could affect its dissolution. Our clinical observation indicated that smaller deposits were more likely to disappear than larger ones. In addition, the age of the participants was not evenly distributed among groups, and this could have added some bias since the temperature of the central cornea has been shown to decrease, while corneal opacities increase with age (Versura et al., 2015; Evans et al., 2018). Since the exact mechanism of action behind our findings remains unknown, more extensive research on the subject is needed that could use the present study as a guide in designing and implementing larger-scale efficacy studies for CCD in dogs. ConclusionTopical treatment with 1%CsA eyedrops is beneficial in reducing CCD in dogs, significantly improving COD in the majority of cases. The probability of reducing COD with 1%CsA applied twice daily seems to be higher than once daily. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThere was no funding source for this study. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. Author contributionsKevin Arteaga, Manuela Crasta, M. Teresa Peña, and Marta Leiva did the diagnosis, provided key information, and analyzed the data. Kevin Arteaga and Manuela Crasta designed the manuscript, wrote the original draft, and revised it critically for important intellectual content. All the authors had direct patient contact, revised, edited the manuscript, and gave their final approval for the version to be published. ReferencesAcheampong, A., Shackleton, M., Lam, S., Rudewicz, P. and Tang-Liu, D. 1998. Cyclosporine distribution into the and systemic blood following topical dosing of cyclosporine to rabbit, dog, and human eyes. In Lacrimal gland, tear film, and dry eye syndromes 2. Advances in experimental medicine and biology. Eds., Sullivan, D.A., Dartt, D.A. and Meneray, M.A. Boston, MA: Springer, vol. 2, pp: 1001–1004. Arteaga, K., Arnone, E. and Crasta, M. 2021. Vitamin E as a safe and effective vehicle for 1% cyclosporine eye drops to treat chronic non-ulcerative keratopathies in dogs and cats. Int. J. Vet. Sci. 11(2), 236–242. Aydemir, O., Celebi, S. and Yilmaz, T. 2004. Protective effects of vitamin E forms (alpha-tocopherol, gamma-tocopherol and d-alpha-tocopherol polyethylene glycol 1,000 succinate) on retinal edema during ischemia-reperfusion injury in the guinea pig retina. Int. Ophthalmol. 25(5–6), 283–289. Barsotti, G., Pasquini, A., Busillo, L., Senese, M., Cardini, G. and Guidi, G. 2008. Corneal crystalline stromal dystrophy and lipidic metabolism in the dog. Vet. Res. Commun. 32(Suppl. 1), S227–S229. Bilgihan, K., Adiguzel, U., Sezer, C., Akyol, G. and Hasanreisoglu, B. 2001. Effects of topical vitamin E on keratocyte apoptosis after traditional photorefractive keratectomy. Ophthalmologica 215(3), 192–196. Bonfioli, A.A., Damico, F.M., Curi, A.L. and Orefice, F. 2005. Intermediate uveitis. Semin. Ophthalmol. 20, 147–154. Carrington, S.D., Bedford, P.G., Guillon, J.P. and Woodward, E.G. 1989. Biomicroscopy of the tear film: the tear film of the pekingese dog. Vet. Rec. 124(13), 323–328. Chen, Q., Ji, C., Zheng, R., Yang, L., Ren, J., Li, Y., Han, Y., Zhou, P., Liu, Z. and Qiu, Y. 2020. N-Palmitoylethanolamine maintains local lipid homeostasis to relieve sleep deprivation-induced dry eye syndrome. Front. Pharmacol. 10, 1622. Cooley, P.L. and Dice, P.F. 1990. Corneal dystrophy in the dog and cat. Vet. Clin. North Am. Small Anim. Pract. 20(3), 681–692. Craig, J.P., Singh, I., Tomlinson, A., Morgan, P.B. and Efron, N. 2000. The role of tear physiology in ocular surface temperature. Eye (Lond) 14(4), 635–641. Crispin, S. 2002. Ocular lipid deposition and hyperlipoproteinaemia. Prog. Retin. Eye Res. 21(2), 169–224. Dinesh, P.T. and Pookode, W. 2019. II-Dry eye disease and pigmentary keratitis in brachycephalic dogs-a brief review. JIVA 17(2), 18–22. Duszak, R.S. 2007. Diagnosis and management of Thygeson’s superficial punctate keratitis. Optometry 78, 333–338. Evans, C.J, Dudakova, L., Skalicka, P., Mahelkova, G., Horinek, A., Hardcastle, A.J., Tuft, S.J. and Liskova, P. 2018. Schnyder corneal dystrophy and associated phenotypes caused by novel and recurrent mutations in the UBIAD1 gene. BMC Ophthalmol. 18(1), 1–7. Fielder, A.R., Winder, A.F., Cooke, E.D. and Bowcock, S.A. 1981. Arcus senilis and corneal temperature in man. In Proceedings of the European congress of ophthalmology. Ed., Trevor-Roper, P., London, UK: Royal Society of Medicine, pp: 1015–1020. Fujishima, H., Toda, I., Yamada, M., Sato, N. and Tsubota, K. 1996. Corneal temperature in patients with dry eye evaluated by infrared radiation thermometry. Br. J. Ophthalmol. 80(1), 29–32. Fujishima, H., Yagi, Y., Shimazaki, J. and Tsubota, K. 1997. Effects of artificial tear temperature on corneal sensation and subjective comfort. Cornea 16(6), 630–634. Gregory, C.R., Hietala, S.K., Pedersen, N.C., Gregory, T.A., Floyd-Hawkins, K.A. and Patz, J.D. 1989. Cyclosporine pharmacokinetics in cats following topical ocular administration. Transplantation 47(3), 516–519. Jayne, S.W. 2009. Schnyder corneal dystrophy. Curr. Opin. Ophthalmol. 20(4), 292–298. Kamao, T., Yamaguchi, M., Kaeasaki, S., Mizoue, S., Shiraishi, A. and Ohashi, Y. 2011. Screening for dry eye with newly developed ocular surface thermographer. Am. J. Ophthalmol. 151, 782–791. Kaswan, R.L., Salisbury, M. and Ward, D.A. 1989. Spontaneous canine keratoconjunctivitis sicca. Arch. Ophtalmol. 107, 1210–1216. McCarthy, J.M., Dubord, P.J., Chalmers, A., Kassen, B.O. and Rangno, K.K. 1992. Cyclosporine A for the treatment of necrotizing scleritis and corneal melting in patients with rheumatoid arthritis. J. Rheumatol. 19, 1358–1361. Moore, C.P., McHug, J., Thorne, J.G. and Phillips, T.E. 2001. Effect of cyclosporine on conjunctival mucin in a canine keratoconjunctivitis sicca model. Invest. Ophthalmol. Vis. Sci. 42(3), 653–659. Mori, A., Oguchi, Y., Okusawa, Y., Ono, M., Fujishima, H. and Tsubota, K. 1997. Use of high-speed, high-resolution thermography to evaluate tear film layer. Am. J. Ophthalmol. 124, 729–735. Olchawa, M.M., Eenreiter, A.M., Pilat, A.K., Skumatz, C.M.B., Niziolek-Kierecka, M., Burke, J.M. and Sarna, T.J. 2015. Zeaxanthin and α-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells. Free Radic. Biol. Med. 89, 873–882. Packer, R.M.A., Hendricks, A. and Burn, C.C. 2015. Impact of facial conformation on canine health: corneal ulceration. PLoS One 13, 10. Phillips, T.E., McHug, J. and Moore, C.P. 2000 Cyclosporine has a direct effect on differentiation of a mucin-secreting cell line. J. Cell Physiol. 184(3), 400–408. Radziejewski, K. and Balicki, I. 2016. Comparative clinical evaluation of tacrolimus and cyclosporine eye drops for the treatment of canine keratoconjunctivitis sicca. Acta Vet. Hung. 64(3), 313–329. Reinhard, T. and Sundmacher, R. 1999. Topical cyclosporin A in Thygeson’s superficial punctate keratitis. Graefes. Arch. Clin. Exp. Ophthalmol. 237, 109–112. Ribeiro, A., Sandez-Maco, I., Casas, M., Alvarez-Pérez, S., Alvarez-Lorenzo, C. and Concheiro, A. 2013. Poloxamine micellar solubilization of α-tocopherol for topical ocular treatment. Colloids Surf. B Biointerfaces 103, 550–557. Spadavecchia, L., Fanelli, P., Tesse, R., Brunetti, L., Cardinale, F., Bellizzi, M., Rizzo, G., Procoli, U., Bellizzi, G. and Armenio, L. 2006. Efficacy of 1.25% and 1% topical cyclosporine in the treatment of severe vernal keratoconjunctivitis in childhood. Pediatr. Allergy Immunol. 17, 527–532. Spangler, W.L., Waring, G.O. and Morrin, L.A. 1982. Oval lipid corneal opacities in beagles. Vet. Pathol. 19(2), 150–159. Sundmacher, R., Hillenkamp, J. and Reinhard, T. 2001. Prospects for therapy and prevention of adenovirus keratoconjunctivitis. Ophthalmologe 98, 991–1007. Tkáčová, M., Živčák, J. and Foffová, P. 2011. A reference for human eye surface temperature measurements in diagnostic process of ophthalmologic diseasesal. Benign Familial. Measurement. In Proceedings of the 8th International Conference, Smolenice, Slovakia, pp: 406–409. Tredici, C., Fasciani, R., Villano, A., Gambini, G. and Caporossi, A. 2020. Efficacy of eye drops containing crosslinked hyaluronic acid and CoQ10 in restoring ocular health exposed to chlorinated water. Eur. J. Ophthalmol. 30(3), 430–438. Versura, P., Giannaccare, G., Fresina, M. and Campos, E.C. 2015. Subjective discomfort symptoms are related to low corneal temperature in patients with evaporative dry eye. Cornea 34(9), 1079–1085. Whitley, D.R. and Ralph, E.H. 2021. Diseases and surgery of the canine cornea and sclera. In Veterinary ophthalmology, 6th edn (ed. Gelatt KN). Ames, IA: Blackwell Publishing, pp: 1082–1172. Winder, A.F. 1994. Disorders of lipid and lipoprotein metabolism. In Pathobiology of ocular disease: a dynamic approach. Eds., Garner, A., Klintworth, G.K., 2nd edition. New York, NY: Marcel Dekker, pp: 1099–1121. Xin, J., Tang, J., Bu, M., Sun, Y., Wang, X., Wu, L. and Liu, H. 2016. A novel eye drop of alpha tocopherol to prevent ocular oxidant damage: improve the stability and ocular efficacy. Drug Dev. Ind. Pharm. 42(4), 525–534. Zapata, G.L., Guajardo, M. and Terrasa, A.M. 2008. The in vitro protective effect of alpha-tocopherol on oxidative injury in the dog. Vet. J. 177(2), 266–272. | ||
| How to Cite this Article |
| Pubmed Style MC, KA, Peña T, Leiva M. Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Vet J. 2023; 13(9): 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 Web Style MC, KA, Peña T, Leiva M. Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. https://www.openveterinaryjournal.com/?mno=146904 [Access: May 17, 2024]. doi:10.5455/OVJ.2023.v13.i9.12 AMA (American Medical Association) Style MC, KA, Peña T, Leiva M. Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Vet J. 2023; 13(9): 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 Vancouver/ICMJE Style MC, KA, Peña T, Leiva M. Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Vet J. (2023), [cited May 17, 2024]; 13(9): 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 Harvard Style , M. C., , . K. A., Peña, . T. & Leiva, . M. (2023) Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Vet J, 13 (9), 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 Turabian Style , Manuela Crasta, Kevin Arteaga, Teresa Peña, and Marta Leiva. 2023. Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Veterinary Journal, 13 (9), 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 Chicago Style , Manuela Crasta, Kevin Arteaga, Teresa Peña, and Marta Leiva. "Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs." Open Veterinary Journal 13 (2023), 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 MLA (The Modern Language Association) Style , Manuela Crasta, Kevin Arteaga, Teresa Peña, and Marta Leiva. "Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs." Open Veterinary Journal 13.9 (2023), 1167-1174. Print. doi:10.5455/OVJ.2023.v13.i9.12 APA (American Psychological Association) Style , M. C., , . K. A., Peña, . T. & Leiva, . M. (2023) Topical 1% cyclosporine eyedrops for the treatment of crystalline corneal dystrophy in dogs. Open Veterinary Journal, 13 (9), 1167-1174. doi:10.5455/OVJ.2023.v13.i9.12 |