| Research Article | ||
Open Vet J. 2023; 13(8): 965-976 Open Veterinary Journal, (2023), Vol. 13(8): 965-976 Original Research Echocardiographic values in healthy Pugs: Effect of body weight, age, and sexGiovanni Romito1*, Prisca Castagna2, Maria Chiara Sabetti3, Michela Ablondi3 and Mario Cipone11Department of Veterinary Medical Sciences, Alma Mater Studiorum—University of Bologna, Ozzano dell’Emilia, Italy 2Clinica Veterinaria San Emilio, Ozzano dell’Emilia, Italy 3Department of Veterinary Sciences, University of Parma, Parma, Italy *Corresponding Author: Giovanni Romito. Department of Veterinary Medical Sciences, Alma Mater Studiorum—University of Bologna, Ozzano dell’Emilia, Italy. Email: giovanni.romito2 [at] unibo.it Submitted: 15/03/2023 Accepted: 12/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
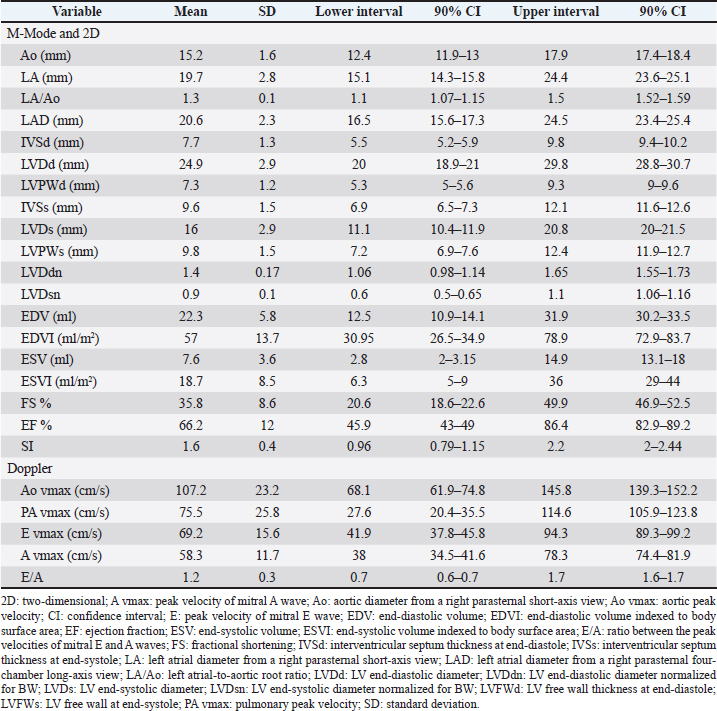
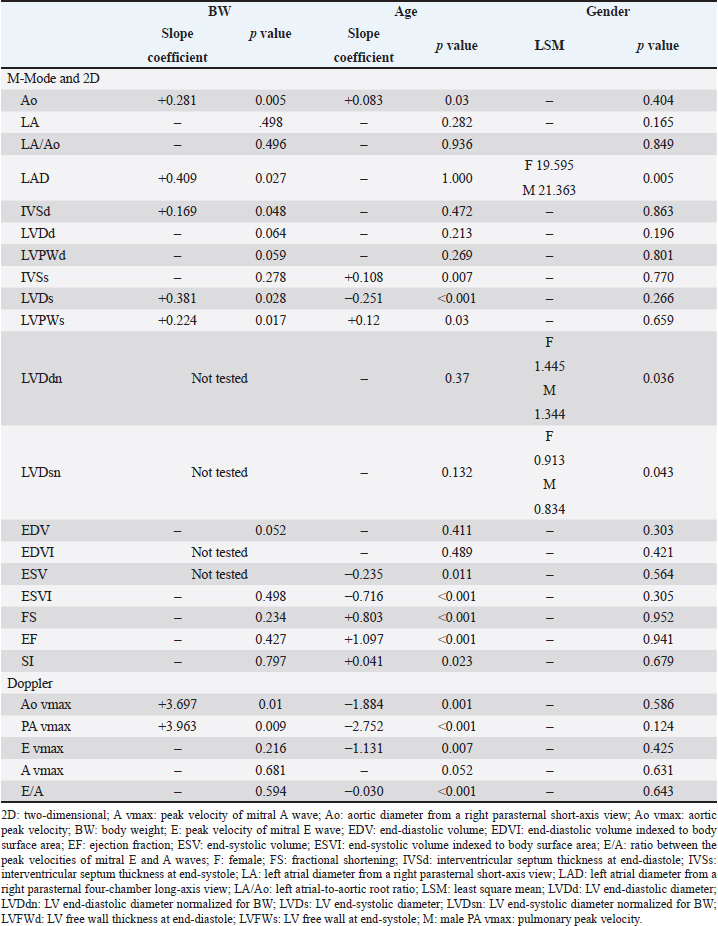
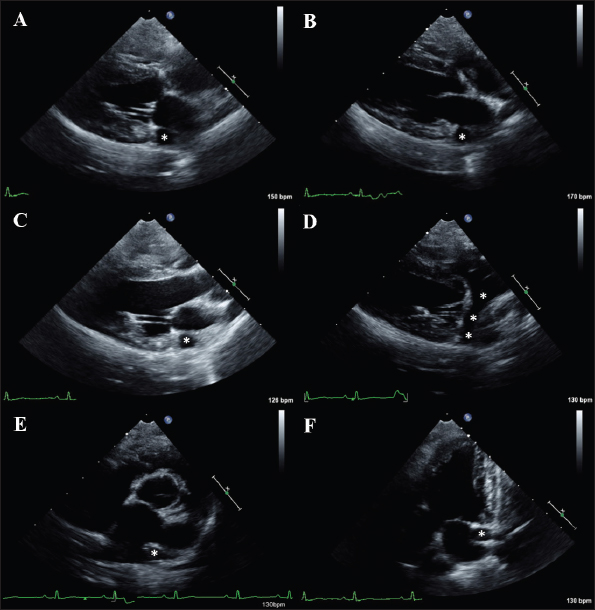
AbstractBackground: Transthoracic echocardiography represents the main noninvasive technique for evaluating cardiac morphology and function in dogs. In dogs with particular somatotypes, such as brachymorphic dogs, breed-specific echocardiographic values are needed for a proper echocardiographic interpretation. Nowadays, the Pug represents one of the most popular brachymorphic canine breeds worldwide. However, data on echocardiographic measurements in this breed are currently limited. Aim: We aimed to determine echocardiographic values in a population of apparently healthy Pugs, and to assess the possible effects of body weight (BW), age, and sex on selected echocardiographic variables, with particular emphasis on those related to the left-sided cardiac chambers. Methods: Apparently healthy Pugs underwent a full physical examination, a 1-minute six-lead electrocardiogram, and a complete transthoracic echocardiography. Twenty-four echocardiographic variables were measured by combining M-mode, two-dimensional and Doppler modalities, and relative values were determined by applying the statistical procedures recommended by the Clinical and Laboratory Standards Institute. Moreover, the effect of selected demographic variables on echocardiographic measurements was tested using a linear mixed model. Results: The investigation included 86 Pugs. Echocardiographic values were provided for each variable and compared with previous veterinary literature. A statistically significant effect of BW, age, and sex was documented for several of the tested variables. Doppler examination demonstrated a trivial pulmonary regurgitation in 24/86 (27.9%) Pugs. Moreover, a persistent left cranial vena cava was suspected in 4/86 (4.7%) dogs. Conclusion: Echocardiographic features of the Pug were addressed and echocardiographic values were made available for clinical use. Because our findings were obtained using a standardized echocardiographic analysis in a population of 86 healthy Pugs, they may act as a reliable guide for an accurate echocardiographic interpretation in this breed. Keywords: Brachymorphic, Persistent left cranial vena cava, Pulmonary regurgitation, Reference interval, Transthoracic echocardiography. IntroductionTransthoracic echocardiography represents the main technique for evaluating cardiac morphology and function because it is non-invasive, widely available, and relatively easy to perform. An essential step for a proper echocardiographic interpretation is the analysis of the recorded measurements and their comparison with reference intervals (RIs). In dogs, the most used echocardiographic RIs derive from heterogeneous populations including subjects of different breeds (O’Grady and Bonagura, 1986; Brown et al., 2003; Cornell et al., 2004; Hall et al., 2008; Esser et al., 2020; Wess et al., 2021). Nevertheless, it should be acknowledged that these ranges represent general cutoffs that may be applicable to many but not all dogs since many variables, including breed as well as somatotype, body weight (BW), age, and sex can result in deviations of cardiac measurements from the normal RIs (Boon et al., 1983; Morrison et al., 1992; Crippa et al., 1992; Bayon et al., 1994; Lonsdale et al., 1998; Della Torre et al., 2000; Cornell et al., 2004; Jacobson et al., 2013; Esser et al., 2020; Patata et al., 2021; Wess et al., 2021). For this reason, studies providing breed-specific echocardiographic RI are needed, especially those involving popular breeds. Nowadays, the Pug represents one of the most diffuse brachymorphic canine breeds worldwide (American Kennel Club, 2022; Ente Nazionale Cinofilia Italiana, 2022; O’Neill et al., 2022; The Kennel Club, 2022). Concomitant to this growing popularity, the presentation of Pugs with cardiac diseases seems also to have increased (Koie et al., 2000; Fukushima et al., 2011; Kander et al., 2015; Guglielmini et al., 2016; Winter et al., 2019; Schreiber et al., 2022). Accordingly, Pugs frequently undergo cardiologic examinations. Regrettably, to date, only a single study on echocardiographic variables is available for this breed (Wiegel et al., 2022). Moreover, the number and type of echocardiographic variables addressed in that investigation were relatively limited, and the statistical reliability of RIs provided could be affected by the small sample size (Wiegel et al., 2022). Given the above, additional data obtained from a larger study sample and including additional echocardiographic measurements may be highly useful in the clinical setting. Therefore, this study aimed to determine echocardiographic values in a large population of apparently healthy Pugs and to assess the possible effects of BW, age, and sex on selected echocardiographic variables, with particular emphasis on those related to the left-sided cardiac chambers. Material and MethodsFor the purpose of this retrospective observational study, medical records of healthy dogs that underwent an echocardiogram as part of their diagnostic evaluation at the authors’ institutions between January 2014 and October 2022 were reviewed by a board-certified cardiologist (G. R.). Reasons for echocardiographic examination in an apparently healthy subject could include evaluation preceding operation before elective surgeries (e.g., castration, spaying) or screening for specific cardiac disorders in dogs related to subjects suffering from heart diseases for which a genetic transmission between relatives is known (e.g., in the case of other members of the same litter affected by pulmonic stenosis). To be included, dogs needed to be at least 1 year old and to have a complete case record, including signalment, history, clinical findings, and cardiac investigation. The latter had to include at least a transthoracic echocardiography (details below) and a 1-minute six-lead electrocardiogram (leads I, II, III, aVR, aVL, and aVF), which had to be performed on the same day according to standardized techniques and interpreted as previously described (Thomas et al., 1993; Romito et al., 2022a, 2022b, 2022c). Dogs were not considered if they showed one or more of the following criteria: a) heart murmur, gallop sound, or electrocardiographic abnormality; b) recent or current evidence of systemic illness based on history or physical examination (except brachycephalic obstructive airway syndrome given the predisposition of this breed to such condition); c) ongoing medications affecting the cardiovascular system; d) lack of cooperation during the echocardiographic examination, leading to suboptimal image acquisition; e) females that were in estrus, pregnant, or lactating; or e) significant cardiac abnormalities identified on two-dimensional (2D), M-Mode, and/or Doppler mode. For this study, trivial valve regurgitations (i.e., barely detectable jets of a few discrete color pixels close to the valvular coaptation point not always detectable throughout systole (in the case of mitral or tricuspid regurgitation) or diastole (in the case of aortic or pulmonary regurgitation) associated neither with audible murmurs on cardiac auscultation nor with any structural valve abnormalities on echocardiography were considered clinically non-significant, and these dogs were not excluded from the study (Rishniw and Erb, 2000a; Quiñones et al., 2002; Zoghbi et al., 2003). With specific concern to echocardiography, all examinations were performed by a board-certified cardiologist (G. R.) using two ultrasound unites (iE33 ultrasound system, Philips Healthcare, Monza, Italy; Mindary M9, Mindray Medical Italy S.R.L., Milano, Italy) equipped with phased array transducers of various frequencies (1–5 MHz; 3–8 MHz). Echocardiograms were acquired in right and left lateral recumbency in a quiet, dark room while the unsedated dogs were restrained gently on an echocardiographic table. Variables obtained in standard 2D, M-Mode, and Doppler were measured following the published methodology in the veterinary literature (Thomas et al., 1993; de Madron, 2016a, 2016b). Specifically, left ventricular (LV) measurements were obtained using the 2D-guided M-mode from the right parasternal short-axis view at the level of the papillary muscles. Measurements were made of the LV end-diastolic (LVDd) and end-systolic (LVDs) diameters as well as LV free wall and interventricular septum thicknesses at end-diastole and end-systole. LV systolic and diastolic diameters were then used for the respective calculation of end-diastolic and end-systolic volumes (EDV and ESV, respectively) using the Teichholz formula (Borgarelli et al., 2007). Moreover, LVDd and LVDs were also normalized for BW as previously described (LVDdn and LVDsn, respectively) (Cornell et al., 2004). LV fractional shortening (FS%) was calculated from LV diameters using the following formula: FS%=[(LVDd − LVDs)/LVDd] × 100. LV ejection fraction (EF%) was calculated from LV volumes using the following formula: EF%=[(EDV − ESV)/EDV] × 100. The EDV and ESV were indexed to body surface area (EDVI and ESVI, respectively), which was derived from BW using the following equation: 0.101 × BW (kg)2/3 . The LV sphericity index (SI) was obtained by dividing the base-to-apex length at end-diastole measured from the right parasternal four-chamber long-axis view using 2D echocardiography by LVDd (Holler and Wess, 2014). Left atrial (LA) and aortic root (Ao) diameters were measured in 2D echocardiography from the right parasternal short-axis view at the level of the heart base in early diastole, and the left atrium to aorta ratio was calculated (LA/Ao) (Rishniw and Erb, 2000b; Rishniw et al., 2019). Moreover, LA diameter (LAD) was obtained from the right parasternal four-chamber long-axis view by measuring the distance between the interatrial septum and the free wall using a line parallel to the mitral annulus at the end-systole (Rishniw and Erb, 2000b; Marchesotti et al., 2019). The maximal systolic pulmonary and Ao flow velocities were determined by pulsed-wave Doppler using the right parasternal transaortic short-axis and left apical five-chamber views, respectively. Early and late diastolic mitral inflow velocities (mitral E and A waves) were also measured from the left apical four-chamber view, and the E to A ratio was calculated. Finally, all cardiac valves were assessed using both 2D and color-flow Doppler modes, both in right and left recumbency, to exclude valve diseases. For each variable, a mean of three measurements was determined from three consecutive cardiac cycles on the same frame. Moreover, to assess measurement variability, six dogs enrolled in the study were randomly selected. For each dog, one single echocardiographic study was then chosen; thus, a total of six different echocardiographic studies were analyzed. For interobserver variability, two operators with different levels of experience in echocardiography [i.e., a board-certified cardiologist (GR) and a veterinarian with a 5-year experience in veterinary cardiology (PC)] analyzed echocardiographic images and videos and performed measurements of the aforesaid M-Mode, 2D, and Doppler variables. For this purpose, attention was made to analyze the same cardiac cycle for each loop. All echocardiographic data were collected into electronic spreadsheets (Microsoft Excel, version 2016, Microsoft Corporation, Redmond, WA) and then imported into statistical software packages (MedCalc Statistical Software version 19.5.1, Ostend, Belgium; IBM SPSS Statistics 22, Turkey) for further analysis. All continuous variables were tested for their distribution with a Shapiro-Wilk normality test and assessed by examining the histogram. Initial descriptive statistics included mean ± standard deviation for normally distributed data and median and range (minimum to maximum) for data that were not normally distributed. Categorical variables were reported as proportions. Outliers were visualized based on box plots and identified based on Tukey’s method (CLSI, 2008; Friedrichs et al., 2012). The fixed effect of sex as well as the effect of covariates BW and age on echocardiographic variables were tested using the following linear mixed model: Y=μ + Sex + aAge + bBW + ε. In this model, Y is the value of the echocardiographic variable, μ is a constant term, sex is the fixed effect of sex divided into two classes (male and female), age and BW are continuous variables, a and b are the slope coefficients for age and BW, and ε is the residual term. Since BW was already used to normalize/index LVDdn and LVDsn as well as EDVI and ESVI, a reduced model was purposefully used for these variables including age and sex only. A value of p < 0.05 was considered significant. Echocardiographic values of selected variables were determined using the robust method and confidence intervals about their limits were bootstrapped as recommended when the reference sample is between 40 and 120 subjects (CLSI, 2008; Friedrichs et al., 2012). The 2.5th and 97.5th percentiles were defined as the lower and upper limits, respectively. In addition, the 90% confidence intervals around these limits were also calculated. Concerning the inter-observer variability, it was then quantified as the coefficient of variation (CV) by use of the equation CV=[(mean difference between measurement/mean of measurements)/100] and expressed as a percentage. The degree of variability was defined as follows: CV < 5%, very low variability; 5%–15%, low variability; 16%–25%, moderate variability; or >25%, high variability (Baron Toaldo et al., 2017). Ethical approvalThis was a retrospective study performed on nonexperimental animals that exclusively underwent selected noninvasive diagnostic procedures made necessary by their clinical condition/history (e.g., echocardiography). However, all owners of dogs enrolled in the study gave informed consent for all of the investigations. ResultsThe study sample included 86 apparently healthy Pugs. Thirty-six were females (30 entire and 6 spayed) and 50 males (40 entire and 10 castrated). The median age and BW were 8 years (1–16 years) and 8.1 kg (3–13 kg), respectively. The RIs for the echocardiographic variables included in the study are reported in Table 1. The investigation of outliers revealed outlier dogs for LVDd (i.e., a value of 36.7), LVDdn (i.e., a value of 1.86 and one of 2.01), ESV (i.e., a value of 18 ml/m2), and ESVI (i.e., a value of 41 ml/m2 and one of 44 ml/m2). Since echocardiography did not reveal any pathological sign and the aforesaid values were not the results of erroneous measurements, they were not excluded from the determination of echocardiographic values. As shown in Table 2, a statistically significant effect of BW, age, and sex was observed for 7, 13, and 3 variables, respectively. In 24/86 (27.9%) dogs, a trivial pulmonary regurgitation (i.e., a barely detectable jet of a few discrete color pixels on the ventricular side of the valvular coaptation point that persists for a brief time in diastole) was found. Moreover, based on the combination of 2D and Doppler findings, a persistent left cranial vena cava (PLCVC) was suspected in 4/86 (4.7%) dogs. Such a suspect relied on the identification of an accessory vascular structure at the level of the LA lateral wall that clearly entered a subjectively mildly dilated coronary sinus to reach the right atrium (de Fornel et al., 2001; Gonzalez-Juanatey et al., 2004) (Fig. 1). The inter-observer measurement variability was low for the M-Mode and 2D variables, with CV values always below 16%. The inter-observed measurement variability was very low for Doppler variables, with CV values always below 5%. Table 1. Descriptive statistics and values for echocardiographic variables in healthy Pugs.
DiscussionThis study represents the largest cardiac investigation in the Pug, with the following findings: (1) some echocardiographic measurements of healthy Pugs diverge from generic RIs; (2) BW, age and sex influence several echocardiographic variables in this breed; and (3) identification of a trivial pulmonary regurgitation and echocardiographic signs consistent with PLCVC appear to be not uncommon in healthy Pugs. Concerning 2D dimensions of Ao and LA obtained from a right parasternal short-axis view, the values documented in our study population were similar to those previously reported in a smaller population of apparently healthy Pugs (Wiegel et al., 2022). Moreover, interestingly, our mean LA/Ao value (1.3) was within the generic RI, and no dog had a LA/Ao above the upper generic cut-off [<1.6 (Rishniw and Erb, 2000b; Rishniw et al., 2019); upper value from the present investigation 1.59]. This finding suggests that the generic rule stating that a LA/Ao ≥ 1.6 should be interpreted as a sign of LA dilatation (Boswood et al., 2016) may apply also to Pugs. In addition to LA/Ao, we also investigated the LAD from a right parasternal four-chamber long-axis view, with the aim of providing echocardiographic values for an alternative/complementary LA parameter that may overcome some of the limitations of LA/Ao. Indeed, potential limitations of LA/Ao include the difficulties in defining the path of aortic measurement relative to valve sinuses, in excluding pulmonary veins from the LA measurement, and in consistently timing LA measurements during the cardiac cycle. Moreover, if the imagining plane of Ao is not correct, the resulting ratio can under- or overestimate LA size, especially in the case of less experienced operators. Finally, significant inter-operator measurement variability of the LA/Ao has been reported, with possible misdiagnosis of LA enlargement (Rishniw and Erb, 2000b; Hansson et al., 2002; Rishniw, 2016; Strohm et al., 2018). In contrast, LAD is a relatively simple measurement that does not need any ratio, is free from any interference of Ao or pulmonary veins, and is highly reproducible with little intra- and interobserver measurement variability (Marchesotti et al., 2019). Intriguingly, when taking into consideration the median BW from our study population (i.e., 8.1 kg) and we compare our results with the generic RIs previously provided for dogs weighting 8 kg (Marchesotti et al., 2019), we found that our LAD values were overall slightly lower than previously reported. For example, our mean value (i.e., 20.6 mm) coincided with the lower value from the normality range reported by Marchesotti et al. (2019) (i.e., 20.6–30.8). Moreover, when we compared LAD values from dogs with a relatively low or high BW, the results tended to diverge from the generic RIs even more clearly. For example, in our study population, a Pug of 5 kg had a LAD of 15.4 mm, whereas the previously reported generic RI for this BW is 17.7–26.5 (Marchesotti et al., 2019). Moreover, 6/86 (7%) Pugs weighting 10–12 kg had a LAD value <22 mm (i.e., 18.1, 18.6, 19.2, 19.5, 20, 21.5 mm), while the generic RI for dogs of 10 kg is 22.1–33.1 (Marchesotti et al., 2019). The reasons for such a divergence are outside the scope of the present study; however, the fact that only 9/330 (2.7%) of the healthy dogs used by Marchesotti et al. (2019) for the estimation of generic LAD RIs were Pugs may have played a role. The aforesaid findings suggest that breed-specific cut-off should be preferred for LAD interpretation in Pugs. Table 2. Slope coefficients for the continuous variables (BW and age) and least square mean for the categorical variable (gender) in the model used for statistical analysis of echocardiographic variables assessed in the whole study population. The slope coefficients and least square mean are only given when the effect is statistically significant.
Fig. 1. Two-dimensional images of a Pug from the present study showing echocardiographic signs consistent with a PLCVC. (A) Echocardiographic image obtained from a right parasternal four-chamber long-axis view in systole. (B) Echocardiographic image obtained from a right parasternal four-chamber long-axis view in diastole. (C) Echocardiographic image obtained from a right parasternal five-chamber view. (D) Echocardiographic image obtained from an oblique right parasternal four-chamber long-axis view optimized to observe the longitudinal section of the PLCVC. (E). Echocardiographic image obtained from a right parasternal short-axis view at the level of the aortic root. (F) Echocardiographic image obtained from a left parasternal five-chamber view. In panels (A–F), it is possible to observe the transverse section of the PLCVC. Note this accessory vascular structure appears as a round, anechoic structure adjacent to the lateral LA wall (white asterisks). In panel (D), the optimized view allows to follow the PLCVC along its course. Note that the accessory vessel enters a subjectively mildly dilated coronary sinus to reach the right atrium (with asterisks). Concerning the absolute LV dimensions, the mean values of our measurements (i.e., LVDd, LVDs, LV free wall, and interventricular septum thicknesses at end-diastole and end-systole, EDV, ESV) were overall similar to those previously described in this breed (Wiegel et al., 2022) as well as to generic RIs (Cornell et al., 2004; Esser et al., 2020). Concerning the LV diameters normalized to BW, all Pugs had an LVDdn within the upper generic limit of normality [i.e., 97.5th percentile, 1.85 (Cornell et al., 2004)]. In contrast, in the case of LVDsn, 2/86 (2.3%) dogs showed a value above the generic cut-off [i.e., 97.5th percentile, 1.14 (Cornell et al., 2004); upper values detected in our study population: 1.15 and 1.16]. Concerning the LV volumes indexed to body surface area, the mean EDVI and ESVI values were within the upper generic limits of normality [i.e., EDVI < 100 ml/m2 and ESVI < 30 ml/m2 (Lombard, 1984)]. However, it should be noted that 8/86 (9.3%) dogs had an ESVI value above the generic cut-off, with a maximum value of 44 ml/m2. Concerning SI, our mean value (i.e., 1.6) was below the generic cut-off proposed for the diagnosis of dilated cardiomyopathy in dogs [i.e., <1.65 (Dukes-McEwan et al., 2003)]. However, interestingly, also a previous investigation in the Pug reported an SI median value below the aforesaid cut-off [i.e., 1.62 (Wiegel et al., 2022)]. Moreover, an even lower value of SI than that documented in the present study has recently been reported in healthy English Bulldogs [i.e., 1.31 (Patata et al., 2021)]. Combining our findings and those from previous studies in Pugs and English Bulldogs, it can be hypothesized that healthy brachymorphic dogs may have a physiologically more rounded heart than mesomorphic and dolicomorphic breeds. Concerning the LV functional parameters, our mean EF% and FS% values were within the generic limits of normality [i.e., >50% and >25%, respectively (Dukes-McEwan et al., 2003)]. However, it should be noted that 9/86 (10.5%) dogs had an EF% value below the generic cut-off, with a minimum value of 43%. Similarly, 6/86 (7.2%) dogs had an FS% value below the generic cut-off, with a minimum value of 21%. Collectively, the aforesaid M-Mode and 2D findings suggest that generic RIs may be appropriate for interpretation of the LV dimensions and function in many, but not all Pugs, and that breed-specific cut-offs should be preferred to avoid misdiagnosis (e.g., erroneous diagnosis of LV systolic dysfunction). Concerning Doppler variables, the mean values of mitral E and A wave, Ao, and pulmonary peak velocities were within previously reported RIs (Misbach et al., 2014; Wiegel et al., 2022). Moreover, it should be noted that no dog showed an Ao or pulmonary peak velocity above the cut-off that is generically used to diagnose stenotic diseases [i.e., in all cases, the Ao and pulmonary peak velocities were <2 m/s (Bussadori et al., 2000)]. Concerning the E/A, our mean value (i.e., 1.2) was within the expected physiologic range [i.e., 1–2 (Schober and Fuentes, 2001; Misbach et al., 2014; Wiegel et al., 2022)]. Nevertheless, 17/86 (19.8%) dogs showed an E/A < 1, with a minimum value of 0.6. Intriguingly, all these dogs were older than nine years. This finding is in line with previous literature and should not be necessarily interpreted as a pathological sign of diastolic dysfunction. Indeed, it has been demonstrated that the aging heart has increased ventricular stiffness and delayed relaxation, and that the critical age of physiologic change from normal filling to a delayed relaxation pattern is approximately at around 10 years in dogs (Schober and Fuentes, 2001). An additional interesting finding from the Doppler analysis of our study population, is the relatively frequent identification of a trivial pulmonary regurgitation. Indeed, we documented such an echocardiographic sign in 24/86 (27.9%) dogs with otherwise normal pulmonary valve. This finding suggests that the identification of trivial pulmonary regurgitation in healthy Pugs should not be systematically interpreted as a pathological finding. Moreover, this result appears overall in line with previous studies on subjects from different breeds, documenting a high prevalence of trivial and mild pulmonic regurgitations in healthy dogs [i.e., 54%–93% (Brown et al., 1991; Yuill and O’Grady, 1991; Nakayama et al., 1994; Rishniw and Erb, 2000a)]. Concerning the effects of demographic variables on echocardiographic measurements, we demonstrated that BW has an effect on several LA and LV parameters. Interestingly, an effect on many LV echocardiographic variables was demonstrated also for age. Moreover, sex influenced LVDdn, LVDsn, and LAD. Overall, these results agree with previous echocardiographic studies in different breeds (Rishniw and Erb, 2000b; Misbach et al., 2014; Giraut et al., 2019; Visser et al., 2019; Stack et al., 2020; Vatne et al., 2021; Vurucu et al., 2021). Concerning inter-operator variability, our analysis demonstrated that the measurements analyzed in this study were reproducible even when two operators with different levels of expertise in cardiology were compared. Although clinically useful, this result is not completely surprising as the echocardiographic variables selected for the present research are conventional ones (i.e., they represent the measurements that are evaluated during a standard echocardiographic examination in dogs). Therefore, the low values of inter-operator variability could be explained by the familiarity that operators have with the echocardiographic measures analyzed herein. A last intriguing finding from this study concerns the identification of echocardiographic signs typical for PLCVC in 4/86 (4.7%) dogs. Indeed, in these dogs, an accessory vascular structure adjacent to the LA lateral wall that entered a subjectively mildly dilated coronary sinus to reach the right atrium was documented. The coronary sinus is the main cardiac vein and normally opens in the right atrium between the caudal vena opening and the tricuspid valve. The dilation of the coronary sinus always raises the suspicion of PLCVC, as, normally, the coronary sinus is not identified via echocardiography. It is important to consider that the dilation of the coronary sinus is not an exclusive feature of the PLCVC. The list of differential diagnoses for this echocardiographic finding includes conditions leading to a pathological increase in right atrial pressure (e.g., pulmonary hypertension/stenosis). However, when the aforesaid echocardiographic signs are detected in a healthy patient with an otherwise normal heart, the PLCVC represents the main differential diagnosis (de Fornel et al., 2001; Gonzalez-Juanatey et al., 2004). The PLCVC derives from a failure of the obliteration of the left anterior cardinal vein and is usually an incidental finding in dogs (Buchanan, 1963; de Fornel et al., 2001; Choi et al., 2016). When this accessory vascular structure drains into the right atrium and is not associated with additional cardiac abnormalities (as in the cases described herein), no hemodynamic consequences are expected (this explains why we decided to maintain Pugs with suspected PLCVC among healthy dogs for RI analysis). Therefore, the life quality and life expectancy are typically not influenced by this heart malformation when it is isolated (de Fornel et al., 2011; Choi et al., 2016). In dogs, a breed predisposition has previously been suspected for the Shi-tzu (Choi et al., 2016); however, no prior studies have hypothesized a predisposition to PLCVC in Pugs. This study has some limitations. First, the study was retrospective and the intra-observer measurement variability was not assessed. However, we have analyzed the inter-operator variability, demonstrating that the echocardiographic variables selected for this study can be acquired with a clinically acceptable variability (i.e., CV always <16%). Second, the study did include neither numerous centers nor numerous operators; therefore, our results may be not completely representative of the actual measurements that could be acquired by operators with different levels of expertise worldwide. This explains why some cardiologists believe that large, multi-center studies should be preferentially used for generating RIs. Third, although the number of healthy dogs we enrolled was higher than that of many previous canine studies aimed at defining echocardiographic RIs (Lombard, 1984; Yuill and O’Grady, 1991; Crippa et al., 1992; Morrison et al., 1992; Bayon et al., 1994; Lonsdale et al., 1998; Della Torre et al., 2000; Rishniw and Erb, 2000b; Hansson et al., 2002; Brown et al., 2003; Jacobson et al., 2013; Strohm et al., 2018; Patata et al., 2021; Wiegel et al., 2022), we did not reach the recommended sample size when defining RIs [i.e., ≥120 subjects (Friedrichs et al., 2012)]. Fourth, we did not evaluate right ventricular echocardiographic variables, which might have been useful in Pugs because right-sided heart diseases have been reported in this breed (e.g., double-chambered right ventricle, pulmonic stenosis, cor triatriatum dexter) (Fukushima et al., 2011; Kander et al., 2015; Schreiber et al., 2022). Fifth, we did not evaluate the possible relationship between the presence/severity of brachycephalic syndrome and the echocardiographic variables in our study population. However, it should be noted that a previous investigation found no significant effects of clinical symptoms of upper airway disease on echocardiographic measurements in Pugs (Wiegel et al., 2022). Sixth, we not considered the possible effect of the neutered/spayed status on echocardiographic variables. Seventh, our analysis of aortic flow did not include the subxiphoid approach. Similarly, our analysis of pulmonic flow was simply obtained from the right recumbency, whereas the left one was not used for further analysis. Eighth, only the LA/Ao and LAD were used for the echocardiographic analysis of LA. The use of complementary measurements, such as the LA size normalized on BW (Visser et al., 2019) or on aortic diameter (Rishniw and Erb, 2000b; Rishniw et al., 2019) could have provided additional useful information. Ninth, we calculated LV volumes and EF% using a M-Mode technique (i.e., the Teichholz formula), not the Simpson's modified 2D method, and this may represent a further source of bias. Currently, a conclusive consensus on which technique should be preferred for EDV, ESV, and EF% in dogs is not available. However, it is important to underline that, although in dogs with left-sided heart diseases, the values obtained with the Teichholz formula and the Simpson’s modified method significantly diverge, in healthy dogs these techniques tend to provide often similar results (Meyer et al., 2013; Smets et al., 2014; Scollan et al., 2016). Additional potential confounding factors may be represented by the way we normalized LVDd and LVDs for BW. Indeed, we used the method proposed by Cornell et al. (2004). However, different research groups have provided different exponentials for the normalization of LV dimensions (Esser et al., 2020; Rishniw and Brown, 2022). As different results can be obtained according to the exponential employed, our results should be cautiously interpreted in the light of the methodology we used. Lastly, as previously done in several other canine publications (Lonsdale et al., 1998; Jacobson et al., 2013; Stack et al., 2020; Vurucu et al., 2021), we did not evaluate the possible effect of heart rate on the echocardiographic values. This was primarily due to the fact that not all Pugs tolerated the electrocardiographic clips during the echocardiographic examination. In conclusion, this study provides echocardiographic RIs in a cohort of healthy adult Pugs and explores the effect of BW, age, and sex on several echocardiographic variables. Because our findings were obtained using a standardized echocardiographic analysis in a large population of healthy Pugs, they likely represent a reliable and broadly applicable guide for echocardiographic interpretation in this breed. AcknowledgmentsNone. Author contributionsThe study was co-authored by all authors. The final manuscript was read and approved by all authors. FundingThis research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors. Conflict of interestThe authors have nothing to disclose. Data availabilityThe datasets generated and/or analyzed during the current study are not publicly available due to the potential to compromise participant consent or confidentiality but are available from the corresponding author upon reasonable request. ReferencesAmerican Kennel Club. 2022. Available via https://www.akc.org/most-popular-breeds/ (Accessed 4 October 2022). Baron Toaldo, M., Romito, G., Guglielmini, C., Pelle, N.G., Contiero, B. and Cipone, M. 2017. Assessment of left atrial deformation and function by 2-dimensional speckle tracking echocardiography in healthy dogs and dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 31, 641–649. Bayon, A., Palacio, M.J. and Montes, M. 1994. M-mode echocardiography study in growing Spanish mastiffs. J. Small. Anim. Pract. 35, 473–479. Boon, J., Wingfield, W.E. and Miller, C.W.1983. Echocardiographic indices in the normal dog. Vet. Radiol. Ultrasound. 4, 214–221. Borgarelli, M., Tarducci, A., Zanatta, R. and Haggstrom, J. 2007. Decreased systolic function and inadequate hypertrophy in large and small breed dogs with chronic mitral valve insufficiency. J. Vet. Intern. Med. 21, 61–67. Boswood, A., Häggström, J., Gordon, S.G., Wess, G., Stepien, R.L., Oyama, M.A., Keene, B.W., Bonagura, J., MacDonald, K.A., Patteson, M., Smith, S., Fox, P.R., Sanderson, K., Woolley, R., Szatmári, V., Menaut, P., Church, W.M., O'Sullivan, M.L., Jaudon, J.P., Kresken, J.G., Rush, J., Barrett, K.A., Rosenthal, S.L., Saunders, A.B., Ljungvall, I., Deinert, M., Bomassi, E., Estrada, A.H., Fernandez Del Palacio, M.J., Moise, N.S., Abbott, J.A., Fujii, Y., Spier, A., Luethy, M.W., Santilli, R.A., Uechi, M., Tidholm, A. and Watson, P. 2016. Effect of Pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study-A randomized clinical trial. J. Vet. Intern. Med. 30, 1765–1779. Brown, D.J., Knight, D.H. and King, R.R. 1991. Use of pulsed-wave Doppler echocardiography to determine aortic and pulmonary velocity and flow variables in clinically normal dogs. Am. J. Vet. Res. 52, 543–550. Brown, D.J., Rush, J.E., MacGregor, J., Ross, J.N. Jr, Brewer, B. and Rand WM. 2003. M-mode echocardiographic ratio indices in normal dogs, cats, and horses: a novel quantitative method. J Vet. Intern. Med. 17, 653–662. Buchanan, J.W. 1963. Persistent left cranial vena cava in dogs: angiocardiography, significance, and coexisting anomalies. J. Am. Vet. Rad. Soc. 4, 1–8. Bussadori, C., Amberger, C., Le Bobinnec, G. and Lombard, C.W. 2000. Guidelines for the echocardiographic studies of suspected subaortic and pulmonic stenosis. J. Vet. Cardiol. 2, 15–22. Choi, S.Y., Song, Y.M., Lee, Y.W. and Choi, H.J. 2016. Imaging characteristics of persistent left cranial vena cava incidentally diagnosed with computed tomography in dogs. J. Vet. Med. Sci. 78, 1601–1606. CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, 3rd edition. CLSI document EP28-A3c. Wayne, PA: Clinical Laboratory Standards Institute, 2008. Available via https://clsi.org/media/1421/ep28a3c_sample.pdf (Accessed 4 October 2022). Cornell, C.C., Kittleson, M.D., Della Torre, P., Häggström, J., Lombard, C.W., Pedersen, H.D., Vollmar, A. and Wey A. 2004. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 18, 311–321. Crippa, L., Ferro, E., Melloni, E., Brambilla, P. and Cavalletti, E. 1992. Echocardiographic parameters and indices in the normal Beagle dog. Lab. Anim. 26, 190–195. de Fornel, P., Rosenberg, D., Rollois, M., Chetboul, V. and Pouchelon, J.L. 2001. Clinical vignette Fortuitous diagnosis of a persistent left cranial vena by color flow Doppler echocardiography in a dog. J. Vet. Cardiol. 3, 23–25. de Madron, E. 2016a. Normal views: 2D, TM, spectral, anc color Doppler. In Clinical echocardiography of the dog and cat. Eds., Chetboul, V., Bussadori, C. and E. de Madron. St. Louis, MO: Elsevier, pp: 3–19. de Madron, E. 2016b. Normal echocardiographic values: TM, 2D, and Doppler spectral modes. In Clinical echocardiography of the dog and cat. Eds., Chetboul, V., Bussadori, C. and E. de Madron. St. Louis, MO: Elsevier, pp: 21–37. Della Torre, P.K., Kirby, A.C., Church, D.B. and Malik, R. 2000. Echocardiographic measurements in greyhounds, whippets and Italian greyhounds dogs with a similar conformation but different size. Aust. Vet. J. 78, 49–55. Dukes-McEwan, J., Borgarelli, M., Tidholm, A., Vollmar, A.C. and Häggström, J., ESVC Taskforce for Canine Dilated Cardiomyopathy. 2003. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J. Vet. Cardiol. 5, 7–19. Ente Nazionale Cinofilia Italiana. 2022. Available via https://www.enci.it/libro-genealogico/razze/carlino (Accessed 4 October 2022). Esser, L.C., Borkovec, M., Bauer, A., Häggström J. and Wess, G. 2020. Left ventricular M-mode prediction intervals in 7651 dogs: population-wide and selected breed-specific values. J. Vet. Intern. Med. 34, 2242–2252. Friedrichs, K.R., Harr, K.E., Freeman, K.P., Szladovits, B., Walton, R.M., Barnhart, K.F. and Blanco-Chavez, J., American Society for Veterinary Clinical Pathology. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 41, 441–453. Fukushima, R., Tanaka, R., Suzuki, S., Hamabe, R., Machida, N., Nakao, S., Saida, Y., Takashima, K., Matsumoto, H., Koyama, H., Hirose, H. and Yamane, Y. 2011. Epidemiological and morphological studies of double-chambered right ventricle in dogs. J. Vet. Med. Sci. 73, 1287–1293. Giraut, S., Häggström, J., Koskinen, L.L.E., Lohi, H. and Wiberg, M. 2019. Breed-specific reference ranges for standard echocardiographic measurements in salukis. J. Small. Anim. Pract. 60, 374–378. Gonzalez-Juanatey, C., Testa, A., Vidan, J., Izquierdo, R., Garcia-Castelo, A., Daniel, C. and Armesto V. 2004. Persistent left superior vena cava draining into the coronary sinus: report of 10 cases and literature review. Clin. Cardiol. 27, 515–518. Guglielmini, C., Baron Toaldo, M., Quinci, M., Romito, G., Luciani, A., Cipone, M., Drigo, M. and Diana, A. 2016. Sensitivity, specificity, and interobserver variability of survey thoracic radiography for the detection of heart base masses in dogs. J. Am. Vet. Med. Assoc. 248 1391–1398. Hall, D.J., Cornell, C.C., Crawford, S. and Brown, D.J. 2008. Meta-analysis of normal canine echocardiographic dimensional data using ratio indices. J. Vet. Cardiol. 10, 11–23. Hansson, K., Haggstrom, J., Kvart, C. and Lord, P. 2002. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound. 43, 568–575. Holler, P.J. and Wess, G. 2014. Sphericity index and E-point-to-septal-separation (EPSS) to diagnose dilated cardiomyopathy in Doberman Pinschers. J. Vet. Intern. Med. 28 123–129. Jacobson, J.H., Boon, J.A. and Bright, J.M. 2013. An echocardiographic study of healthy Border Collies with normal reference ranges for the breed. J. Vet. Cardiol. 15, 123–130. Kander, M,, Pasławska, U., Staszczyk, M., Cepiel, A., Pasławski, R., Mazur, G. and Noszczyk-Nowak, A. 2015. Retrospective analysis of co-occurrence of congenital aortic stenosis and pulmonary artery stenosis in dogs. Pol. J. Vet. Sci. 18, 841–845. Koie, H., Kurotobi, E.N. and Sakai, T. 2000. Double-chambered right ventricle in a dog. J. Vet. Med. Sci. 62, 651–653. Lombard, C.W. 1984. Normal values of the canine M-mode echocardiogram. Am. J. Vet. Res. 45, 2015–2018. Lonsdale, R.A., Labuc, R.H. and Robertson, I.D. 1998. Echocardiographic parameters in training compared with non-training greyhounds. Vet. Radiol. Ultrasound. 39, 325–330. Marchesotti, F., Vezzosi, T., Tognetti, R., Marchetti, F., Patata, V., Contiero, B., Zini, E. and Domenech, O. 2019. Left atrial anteroposterior diameter in dogs: reference interval, allometric scaling, and agreement with the left atrial-to-aortic root ratio. J. Vet. Med. Sci. 81, 1655–1662. Meyer, J., Wefstaedt, P., Dziallas, P., Beyerbach, M., Nolte, I. and Hungerbühler, S.O. 2013. Assessment of left ventricular volumes by use of one-, two-, and three-dimensional echocardiography versus magnetic resonance imaging in healthy dogs. Am. J. Vet. Res. 74, 1223–1230. Misbach, C., Lefebvre, H.P., Concordet, D., Gouni, V., Trehiou-Sechi, E., Petit, A.M., Damoiseaux, C., Leverrier, A., Pouchelon, J.L. and Chetboul, V. 2014. Echocardiography and conventional Doppler examination in clinically healthy adult Cavalier King Charles Spaniels: effect of body weight, age, and gender, and establishment of reference intervals. J. Vet. Cardiol. 16, 91–100. Morrison, S.A., Moise, N. and Scarlett, J. 1992. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J. Vet. Intern. Med. 6, 220–224. Nakayama, T., Wakao, Y., Takiguchi, S., Uechi, M., Tanaka, K. and Takahashi, M. 1994. Prevalence of valvular regurgitation in normal Beagle dogs detected by color Doppler echocardiography. J. Vet. Med. Sci. 56, 973–975. O’Grady, M.R., Bonagura, J.D., Powers, J.D. and Herring, D.S. 1986. Quantitative cross-sectional echocardiography in the normal dog. Vet. Radiol. 27, 34–49. O'Neill, D.G., Sahota, J., Brodbelt, D.C., Church, D.B., Packer, R.M.A. and Pegram, C. 2022. Health of Pug dogs in the UK: disorder predispositions and protections. Canine Med. Genet. 9, 4. Patata, V., Vezzosi, T., Marchesotti, F. and Domenech, O. 2021. Echocardiographic parameters in 50 healthy English bulldogs: preliminary reference intervals. J. Vet. Cardiol. 36, 55–63. Quiñones, M.A., Otto, C.M., Stoddard, M., Waggoner, A. and Zoghbi, W.A., Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. 2002. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 15, 167–184. Rishniw, M. and Erb, H.N. 2000a. Prevalence and characterization of pulmonary regurgitation in normal adult dogs. J. Vet. Cardiol. 2, 17–21. Rishniw. M and Erb, H.N. 2000b. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 14, 429–435. Rishniw, M. 2016. Interobserver variability in two-dimensional echocardiographic left atrial measurements is complex. Research Communication, 25th ECVIM-CA Congress, Lisbon, Portugal. Rishniw, M., Caivano, D., Dickson, D., Vatne, L., Harris, J. and Matos, J.N. 2019. Two-dimensional echocardiographic left- atrial-to-aortic ratio in healthy adult dogs: a reexamination of reference intervals. J. Vet. Cardiol. 26, 29–38. Rishniw, M. and Brown, D. 2022. The ACVIM consensus statement definition of left ventricular enlargement in myxomatous mitral valve disease does not always represent left ventricular enlargement. J. Vet. Cardiol. 42, 92–102. Romito, G., Castagna, P., Sabetti, M.C. and Cipone M. 2022a. Physiological shift of the ventricular mean electrical axis in healthy French Bulldogs: a retrospective electrocardiographic analysis of 80 healthy dogs. J. Vet. Cardiol. 42, 34–42. Romito, G., Castagna, P., Pelle, N.G., Testa, F., Sabetti, M.C. and Cipone M. 2022b. The canine T wave: a retrospective analysis on qualitative and quantitative T wave variables obtained in 129 healthy dogs and proposed reference intervals. J. Vet. Cardiol. 42, 52–64. Romito, G., Castagna, P., Pelle, N.G., Testa, F., Sabetti, M.C. and Cipone, M. 2022c. Retrospective evaluation of the ST segment electrocardiographic features in 180 healthy dogs. J. Small. Anim. Pract. 63, 756–762. Schober, K.E. and Fuentes, V.L. 2001. Effects of age, body weight, and heart rate on transmitral and pulmonary venous flow in clinically normal dogs. Am. J. Vet. Res. 62(9), 1447–1454. Schreiber, N., Toaldo, M.B., Wolfer, N., Dennler, M., Corona, D., Henze, I., Kovacevic, A. and Glaus, T. 2022. Long-term palliation of right-sided congestive heart failure after stenting a recurrent cor triatriatum dexter in a 10½-year-old pug. J. Vet. Cardiol. 4, 121–127. Scollan, K.F., Stieger-Vanegas, S.M. and Sisson, D.D. 2016. Assessment of left ventricular volume and function in healthy dogs by use of one-, two-, and three-dimensional echocardiography versus multidetector computed tomography. Am. J. Vet. Res. 77, 1211–1219. Smets, P., Daminet, S. and Wess, G. 2014. Simpson's method of discs for measurement of echocardiographic end-diastolic and end-systolic left ventricular volumes: breed-specific reference ranges in Boxer dogs. J. Vet. Intern. Med. 28, 116–122. Stack, J.P., Fries, R.C., Kruckman, L. and Schaeffer, D.J. 2020. Reference intervals and echocardiographic findings in Leonberger dogs. J. Vet. Cardiol. 29, 22–32. Strohm, L.E., Visser, L.C., Chapel, E.H., Drost, W.T. and Bonagura, J.D. 2018. Two-dimensional, long-axis echocardiographic ratios for assessment of left atrial and ventricular size in dogs. J. Vet. Cardiol. 20,330–342. The Kennel Club. 2022. Breed registration statistics: The Kennel Club Limited. Available via https://www.thekennelclub.org.uk/media-centre/breed-registration-statistics/ (Accessed 4 October 2022). Thomas, W.P., Gaber, C.E., Jacobs, G.J., Kaplan, P.M., Lombard, C.W., Moise, N.S. and Moses BL. 1993. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 7, 247–252. Vatne, L., Dickson, D., Tidholm, A., Caivano, D. and Rishniw M. 2021. The effects of activity, body weight, sex and age on echocardiographic values in English setter dogs. J. Vet. Cardiol. 37, 26–41. Visser, L.C., Ciccozzi, M.M., Sintov, D.J. and Sharpe, A.N. 2019. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: a prospective study proposing reference intervals and assessing repeatability. J. Vet. Intern. Med. 33, 1909–1920. Vurucu, M., Ekinci, G. and Gunes, V. 2021. An echocardiographic study of breed-specific reference ranges in healthy French Bulldogs. Vet. Radiol. Ultrasound 62, 573–582. Wess, G., Bauer, A. and Kopp, A. 2021. Echocardiographic reference intervals for volumetric measurements of the left ventricle using the Simpson's method of discs in 1331 dogs. J. Vet. Intern. Med. 35, 724–738. Wiegel, P.S., Nolte, I., Mach, R., Freise, F. and Bach, J.P. 2022. Reference ranges for standard-echocardiography in pugs and impact of clinical severity of brachycephalic obstructive airway syndrome (BOAS) on echocardiographic parameters. BMC Vet. Res. 18, 282. Winter, R.L., Newhard, D.K., Taylor, A.R., Johnson, J.A. and Baravik-Munsell, E.D. 2019. Balloon valvuloplasty in a dog with congenital bicuspid aortic valve and supravalvar aortic stenosis (atypical Shone's complex). J. Vet. Cardiol. 23, 88–95. Yuill, C.D.M. and O’Grady, M.R. 1991. Doppler-derived velocity of blood flow across the cardiac valves in the normal dog. Can. J. Vet. Res. 55, 185–192. Zoghbi, W.A., Enriquez-Sarano, M., Foster, E., Grayburn, P.A., Kraft, C.D., Levine, R.A., Nihoyannopoulos, P., Otto, C.M., Quinones, M.A., Rakowski, H., Stewart, W.J., Waggoner, A. and Weissman, N.J. and American Society of Echocardiography. 2003. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 16, 777–802. | ||
| How to Cite this Article |
| Pubmed Style Romito G, Castagna P, Sabetti MC, Ablondi M, Cipone M. Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Vet J. 2023; 13(8): 965-976. doi:10.5455/OVJ.2023.v13.i8.2 Web Style Romito G, Castagna P, Sabetti MC, Ablondi M, Cipone M. Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. https://www.openveterinaryjournal.com/?mno=146473 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i8.2 AMA (American Medical Association) Style Romito G, Castagna P, Sabetti MC, Ablondi M, Cipone M. Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Vet J. 2023; 13(8): 965-976. doi:10.5455/OVJ.2023.v13.i8.2 Vancouver/ICMJE Style Romito G, Castagna P, Sabetti MC, Ablondi M, Cipone M. Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Vet J. (2023), [cited July 01, 2025]; 13(8): 965-976. doi:10.5455/OVJ.2023.v13.i8.2 Harvard Style Romito, G., Castagna, . P., Sabetti, . M. C., Ablondi, . M. & Cipone, . M. (2023) Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Vet J, 13 (8), 965-976. doi:10.5455/OVJ.2023.v13.i8.2 Turabian Style Romito, Giovanni, Prisca Castagna, Maria Chiara Sabetti, Michela Ablondi, and Mario Cipone. 2023. Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Veterinary Journal, 13 (8), 965-976. doi:10.5455/OVJ.2023.v13.i8.2 Chicago Style Romito, Giovanni, Prisca Castagna, Maria Chiara Sabetti, Michela Ablondi, and Mario Cipone. "Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex." Open Veterinary Journal 13 (2023), 965-976. doi:10.5455/OVJ.2023.v13.i8.2 MLA (The Modern Language Association) Style Romito, Giovanni, Prisca Castagna, Maria Chiara Sabetti, Michela Ablondi, and Mario Cipone. "Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex." Open Veterinary Journal 13.8 (2023), 965-976. Print. doi:10.5455/OVJ.2023.v13.i8.2 APA (American Psychological Association) Style Romito, G., Castagna, . P., Sabetti, . M. C., Ablondi, . M. & Cipone, . M. (2023) Echocardiographic values in healthy Pugs: Effect of body weight, age, and sex. Open Veterinary Journal, 13 (8), 965-976. doi:10.5455/OVJ.2023.v13.i8.2 |