| Research Article | ||
Open Vet J. 2023; 13(7): 834-838 Open Veterinary Journal, (2023), Vol. 13(7): 834–838 Original Research Prevalence of Varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in LibyaMarwan M. Keshlaf1*, Hamida B. Mirwan1, Salah Ghana2, Salem Mubrok1 and Taher Shaibi21Department of Plant Protection, Faculty of Agriculture, University of Tripoli, Tripoli, Libya 2Department of Zoology, Faculty of Science, University of Tripoli, Tripoli, Libya *Corresponding Author: Marwan M. Keshlaf. Department of Plant Protection, Faculty of Agriculture, University of Tripoli, Tripoli, Libya. Email: m.keshlaf [at] uot.edu.ly Submitted: 04/03/2023 Accepted: 06/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
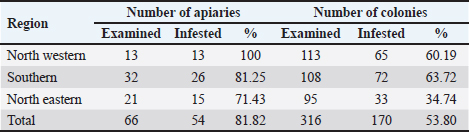
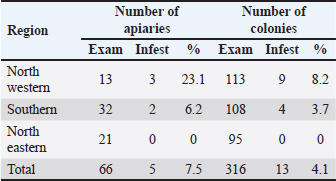
AbstractBackground: Primary key pest affecting the apiculture business in many areas of the globe is the ecto parasite Varroa mite (Varroa destructor), recently, bee lice have become a considerable bee pest. Aim: In this study, the ecto parasites that infest honey bees, were investigated during the spring of 2013. Methods: A total of 66 apiaries were investigated from different geographical regions in Libya: 34 apiaries from the southern region, 21 apiaries from the north-eastern region, and 11 apiaries from the north-western region. Three bee colonies were randomly chosen from each apiary (316 colonies). From each colony, 300 worker bees were taken as samples, put in container filled with 100 ml of alcohol, and transported to the Honey bee Laboratory—Plant Protection Department at the University of Tripoli’s. The parasites were separated from the bees and identified, and the infestation rates were calculated. Results: The study of the parasite distribution clearly showed that Varroa mites were present in all regions. However, the degree of mite density varies not only between apiaries but also between colonies in the same apiary, while the bee louse, Barulla coeca, was detected in 23.1% and 5.8% of apiaries in the western region and southern region of Libya, respectively. On the other hand, all the inspected apiaries in the northern region were not infested. Conclusion: Lack of knowledge about honey bee parasites, poor management practices, and the lack of proper distance between hives of migrated apiaries have been attributed as the possible reason for the spread of these two parasites, especially the Varroa mites in the country. Keywords: Prevalence, Apis mellifera, Varroa destructor, Bruala coeca, Libya. IntroductionMany pathogens and parasites infect honey bee, Apis mellifera, colonies (Rosenkranz et al., 2010). Most parasites and pathogens that harm honey bees are fairly globally distributed (Hepburn and Radloff, 1998). However, it is difficult to define the honey bee health state in Africa (Hepburn and Radloff, 1998; Dietemann et al., 2009). Varroa mites Varroa destructor (Rosenkranz et al., 2010) and bee lice Bruala coeca are pests to honey bees (Ellis, 2008). Varroa destructor lives on the hemolymph of mature and developing bees, it poses a significant danger to beekeepers by spreading diseases to honey bees and reducing their longevity (Fries et al., 2006), while bee louse (B. coeca), a cosmopolitan inhabitant of adult honey bees, is generally regarded as a minor pest (Hepburn, 1978). Although the presence of V. destructor worldwide (Ellis and Munn, 2005), few surveys on honey bee s’ parasites have been undertaken in African countries. According to Crane (1979), Libya had V. destructor in 1976. It was introduced to the Algabal Elakder area (Northeast) through imported bee packages from Bulgaria, where it was established and spread across the country (Keshlaf, 2017). The first mention of B. coeca in Africa was in Tunisia in 1978 (Smith Jr and Caron, 1985), the parasite was then reported in 1981 in Algeria and Egypt (El-Niweiri et al., 2008), and subsequently in Libya and Morocco (Neumann and Elzen, 2004). Beekeeping has been practiced in Libya for a very long time (Crane, 1999), However, beekeeping methods of using modern hives have only recently become popular (Brittan, 1956). Beekeepers in Libya have recently expressed concern over the sharp decline in honey output, the collapse of honey bee colonies, and the negative effects these factors are having on agriculture and food production (Keshlaf, 2017). In this regard, studies on the significance of parasitism on beekeepers by V. destructor and B. coeca were very infrequently conducted. We report here the first survey of the prevalence of V. destructor and B. coeca in Libyan apiaries based on a large-scale sampling of adult bees with different geographic origins. Materials and MethodsTo identify the prevalence of Varroa mite and bee louse in Libya, a survey was performed in March 2013. A total of 66 apiaries from different geographical regions in Libya were investigated. 34 apiaries from the southern region, covering Sabha, Obaray, Brakeshati, Sookna, Hoon, Ghat, Morzik, and Omlaraneb. From the north-eastern region, 21 apiaries were included: Sert, Bangazi, Shehat, Tobrug, Derna Al-Marg, and Al-jabal Al-akdar. 11 apiaries represented the north-western region, including Tripoli, Tajora, WadiRabee, Ain Zara, and Yefrin. At least three bee hives from each apiary were randomly selected, making a total of 316 hives that were examined across all apiaries. Thereafter, approximately 300 adult worker bees were then brushed off the comb and placed immediately into a vial containing about 100 ml of 70% ethanol before being transported to the honey bee laboratory at the Department of Plant Protection, University of Tripoli, Libya, where containers were placed on a shaker for 30 minutes to dislodge ecto parasites. The content was poured over sieves to collect the parasites (De Jong et al., 1982). To distinguish between the bee lice and the Varroa mite, the parasites were then inspected under a microscope at a 40× magnification. Infestation rates were calculated as: the number of the bee lice and the Varroa mite in each sample divided by the number of honey bees in each sample and multiplied by 100. Ethical approvalThis study was approved by the Graduate School of the University of Tripoli, Faculty of Agriculture, Department of Plant Protection. All animal welfare protocols were followed. ResultsDistribution of Varroa mites in LibyaThe overall infestation rate of V. destructor in honey bee colonies was 3.5% in the adult worker bees (i.e., 1,355 mites per 38,657 bees). Our study revealed that V. destructor was found in 81.8% of investigated apiaries and in 53.8% of the inspected colonies (Table 1). All inspected apiaries of the North Western region were infested with Varroa mites (100%), whereas 81.2% and 71.4% of infested apiaries were recorded in the Southern and North Eastern regions, respectively. The North Western region had a higher number of colonies per apiary (8 colonies per apiary) than the Southern and North Eastern regions (4.5 colonies per apiary and 3 colonies per apiary, respectively). The recorded infestation levels varied among locations within the regions (Table 2). Darna and Tobrog showed no infestation, while Morzik showed the highest infestation (15.7%). Distribution of bee lice in LibyaThe prevalence of the bee lice B. coeca infestation on honey bee colonies in the three regions of Libya revealed an overall prevalence of 0.11% in the adult honey bees (i.e., 44 louse per 38,657 bees). Our study revealed that the bee louse, B. coeca, is quite uncommon, having been found only in 7.5% of investigated apiaries and in 4.1% of inspected hives (Table 3). All inspected apiaries in the Northeastern of Libya were lice-free, while only Sookna and Hoon in the Southern region were slightly infected compared to a higher infestation in bee colonies in Tripoli (Table 4). DiscussionThe sampling time (February to March) may have contributed to the low incidence of Varroa mites infestation that was reported in this study compared to previous reports in Libya. Keshlaf and AlFallah (2018) reported higher infestations of Varroa mites on adult worker bees in spring (6.3%) and summer (16.3%). Varroa mites generally increases after the peak of brood production because they reproduce well in brood cells (Alfallah and Mirwan, 2018). The recorded infestation levels seem to be apiary-dependent, rather than the regional factor, since good beekeeping practice might be the reason for the lower infestation rate. However, the low infestation in Darna and Tobrog might be attributed to low sampling, since only one apiary was used from each of these two locations. Varroa mites were the most common honey bee pest in Jordan, according to Al-Chzawi et al. (2009); they were reported in all apiaries, and 90% of the inspected colonies. Similar infection by rates to those in our study. A study carried out in Jordan revealed that the bee louse, Braula orientalis, is quite frequently identified in 45.4% of the inspected colonies and 64.3% of the examined apiaries (Al-Chzawi et al., 2009). The infestation rates varied from 0.3 to 4.6 individuals per 100 Apis mellifera adansonii workers were reported in Benin by Paraïso et al. (2012). Strauss et al. (2013) agreed with what we found, they reported that Apis mellifera scutellata colonies in South Africa were infested (2.1 to 2.3 individuals per 100). According to Gidey et al. (2012), the infestation rates with louse among the adult worker bees were 5%–6% in Wukro Woreda, Ethiopia. Low infestation by B. coeca among investigated honey bees was reported in this study and may be related to the time of sampling (February to March). Barulla coeca populations typically increase after the honey crop, when they have favorable reproduction in honey cap wax (Keshlaf and Mirwan, 2019). Despite bee lice being discovered inside bee colonies all year long, Al-Ghzawi et al. (2009) observed a decline in the infestation rate after December and during the spring, hitting its lowest level in April. Zaitoun and Al-Ghzawi (2008) reported that the infestation rate of B. coeca increased rapidly in May. In comparison, B. coeca populations peaked at various times in other parts of the world, including the spring and fall seasons in Egypt (Hassanein and Abd El-Salam, 1962), and the USA (Smith Jr and Caron, 1984), and Jordan in summer and autumn (Zaitoun and Al-Ghzawi, 2008). The variations in B. coeca populations between these areas could be explained by environmental changes. Strauss et al. (2013) reported that the infection rates of V. destructor and B. coeca that in South Africa varied between the winters of 2010 and 2011. This might be explained by varying climatic circumstances between years and between areas. Table 1. Apiaries and colonies infested with Varroa mites V. destructor in adult worker bee samples from the three regions of Libya.
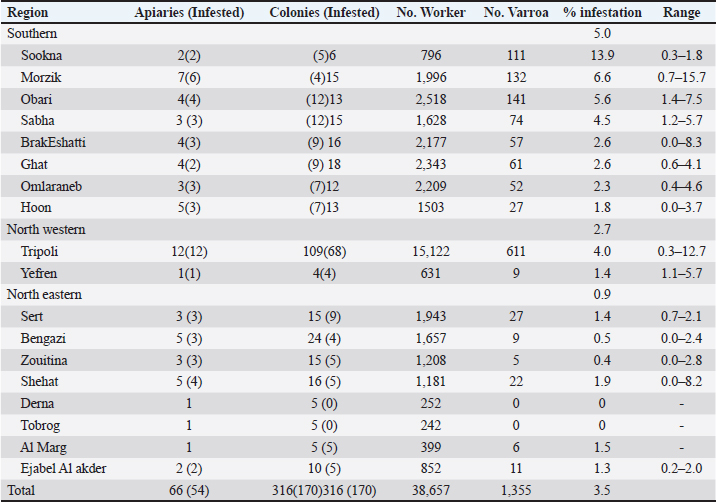
Table 2. Detailed infestation rates Varroa mites V. destructoron adult worker bee samples from 316 selected colonies.
Table 3. Apiaries infested with bee lice B. coeca in adult worker bee samples from the three regions of Libya.
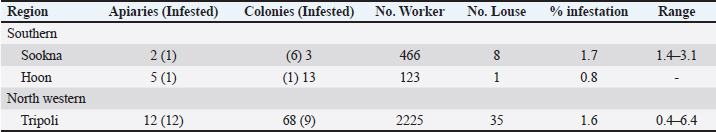
Table 4. Detailed infestation rates of bee lice B. coeca on adult worker bee samples from 316 selected colonies.
A recent experimental study revealed bee louse to be an obvious cause of a decrease in the number of worker bees and honey production (Al-Ghzawi et al., 2009), despite some previous accounts not attributing any negative effects to the presence of B. coeca on the honey bees (Akratanakul, 1986). It is unlikely that there would be a competition between V. destructor and B. coeca for space on adult honey bees or for food. They have different behaviors in regard to space occupied by honey bee workers and food consumed (Bowen-Walker et al., 1997; Ellis, 2008). As well, the lack of a significant association between their seasonal infestation rates decreases the possibility of direct competition (Keshlaf and Mirwan, 2019). By removing infected pupae, hygienic behavior has been described as a colony-level defense strategy against the parasitic mite V. destructor (Boecking and Drescher, 1992; Spivak and Downey, 1998). In a preliminary study, Keshlaf and Alfallah (2019) reported that the hygienic behavior of Libyan honey bees was high and reduced the Varroa mites populations in the colony. However, their results indicated that this behavioral resistance was ineffective with bee lice. This is made even clearer when comparing the two creatures’ life histories; bee lice B. coeca larvae emerge from eggs placed on honey caps and are not reliant on developing honey bee offspring to live through adulthood (Ellis, 2008). In contrast, honey bee larvae that have been sealed is required for the reproduction of Varroa mites V. destructor, and their primary food supply is honey bee hemolymph rather than the food of honey bees as is the case with bee lice B. coeca (Ellis, 2008; Rosenkranz et al., 2010). The migratory beekeeping encouraged the outcross distribution of V. destructor and B. coeca; as a consequence, multiple species may coexist in the same population (Crane, 1990). ConclusionAccording to the results and the low rates of both ecto parasites of this study V. destructor and B. coeca, considered that they have a minor threat to the health of the honey bee population under investigation. However, the numbers found can be regarded as high because V. destructor was significantly more prevalent in the examined populations. Moreover, despite being bee lice B. coeca present in west Libya, it is rarely observed there. Throughout this investigation, integrated pest management is highly recommended to improve the beekeeping industry and pollination services. AcknowledgmentsWe appreciate the assistance of all beekeepers who provided samples and access to apiaries. Thanks to Salah Al-abyed for helping to gather laboratory results. Conflict of interestThe authors have no conflict of interest to declare. FundingThis work was funded by the Libyan Authority for Scientific Research (NSR) and the University of Tripoli. Availability of dataData available on request from the authors. Authors’ contributionsMK and TS: conception, design, and organization of the study; MK, TS, HM, SG and SM: conducted the study; MK, HM and SM: acquisition of data; MK, HM and TS: analysis and interpretation of data; MK, HM and TS: drafting of the manuscript and critiquing the output for important intellectual content. All authors discussed the results and commented on the manuscript. ReferencesAkratanakul, P. 1986. Beekeeping in Asia. Rome, Italy: FAO. Al-Chzawi, A.A.M.A., Zaitoun, S.T. and Shannag, H.K. 2009. Incidence and geographical distribution of honey bee (Apis mellifera L.) pests in Jordan. Ann. Soc. Entomol. Fr. 45(3), 305–308. Al-Ghzawi, A.A.M., Zaitoun, S. and Shannag, H. 2009. Management of Braula orientalis Örösi (Diptera: Braulidae) in honey bee colonies with tobacco smoke under semiarid conditions. Entomol. Res. 39(3), 168–174. Alfallah, H.M. and Mirwan, H.B. 2018. The story of Braula coeca (Bee Lice) in honey bee colonies Apis mellifera L. in Libya. Int. J. Agric. Sci. 5, 34–36. Boecking, O. and Drescher, W. 1992. The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after artificial and natural infestation with Varroa jacobsoni Oud. And to freeze-killed brood. Exp. Appl. Acarol. 16(4), 321–329. Bowen-Walker, P., Martin, S. and Gunn, A. 1997. Preferential distribution of the parasitic mite, Varroa jacobsoni Oud. On overwintering honey bee (Apis mellifera L.) workers and changes in the level of parasitism. Parasitology 114(2), 151–157. Brittan, O. 1956. Introduction of modern beekeeping to Cyrenacia (Libya). Bee Craft 37(12), 145–146. Crane, E. 1979. Fresh news on the Varroa mite. Bee World 60, 8. Crane, E. 1990. Bees and beekeeping: science, practice and world resources. Oxford, UK: Heinemann Newnes. Crane, E. 1999. The world history of beekeeping and honey hunting. Oxfordshire: Routledge. De Jong, D., Roma, D.D.A. and Goncalves, L. 1982. A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honey bees. Apidologie 13(3), 297–306. Dietemann, V., Pirk, C.W.W. and Crewe, R. 2009. Is there a need for conservation of honey bee s in Africa? Apidologie 40(3), 285–295. Ellis, D. 2008. Bee louse, bee fly, Braulid, Braula coeca Nitzsch (Diptera: Braulidae). In Encyclopaedia of entomology. Ed., Capinera, J.L. Berlin, Germany: Springer, pp: 417–419. Ellis, J.D. and Munn, P.A. 2005. The worldwide health status of honey bees. Bee World 86(4), 88–101. El-Niweiri, M.A., El-Sarrag, M.S. and Neumann, P. 2008. Filling the Sudan gap: the northernmost natural distribution limit of small hive beetles. J. Apic. Res. 47(3), 184–185. Fries, I., Imdorf, A. and Rosenkranz, P. 2006. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 37(5), 564–570. Gidey, A., Mulugeta, S. and Fromsa, A. 2012. Prevalence of bee lice Braula coeca (Diptera: Braulidae) and other perceived constraints to honey bee production in Wukro Woreda, Tigray Region Ethiopia. Glob. Vet. 8(6), 631–635. Hassanein, M. and Abd El-Salam, A. 1962. Biological studies on the bee louse, Braula coeca Nitzsch. Bull. Sociktk Entomol. Egypte 46, 87–95. Hepburn, H.R. 1978. The bee louse. S. Afr. Bee. J. 50, 11–12. Hepburn, H.R. and Radloff, S.E. 1998. Honey bees of Africa. Berlin, Germany: Springer. Keshlaf, M. 2017. The past and present status of beekeeping in Libya. J. Apic. Res. 56(3), 190–195. Keshlaf, M. and AlFallah, H. 2018. Efficacy of modified bottom boards to control Varroa mite (Varroa destructor Anderson & Trueman) in honey bee colonies. Int. J. Sci. Res. 8(1), 465–469. Keshlaf, M. and Alfallah, H. 2019. Behavioural resistance of Libyan honey bee against Varroa mite: preliminary study. Int. J. Sci. Res. 8(7), 138–141. Keshlaf, M. and Mirwan, H.B. 2019. Population dynamics of Varroa mites and bee lice in honey bees colonies. Int. J. Sci. Res. 8(8), 1071–1075. Neumann, P. and Elzen, P.J. 2004. The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): gaps in our knowledge of an invasive species. Apidologie 35(3), 229–247. Paraïso, A.A., Agassounon, M., Daouda, I.H., Amevoin, K. and Glitho, I.A. 2012. First record of Braula cœca Nitzsch (Diptera: Braulidae), a parasite of Apis mellifera adansonnii in Benin. Int. J. Sci. Adv. Technol. 2(12), 24–30. Rosenkranz, P., Aumeier, P. and Ziegelmann, B. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103, S96–S119. Smith Jr, I.B. and Caron, D.M. 1984. Distribution of the bee louse Braula coeca Nitzsch in honey bee colonies and its preferences among workers, queens and drones. J. Apic. Res. 23(3), 171–176. Smith Jr, I.B. and Caron, D.M. 1985. Distribution of the bee louse, Braula coeca, in Maryland and worldwide. Am. Bee. J. 125(4), 294–296. Spivak, M. and Downey, D.L. 1998. Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91(1), 64–70. Strauss, U., Human, H., Gauthier, L., Crewe, R.M., Dietemann, V. and Pirk, C.W. 2013. Seasonal prevalence of pathogens and parasites in the savannah honey bee (Apis mellifera scutellata). J. Invertebr. Pathol. 114(1), 45–52. Zaitoun, S. and Al-Ghzawi, A.A.M. 2008. Daily number of bee louse (Braula coeca) in honey bee (Apis mellifera carnica and A. m. syriaca) colonies maintained under semi-arid conditions. Insect. Sci. 15(6), 563–567. | ||
| How to Cite this Article |
| Pubmed Style Keshlaf M, Mirwan HB, Ghana S, Mubrok S, Shaibi T. Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Vet J. 2023; 13(7): 834-838. doi:10.5455/OVJ.2023.v13.i7.4 Web Style Keshlaf M, Mirwan HB, Ghana S, Mubrok S, Shaibi T. Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. https://www.openveterinaryjournal.com/?mno=143404 [Access: July 03, 2025]. doi:10.5455/OVJ.2023.v13.i7.4 AMA (American Medical Association) Style Keshlaf M, Mirwan HB, Ghana S, Mubrok S, Shaibi T. Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Vet J. 2023; 13(7): 834-838. doi:10.5455/OVJ.2023.v13.i7.4 Vancouver/ICMJE Style Keshlaf M, Mirwan HB, Ghana S, Mubrok S, Shaibi T. Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Vet J. (2023), [cited July 03, 2025]; 13(7): 834-838. doi:10.5455/OVJ.2023.v13.i7.4 Harvard Style Keshlaf, M., Mirwan, . H. B., Ghana, . S., Mubrok, . S. & Shaibi, . T. (2023) Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Vet J, 13 (7), 834-838. doi:10.5455/OVJ.2023.v13.i7.4 Turabian Style Keshlaf, Marwan, Hamida B. Mirwan, Salah Ghana, Salem Mubrok, and Taher Shaibi. 2023. Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Veterinary Journal, 13 (7), 834-838. doi:10.5455/OVJ.2023.v13.i7.4 Chicago Style Keshlaf, Marwan, Hamida B. Mirwan, Salah Ghana, Salem Mubrok, and Taher Shaibi. "Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya." Open Veterinary Journal 13 (2023), 834-838. doi:10.5455/OVJ.2023.v13.i7.4 MLA (The Modern Language Association) Style Keshlaf, Marwan, Hamida B. Mirwan, Salah Ghana, Salem Mubrok, and Taher Shaibi. "Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya." Open Veterinary Journal 13.7 (2023), 834-838. Print. doi:10.5455/OVJ.2023.v13.i7.4 APA (American Psychological Association) Style Keshlaf, M., Mirwan, . H. B., Ghana, . S., Mubrok, . S. & Shaibi, . T. (2023) Prevalence of varroa mites (Varroa destructor Anderson & Trueman) and bee lice (Bruala coeca Nitzsch) in honey bee (Apis mellifera L.) colonies in Libya. Open Veterinary Journal, 13 (7), 834-838. doi:10.5455/OVJ.2023.v13.i7.4 |