| Case Report | ||
Open Vet J. 2022; 12(2): 188-191 Open Veterinary Journal, (2022), Vol. 12(2): 188–191 Case Report A long survival case of spinal nephroblastoma in a dogMunekazu Nakaichi1*, Toshie Iseri1, Hiro Horikirizono1, Harumichi Itoh2, Hiroshi Sunahara3, Yuki Nemoto3, Kazuhito Itamoto2 and Kenji Tani31Department of Veterinary Radiology, Joint Faculty of Veterinary Science, Yamaguchi University, Yamaguchi, Japan 2Department of Veterinary Small Animal Clinical Science, Joint Faculty of Veterinary Science, Yamaguchi University, Yamaguchi, Japan 3Department of Veterinary Surgery, Joint Faculty of Veterinary Science, Yamaguchi University, Yamaguchi, Japan *Corresponding Author: Munekazu Nakaichi. Department of Veterinary Radiology, Joint Faculty of Veterinary Science, Yamaguchi University, Yamaguchi, Japan. Email: nakaichi [at] yamaguchi-u.ac.jp Submitted: 30/01/2022 Accepted: 28/02/2022 Published: 19/03/2022 © 2022 Open Veterinary Journal
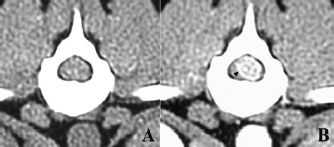
AbstractBackground: Dogs’ nephroblastoma of the spinal cord is a rare neoplastic disease, with few reports of long-term survival after surgery. We experienced that surgical treatment with postoperative radiation therapy for spinal nephroblastoma in a dog resulted in the long-term survival of 11 years. Case Description: The patient presented to our veterinary hospital because of progressive hindlimb paralysis. Based on diagnostic imaging, she was diagnosed with a thoracolumbar spinal cord tumor and was treated with surgery. The gross tumor tissue was removed after laminectomy, followed by postoperative radiation therapy using orthovoltage equipment. The histopathological features of the surgical specimen were consistent with those of previously reported spinal nephroblastoma, although infrequent mitotic figures were observed. The dog recovered well after treatment and resumed her normal walking condition. No tumor recurrence was observed on periodic follow-up magnetic resonance imaging performed 10 and 21 months after surgery. Imaging evaluation for the gradual development of hindlimb weakness was performed 9 years after surgery; however, no recurrence of tumor tissue was observed, and spondylosis deformans, probably induced after laminectomy, were identified as a possible cause. The dog died of aspiration pneumonia 11 years after surgery, independent of spinal nephroblastoma. Conclusion: To date, no clinical cases of canine spinal cord primary nephroblastoma that survived for 11 years after surgery have been reported. This case strongly suggests that providing intensive treatment for canine spinal nephroblastoma is very important. Keywords: Dog, Nephroblastoma, Radiation, Spinal cord, Surgery. IntroductionNephroblastoma in the thoracolumbar spinal cord of young dogs is a rare neoplastic disease thought to arise from ectopic renal tissue entrapped in the spinal cord during the embryonic period (Higgers et al., 2017; Rossmeisl and Pancotto, 2020). Based on previous reports, the clinical features of this disease include the age of onset in younger dogs, the site of tumor development being T10–L2, and clinical signs including hindlimb weakness and/or paralysis (Summers et al., 1988; Jeffery and Phillips, 1995; Macri et al., 1997; Vaughan-Scott et al., 1999; Sale et al., 2004; Nakade et al., 2006; Brewer et al., 2011; Liebel et al., 2011; Tagawa et al., 2020). German shepherds have been reported to be predisposed to spinal nephroblastoma (Brewer et al., 2011; Higgers et al., 2017; Rossmeisl and Pancotto, 2020). Many reports have suggested no sex predisposition; however, 9 out of 11 cases in 1 report were female (Brewer et al., 2011). Although restoration of hindlimb function and walking is possible after surgical treatment (Summers et al., 1988; Jeffery and Phillips, 1995; Macri et al., 1997; Sale et al., 2004; Brewer et al., 2011; Liebel et al., 2011; Tagawa et al., 2020), few long-term surgical outcomes have been reported. We encountered a case of canine spinal nephroblastoma that was treated with surgery and postoperative radiation therapy. As a result, the patient recovered her ability to walk and led an everyday life for approximately 11 years after the operation. We report the treatment and postoperative clinical course of this dog. Case DetailsAn 18-month-old spayed female crossbreed dog presented with progressive hindlimb paralysis over the previous months. She could not stand or walk unaided at her first visit and showed severe pain upon palpation of the thoracolumbar spine. Plain spinal radiography and routine blood tests were normal. Computed tomography of the thoracolumbar spine showed a clear contrast-enhanced mass lesion of the spinal cord at T13, suggesting the presence of spinal tumors; however, the detailed relationship between the tumor tissue and the normal spinal cord was unknown (Fig. 1). Six days later, the dog underwent surgical removal of the presumed tumor under general anesthesia, as the animal’s owner wanted aggressive treatment, including surgery.
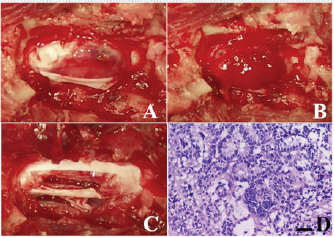
Fig. 1. Computed tomographic findings at T13 of the case. (A) Without contrast medium, (B) with an intravenous administration of contrast medium. A clear contrast-enhanced mass lesion of the spinal cord could be observed in the contrast study (B, arrow head). The dog was fixed in ventral recumbency, and a midline thoracolumbar skin incision was made. The muscles around the T13 spinous process were bluntly detached to expose the spinous process, which was then resected. Then, laminectomy was carried out to expose the spinal dura mater, which appeared slightly red. The dura was incised, and dark red tumor tissue was identified (Fig. 2A). Subsequently, the tumor tissue swelled and protruded outward (Fig. 2B). The protruding tumor tissue seemed well demarcated from the spinal cord, suggesting that the tumor was extramedullary rather than intramedullary. After a portion of the tumor tissue was biopsied, gross total resection was carried out using an ultrasonic surgical aspirator (Sonopet®; StrykerJapan KK, Tokyo, Japan) (Fig. 2C). After tumor removal, the dura was left unsutured, and the muscular, subcutaneous tissue and skin were closed. Antibiotics and corticosteroids were administered orally for 3 weeks after surgery. The dog’s postoperative recovery was unremarkable. The surgical specimen was formalin-fixed and used for routine hematoxylin and eosin (HE) staining. The surgical specimen consisted of extensive necrotic foci and tumor cell growth foci, and many tumor cells proliferated while forming a tubular structure. The cells in the tubular formation had oval nuclei arranged on the basement membrane side and clear cytoplasm on the luminal side. These histopathological features are consistent with previously reported spinal nephroblastoma (Fig. 2D) (Ohta et al., 2009; Brewer et al., 2011; Liebel et al., 2011). Mitotic figures were less frequent at all sites examined. She had a normal appetite on the day after the operation. Two weeks after surgery, the dog gradually was able to walk and resume normal activities. She received postoperative radiation (20 Gyin 5 fractions) using an orthovoltage radiation therapy device (MBR-320, Hitachi Medico, Tokyo, Japan). For the radiation treatment, the dog was anesthetized and placed in ventral recumbency. The spinal cord in which the tumor tissue was present was irradiated with a single dose of 4 Gyusing 4-cm square tubes (300 kVp, 20 mA). Prior to starting radiation therapy, a total dose of 40 Gy was planned; however, due to sufficient recovery of the dog, the owner did not wish for further treatment at the time of 20-Gy irradiation.
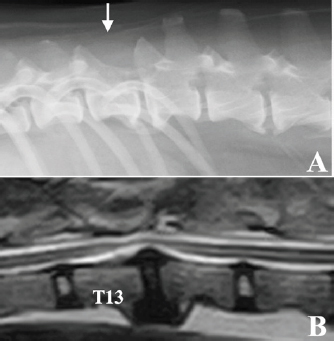
Fig. 2. Intraoperative findings of the case. (A) After durectomy, a dark reddish tumor tissue was identified. (B) After that, the tumor tissue bulged outward and seemed clearly demarcated from normal spinal cord. (C) Gross appearance of the surgical site after tumor removal using an ultrasonic surgical aspirator. (D) Histopathology of the biopsy specimen was consistent with nephroblastoma; HE stain, bar=50 µm. After treatment, the dog lived for approximately 10 years with mild proprioceptive deficits in the left hind limb. No tumor recurrence was observed on periodic follow-up magnetic resonance imaging (MRI) examinations carried out 10 and 21 months after surgery. Nine years after surgery, the dog returned to the hospital to evaluate the thoracolumbar spinal cord due to the gradual development of hindlimb weakness. Plain radiography of the thoracolumbar spine showed spondylosis deformans between T13–L1 and L2–3, with the former being consistent with the surgical site (Fig. 3A). MRI examination showed no recurrent tumor tissue in the thoracolumbar spinal cord; however, there was slight compression of the spinal cord from the ventral side at T13–L1 (Fig. 3B). This was thought to be due to the proliferation of bony tissues associated with intervertebral instability after laminectomy. The dog died 11 years after surgery for reasons unrelated to the tumor. According to the owner, the dog had vomiting of unknown cause for several months and died of aspiration pneumonia. No necropsy was performed. DiscussionRecovery of hindlimb paralysis after surgery for canine spinal nephroblastoma has been previously reported (Jeffery and Phillips, 1995; Macri et al., 1997; Brewer et al., 2011; Liebel et al., 2011; Tagawa et al., 2020). Liebel et al. (2011) noted that patients treated with surgery and/or radiation experienced longer survival than untreated ones. Although some unsuccessful surgical cases have been reported (Vaughan-Scott et al., 1999; Nakade et al., 2006), surgical treatment is generally effective. However, there are very few reports on the long-term survival of canine spinal nephroblastoma patients. Even in some reports describing long-term survivors, most of the follow-up periods were 2 years or less (Macri et al., 1997; Sale et al., 2004; Brewer et al., 2011; Liebel et al., 2011), although one case had survived for more than 3 years at the time of writing (Brewer et al., 2011). There are no detailed follow-up reports on survivors over 3 years. In our case, the spinal cord function of the dog recovered well after surgery, and the dog had lived for an additional 10 years, illustrating that long-term survival with sufficient neurologic function is possible after treatment, even in spinal nephroblastoma cases.
Fig. 3. Radiographic and MRI findings of the thoracolumbar spine of the case 9 years after surgery. (A) Radiographic features of spondylosis deformans were observed in both T13–L1 and L2–3. The spinous process of T13 was missing due to laminectomy (white arrow). (B) MRI (T2-weighted image) showed that the spinal cord was slightly compressed from the ventral side in T13–L1, probably due to the proliferation of bone tissue due to spinal instability. No tumor tissue was observed in the spinal cord. The reason for the good prognosis, in this case, remains unknown; multiple factors are likely involved. The gross appearance of the tumors at the time of surgery was well demarcated from the spinal cord with clear margins, which allowed gross total resection. Although spinal nephroblastoma has been classically reported as an extramedullary lesion (Summers et al., 1988; Vaughan-Scott et al., 1999), some cases have been reported as intramedullary (Jeffery and Phillips, 1995; Macri et al., 1997; Sale et al., 2004; Liebel et al., 2011; Higgers et al., 2017; Tagawa et al., 2020). In a series of 10 canine spinal nephroblastoma cases, Liebel et al. (2011) reported a longer survival in dogs with extramedullary lesions. Intramedullary tumor development may detrimentally affect neurologic outcomes and survival by precluding complete surgical resection, resulting in local recurrence. The most important reason for the long survival in our case was likely the relatively localized presence of extramedullary tumor tissue. Besides the difference in the site of tumor development, the difference in malignancy of the tumor cells is considered one of the factors responsible for the good prognosis in this case. In one report of canine spinal nephroblastoma focusing on tumor cell growth analysis, the case was highly malignant based on its high potential for growth and invasion (Ohta et al., 2009), indicating some differences in malignancy among tumor tissues of the same histopathological type. Recently, intraspinal metastasis of spinal cord nephroblastoma was reported, which is considered a highly malignant finding (Terrell et al., 2000). Since the histopathological examination in our case showed infrequent mitotic figures, this case might have had relatively low malignancy, leading to a good prognosis. Although gross total resection was achieved in this case, residual tumor cells may have remained. Therefore, postoperative treatment is necessary. We elected to perform local radiation therapy because the effectiveness of chemotherapy has not been reported in dogs. In this case, only 20 Gy was applied after surgery at the owner’s request. However, our case cannot draw conclusions regarding postoperative treatment, and further evaluations are needed to determine suitable adjunctive treatments after surgical resection of this disease. This case fell into a serious condition, such as being unable to stand up due to a spinal cord tumor at an early age; however, the dog was able to almost reach the end of her life while maintaining a normal walking ability using a combination of surgery and radiation therapy. This case strongly suggests that providing intensive treatment for canine spinal nephroblastoma is very important. AcknowledgmentThe authors thank the Edanz Group (https://en-author-services.edanz.com/ac) and Editage (www.editage.com) for editing a draft of this manuscript. Conflict of interestNone of the authors of this article has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper. Authors’ contributionsThe diagnostic imaging at the first visit of this case was performed by Munekazu Nakaichi. Surgical treatment and postoperative management of the case were performed by Munekazu Nakaichi, Hiro Horikirien, Harumichi Ito, and Hirosi Sunahara. Postoperative radiation therapy was performed by Toshie Iseri and Yuki Nemoto. Munekzu Nakaichi, Kenji Tani, and Kazuhito Itamoto were involved in the writing of the first draft, and all authors read and approved the final draft. ReferencesBrewer, D.M., Cerda-Gonzalez, S., Dewey, C.W., Diep, A.N., Van Horne, K. and McDonough, S.P. 2011. Spinal cord nephroblastoma in dogs: 11 cases (1985-2007). J. Am. Vet. Med. Assoc. 238, 618–624. Higgers, R.J., Bollen, A.W. and Dickinson, P.J. 2017. Tumors of the nervous system. In Tumors in domestic animals, 5th ed. Ed., Meuten, D.J. Hoboken, NJ: Wiley Blackwell, pp: 834–891. Jeffery, N.D. and Phillips, S.M. 1995. Surgical treatment of intramedullary spinal cord neoplasia in two dogs. J. Small Anim. Pract. 36, 553–557. Liebel, F.X., Rossmeisl, J.H., Jr., Lanz, O.I. and Robertson, J.L. 2011. Canine spinal nephroblastoma: long-term outcomes associated with treatment of 10 cases (1996-2009). Vet. Surg. 40, 244–252. Macri, N.P., Van Alstine, W. and Coolman, R.A. 1997. Canine spinal nephroblastoma. J. Am. Anim. Hosp. Assoc. 33, 302–306. Nakade, T., Inoue, A., Shimazaki, H., Miyoshi, K., Takeuchi, N., Kadosawa, T., Akihara, Y., Taniyama, H. and Ishida, O. 2006. Spinal nephroblastoma in a miniature Dachshund. J. Vet. Med. Sci. 68, 1383–1385. Ohta, G., Kobayashi, M., Sakai, H., Masegi, T. and Yanai, T. 2009. Proliferative potential of a spinal nephroblastoma in a young dog. J. Toxicol. Pathol. 22, 79–82. Rossmeisl, J.H., Jr. and Pancotto, T.E. 2020. Tumors of the nervous system. In Withrow and MacEwen’s small animal clinical oncology, 6th ed. Eds., Vail, D.M., Thamm, D.H. and Liptak, J. St. Louis, MO: Elsevier, pp: 657–674. Sale, C.S., Skerritt, G.C. and Smith, K.C. 2004. Spinal nephroblastoma in a crossbreed dog. J. Small Anim. Pract. 45, 267–271. Summers, B.A., deLahunta, A., McEntee, M. and Kuhajda, F.P. 1988. A novel intradural extramedullary spinal cord tumor in young dogs. Acta. Neuropathol. 75, 402–410. Tagawa, M., Shimbo, G., Tomihari, M., Yanagawa, M., Watanabe, K.I., Horiuchi, N., Kobayashi, Y. and Miyahara, K. 2020. Intramedullary spinal nephroblastoma in a mixed breed dog. J. Vet. Med. Sci. 82, 917–921. Terrell, S.P., Platt, S.R., Chrisman, C.L., Homer, B.L., de Lahunta, A. and Summers, B.A. 2000. Possible intraspinal metastasis of a canine spinal cord nephroblastoma. Vet. Pathol. 37, 94–97. Vaughan-Scott, T., Goldin, J. and Nesbit, J.W. 1999. Spinal nephroblastoma in an Irish wolfhound. J. S. Afr. Vet. Assoc. 70, 25–28. | ||
| How to Cite this Article |
| Pubmed Style Nakaichi M, Iseri T, Horikirizono H, Itoh H, Sunahara H, nemoto Y, Itamoto K, Tani K, . A long survival case of spinal nephroblastoma in a dog. Open Vet J. 2022; 12(2): 188-191. doi:10.5455/OVJ.2022.v12.i2.5 Web Style Nakaichi M, Iseri T, Horikirizono H, Itoh H, Sunahara H, nemoto Y, Itamoto K, Tani K, . A long survival case of spinal nephroblastoma in a dog. https://www.openveterinaryjournal.com/?mno=140121 [Access: April 26, 2024]. doi:10.5455/OVJ.2022.v12.i2.5 AMA (American Medical Association) Style Nakaichi M, Iseri T, Horikirizono H, Itoh H, Sunahara H, nemoto Y, Itamoto K, Tani K, . A long survival case of spinal nephroblastoma in a dog. Open Vet J. 2022; 12(2): 188-191. doi:10.5455/OVJ.2022.v12.i2.5 Vancouver/ICMJE Style Nakaichi M, Iseri T, Horikirizono H, Itoh H, Sunahara H, nemoto Y, Itamoto K, Tani K, . A long survival case of spinal nephroblastoma in a dog. Open Vet J. (2022), [cited April 26, 2024]; 12(2): 188-191. doi:10.5455/OVJ.2022.v12.i2.5 Harvard Style Nakaichi, M., Iseri, T., Horikirizono, H., Itoh, H., Sunahara, H., nemoto, Y., Itamoto, K., Tani, K. & (2022) A long survival case of spinal nephroblastoma in a dog. Open Vet J, 12 (2), 188-191. doi:10.5455/OVJ.2022.v12.i2.5 Turabian Style Nakaichi, Munekazu, Toshie Iseri, Hiro Horikirizono, Harumichi Itoh, Hiroshi Sunahara, Yuki nemoto, Kazuhito Itamoto, Kenji Tani, and . 2022. A long survival case of spinal nephroblastoma in a dog. Open Veterinary Journal, 12 (2), 188-191. doi:10.5455/OVJ.2022.v12.i2.5 Chicago Style Nakaichi, Munekazu, Toshie Iseri, Hiro Horikirizono, Harumichi Itoh, Hiroshi Sunahara, Yuki nemoto, Kazuhito Itamoto, Kenji Tani, and . "A long survival case of spinal nephroblastoma in a dog." Open Veterinary Journal 12 (2022), 188-191. doi:10.5455/OVJ.2022.v12.i2.5 MLA (The Modern Language Association) Style Nakaichi, Munekazu, Toshie Iseri, Hiro Horikirizono, Harumichi Itoh, Hiroshi Sunahara, Yuki nemoto, Kazuhito Itamoto, Kenji Tani, and . "A long survival case of spinal nephroblastoma in a dog." Open Veterinary Journal 12.2 (2022), 188-191. Print. doi:10.5455/OVJ.2022.v12.i2.5 APA (American Psychological Association) Style Nakaichi, M., Iseri, T., Horikirizono, H., Itoh, H., Sunahara, H., nemoto, Y., Itamoto, K., Tani, K. & (2022) A long survival case of spinal nephroblastoma in a dog. Open Veterinary Journal, 12 (2), 188-191. doi:10.5455/OVJ.2022.v12.i2.5 |