| Original Article | ||
Open Vet J. 2023; 13(3): 307-321 Open Veterinary Journal, (2023), Vol. 13(3): 307–321 Original Research Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent pars distalis, pars intermedia and pars nervosaNaief Dahran1 and Wael A. M. Ghonimi2*1Department of Anatomy, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia 2Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Wael A. M. Ghonimi. Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: WAGhonemy [at] vet.zu.edu.eg. Submitted: 25/12/2022 Accepted: 11/02/2023 Published: 10/03/2023 © 2023 Open Veterinary Journal
AbstractBackground: Hypophysis cerebri is considered the master endocrine gland as it plays a critical role in influencing and controlling the vitality of other endocrine organs via several hormones secretion. Aim: The present study was performed to clarify the localization of Wulzen's cone (WC) within sheep hypophysis and cytodifferentiation of the glandular cells filling cone parenchyma with particular emphasis on the cone correlations with adjacent pars distalis (pd), pars intermedia (pi), and pars nervosa (pn). Methods: Pituitaries were collected and processed histologically, then subjected to different combinations of special stains; Br-AB- OFG., PFA-AB-PAS-OG., PAS-Orange G., Orange G- Acid Fuchsin- Light Green, Bielschowsky technique, Masson's trichrome & Gomori's reticulin. Results: A sagittal section through the pituitaries revealed a well-developed cone of glandular cells protruding from the pi like a tongue plate towards the hypophyseal cleft in the neighborhood of the pd and behind the pn. Resembling the pd, various glandular cells were distinguished in the cone; chromophobes and chromophils of acidophils & basophils. The cone is mainly formed from acidophils intermingled with the chromophobes. Meanwhile, basophils were primarily localized at the most anterior & posterior parts of the cone. In front of the cone, pd were localized, resembling a wing-shaped and filled with several categorized glandular cells; chromophobes and chromophils. Upper to the cone, pi were localized and composed mainly of weakly basophilic cuboidal or polygonal cells arranged in parallel cords or follicles. Behind the cone, pn was localized as a ventral outpouching of the brain floor-like water drop. Unlike the cone, it was devoid of any glandular secretory cells or nerve cells but consisted mainly of unmyelinated nerve fibers, herring bodies, and pituicytes. Conclusion: WC is present and well-developed in sheep adenohypophysis. Various glandular cells were distinguished, filling the cone, chromophobes, and chromophils of acidophils & basophils that were typically similar to the glandular cells of pd but with different distributions. Keywords: Cephalic cone, Wulzen cone, Pars distalis, Pars intermedia, Pars nervosa. IntroductionThe pituitary gland plays a central role in regulating various physiological processes of the body through its interplay between the endocrine and nervous systems. It is considered a master gland because its secretion controls the activity of most other hormone-secreting glands. It senses the needs of the body and sends signals to various glands and organs via the body to control and regulate their function. Also, it secretes different hormones into the bloodstream that act as messengers, transmitting information from the hypophysis to far cells and controlling their activity (Martinez-Lage, 2011; Marieb and Hoehn, 2012). Due to its dual origin, the hypophysis cerebri is categorized into two glands; anterior hypophysis cerebri (adenohypophysis) derives from oral ectoderm, and posterior hypophysis cerebri (neurohypophysis) derives from neural ectoderm that anatomically linked but functionally different (Getty, 1975). Adenohypophysis is subdivided into the pars distalis (pd), pars intermedia (pi), and pars tuberalis. Meanwhile, neurohypophysis is subdivided into the infundibular stem, median eminence, and pars nervosa (pn), neural lobe (Eurell and Frappier, 2006). An intraglandular cleft affects a partial separation between pi and distalis. Through this cleft, a cephalic cone of glandular cells is frequently projected from the pi toward the pd, passing through the intraglandular cleft (Getty, 1975). Firstly, this cone was identified by Rosalind Wulzen and currently bears his name, Wulzen cone (Wulzen, 1914), which is described as a cone-shaped structure connected to the intermediate part of the bovine adenohypophysis. This structure was apparent about "one-third of the distance from the dorsal to the ventral end of the residual lumen. It houses "acidophilic" cells comparable to those in the anterior lobe (Wulzen, 1914). A minor amount of literature is available on the Wulzen’s cone (WC) of sheep pituitary gland. Hence the present study was performed to clarify the localization of the cephalic cone within the sheep hypophysis and cytodifferentiation of the glandular cells filling the cone parenchyma with particular emphasis on the cone correlations with adjacent pd, pi, and pn. Material and MethodsCollection of samplesPituitary glands of ten apparently healthy, freshly slaughtered mature male Egyptian Baladi sheep, Ovis aries, over 1-year-old, were collected from the Zagazig abattoir in Sharkia province, Egypt, for the histological studies. Tissue preparationFor histological and histochemical investigation The pituitaries were quickly removed after the slaughter and section of the head. They were parasagittally sectioned in two parts and immediately fixed in 10% buffered neutral formalin and Bouin’s fluid. The fixed specimens were processed using the standard histological techniques, dehydrated in ascending grades of ethanol series, cleared in benzene, and embedded in paraffin. Next, 4 to 6 μm thick sagittal sections were prepared and mounted on glass slides. These were dewaxed in xylene, hydrated in descending grades of ethanol series, and stained with the following stains: 1. Harris’s Hematoxylin and Eosin (H&E) for general structure. 2. Periodic Acid Schiff (PAS)- Orange G method for detection of basophils and acidophils, respectively (Pearse, 1985). 3. Orange G- Acid Fuchsin- Light Green (OFG) combination dyes for detecting acidophils, basophils, and collagen fibers, respectively. 4. Bromin Alcian Blue Orange G Fuchsin (Br-Ab- OFG) method for detecting basophils, acidophils, and chromophobes. This method is the development and improvement of the PFA-AB-PAS-OG (Performic acid- Alcian Blue - Periodic Acid Schiff- Orange G). This can distinguish two types of basophil cells (BC), S cells and R cells, through alcian blue staining. Firstly, S cells stained blue due to having sulfur-containing amino acids. Meanwhile, R cells stained magenta due to their ability to resist oxidation. Moreover, alcian blue can stain neurosecretory substances in the posterior pituitary as keratin and mast cell granules (Slidders, 1961). 5. PFA AB PFA-AB-PAS-OG method for detection of basophils, acidophils, and chromophobes. 6. Blue Masson’s trichrome stain for the detection of collagen fibers and to distinguish acidophils from basophils. 7. Gomori’s reticulin stain for detecting reticular fibers, nerve fibers, and argyrophilic cells (neuroendocrine cells which react with silver stain). 8. Bielschowsky technique is a silver staining method used in histochemistry to visualize nerve fibers (Stahnisch, 2015). The methods of processing and staining were carried out according to Bancroft and Gamble (2008). For immunocytochemistry The immunocytochemical study was performed to detect and identify gonadotrophic cells (GC). It was carried out on the paraffin sections by the streptavidin-biotin complex technique. In addition, deparaffinized sections were stained by an indirect immunoperoxidase technique (Polak and Noorden, 1997). 5 µm sections were cut, placed on positively charged slides, and aired dried at room temperature. The paraffin sections were deparaffinized and rehydrated with a series of descending grades of ethanol and then washed with phosphate buffer saline (PBS) 3 times for 5 minutes each (3 × 5 minutes each). Then, the sections were immersed in 0.1 M citrate buffer solution (pH=6) for 10 minutes using a microwave (600 Watt), where the antigen retrieval was carried out. Sections were washed with PBS 3 × 5 minutes each and incubated with 3% hydrogen peroxide (H2O2) in absolute methanol for 30 minutes at 4°C to eliminate the endogenous peroxidase activity. In addition, sections were incubated with a blocking solution formed from PBS containing 10% (normal goat serum; life Technology, Germany) and 0.1% Triton-X-100 (Sigma, Germany) to minimize nonspecific labeling. The sections were incubated overnight at 4°C with the specific primary antibodies diluted in blocking solution [rabbit polyclonal anti-follicular stimulating hormone (FSH), 1:1,000, and rabbit polyclonal anti-luteinizing hormone (LH), dilution 1:1,000, Biogenesis Ltd., Sandown, NH]. For negative control sections, PBS was used instead of the primary antibody. Then the sections were washed in PBS for 3 × 5 minutes each. The biotin-conjugated secondary antibody was incubated at room temperature for 1 hour in PBS containing biotinylated goat anti-rabbit IgG (1:300; Molecular probes). Then the sections were washed in PBS for 3 × 5 minutes each and subsequently incubated with streptavidin-peroxidase for 30 minutes at approximately 25°C. The final chromogen (streptavidin-biotin complex; immunopositive reactions) were developed and visualized using a 3,3′-diaminobenzidine tetrahydrochloride- H2O2 solution, pH 7.0, for 3 minutes according to the package insert, which produces an insoluble brown pigment. The sections were then counterstained with Harris hematoxylin, dehydrated with ascending grades of ethanol, cleared with Rothihistol, and mounted using entellan (Merck, Germany). Most of the stained sections were photographed by a digital camera (Canon) connected to a light microscope (Zeiss) in the Histology and Cytology department, Zagazig University, Zagazig, Egypt. And also, some stained sections were photographed using a digital Dsc-W 130 super steady cyber shot camera connected to an SK W/5× Correct microscope from the parasitology department, Zagazig University, Zagazig, Egypt. Ethical approval Not required for this type of study. ResultsThe pituitary gland (hypophysis cerebri) is considered the master endocrine gland as it plays a critical role in influencing and controlling the activities of other endocrine glands via several hormones secretion. However, the hypophysis itself is under the control of the hypothalamus. Histologically, the pituitary gland was surrounded by a thin fibrous connective tissue capsule derived from the dura mater (Fig. 1a). The loose connective tissue between the capsule and sphenoid bone housing dense plexus of blood vessels (BVs) that are surrounding the pituitary gland. From all the examined sections, these plexus were observed to be composed mainly of networks of medium-sized arteries with well-developed undulating internal elastic lamina and thick muscular walls (Fig. 1a–c). The hypophysis parenchyma has two distinct zones, the glandular zone; adenohypophysis, and the neural area; neurohypophysis, which has different histological and cytological components. But, from the sagittal sections, the sheep pituitary gland appeared to consist of distinct lobes; some of them were well-developed, like the pi that occupies the most central portion of the section in the form of a cord. Distal to the pi, a cone of glandular epithelial cells; WC protruded as a tongue plate of pi or bulb-shaped structure towards the lumen of the hypophyseal cleft. In front of the cone and pi, wing-shaped glandular epithelial cells, pd, were localized, occupying the most anterior part of the gland (Fig. 1a). Meanwhile, behind the cone and pi, pale eosinophilic area; pn was localized occupying the most posterior part of the gland as water- drop shape and devoid from any glandular cells (Figs. 1a & 9a and b).
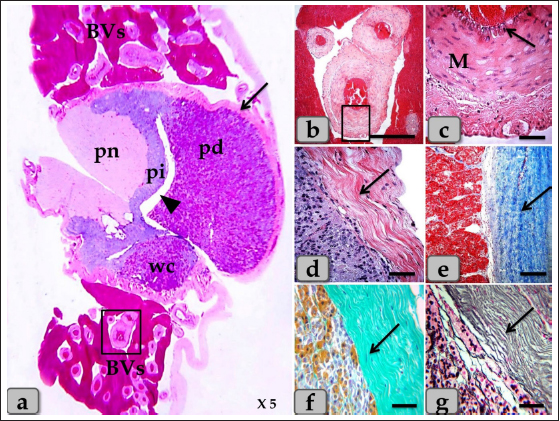
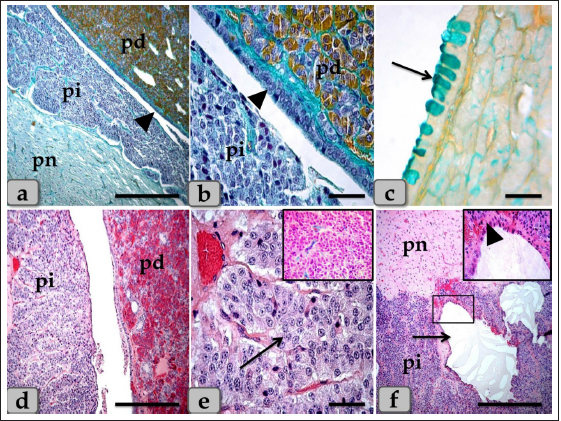
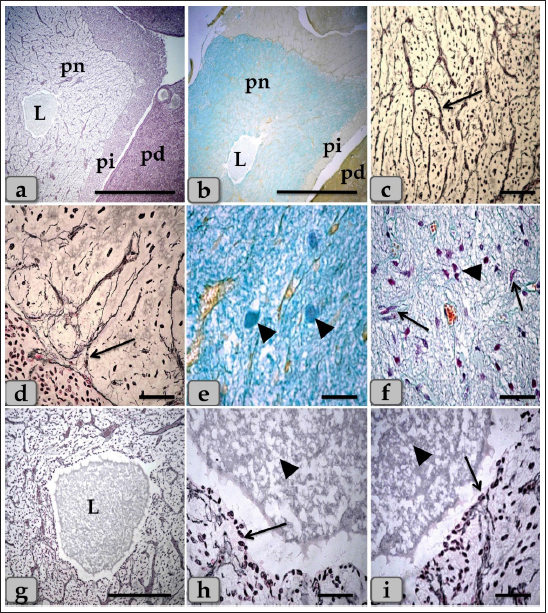
Fig. 1. (a) A panoramic view (sagittal section) of the sheep pituitary gland showing the gland capsule (arrow), pd, pi, pi WC, pn, hypophyseal cleft (arrowhead) and BVs. (b&c) Higher magnifications of the (a) show numerous medium-sized arteries with a thick muscular wall (M) and well-developed undulating internal elastic lamina (arrow). (d) showing the fibrous capsule that rich with fibroblast cells (arrow). (e&f) showing the collagen fibers of the capsule (arrow). (g) showing the reticular fibers of the capsule (arrow). Stain: (a–d) H&E, (e) Blue Masson’s trichrome, (f) OFG, & (g) Gomori’s reticulin. Scale bars: b=300 µm & c–g=40 µm. (The magnification power of (a) is ×5 under SK W/5× Correct microscope). In front of the cone, the pd was the largest part of the whole gland, wing-shaped, forming the main bulk of the adenohypophysis and surrounding the pi except the top of the gland. It was covered with a relatively thick fibrous connective tissue capsule (Fig. 1d) that is mainly formed from collagen (Fig. 1e and f) & reticular fibers (Fig. 1g) and rich with fibroblast cells (Fig. 1d) The pd is considered the glandular epithelial portion of the pituitary gland as its parenchyma consists mainly of different glandular epithelial cells that are arranged in clusters, nests (Fig. 2a–e), and sometimes irregular branching cords (Fig. 1f). These glandular cells were supported and laterally separated from each other’s by a thin loose connective tissue septa; mainly collagen (Fig. 2d) & delicate reticular fibers (Fig. 4c) and rich with many sinusoidal blood capillaries (Fig. 3c) to support & supply the gland, in addition, to conduct the secretory material, hormones out of the gland. According to the H&E and special stains affinities of the secretory material within the cells, the pd revealed three different cell types; chromophobe cells (CC), acidophil cells (AC) & BC. CC were small-sized, rounded, or polygonal shaped and arranged in clusters. With H&E and special stains, their cytoplasm was devoid of any secretory granules, so it appeared lighter or colorless, which was considered the most characteristic feature of these cells. Their nuclei were oval or rounded with a larger N/C ratio (Nucleus/ Cell ratio) and were centrally located with distinct nucleolus (Fig. 2a–c). They are a reserve population of cells (stem-like cells) from which any of the various fully granulated cells can be formed, so they are considered the inactive form of chromophil cells. AC were larger than the chromophobes; most were located in the central and lateral parts of pd. They were round or ovoid-shaped, sometimes triangles, and had medium-sized in between chromophobes and basophils. Their nuclei were large, vesicular, rounded, or oval, centrally located with distinct nucleolus (Fig. 2a–d). Its cytoplasm was voluminous and contained large-sized granules that were acidophilic with H&E (Fig. 2a and b), red with Masson’s trichrome (Fig. 2c and d), and orange with orange G (Figs. 2e and f & 3a and b) and so, were described as orangeophils but negative with PAS reaction (as, no carbohydrates). With special stains like Br-Ab- OFG, the acidophils could be distinguished into two types; Somatotrophic and Lactotrophic cells (LC). Somatotrophic cells (SC) were the most common cell type in the pd. They were distributed either singly or commonly in clusters throughout the pd. Their shape varied from rounded, ovoid, or polygonal. Their nuclei were vesicular, rounded or oval shaped, and centrally located with the distinct nucleolus. Its cytoplasm appeared granulated and had numerous large, spherical secretory granules that stained acidophilic with H&E (Fig. 2a and b), red with Masson’s trichrome (Fig. 2c and d), and orange with orange G (Figs. 2e and f & 3a and b) but failed to stain with PAS. LC, Mammotrophic cells, were typically similar to SC in shape and staining affinity but relatively more minor in size and were arranged in clusters mainly but could be found scattered individually within the pd (Fig. 2). BC were relatively few than acidophils but larger in size. They were more concentrated in the central portion of the pd. According to the reactivity of BC to Br-AB- OFG, they were classified into two main classes; S-cells were basophils whose secretory granules contained Alcian blue demonstrated sulfur-containing amino acid that stained dark green blue and included Adrenocorticotrophs. R-cells were basophils whose secretory granules resist oxidation & Alcian blue but stained magenta red, including thyrotrophs cells and gonadotrophs cells. Basophils were rounded, oval, or polygonal shaped with oval or rounded nuclei. Its cytoplasm had small-sized secretory granules and had a slightly basophilic appearance with H&E (Fig. 2a and b), dark blue with alcian blue stain (Figs. 2e & 3a and b), and magenta or purple with PAS (Fig. 3c and d) because of the high content of glycoprotein, and subsequently, with special dyes, the basophil could be differentiated to three different types; Thyrotrophic cells (TC), Adrenocorticotrophic cells (ACTH) & GC. ACTH were relatively few, smaller-sized than other basophils, and scattered throughout the pd. It varied in shape from elliptical, pyramidal to stellate or polygonal, sometimes, possessed processes that ended on the walls of sinusoids. Their nuclei were round or oval shaped, central position with prominent nucleoli, and with a small N/C ratio. Their cytoplasmic was basophilic and stained dark green-blue after the Br-AB-OFG technique as they were rich with sulfur-containing amino acid (Figs. 2e & 3a and b). TC, unlike other kinds of cells, were more frequently present in clusters or cords, which were localized mainly at the center of pd. They were larger stellate, pyramidal, polygonal, or rounded in shape. The nuclei were oval or rounded with a small N/C ratio. Their cytoplasm was relatively more voluminous and contained excellent cytoplasmic granules that stained magenta red with Br-AB-OFG methods (Figs. 2f & 3a and b) and magenta with PAS (Fig. 3c and d). GC, these cells secret both FSH and LH under different conditions. Immunohistochemical stains against anti-FSH antibody (Fig. 3e) and anti-LH antibody (Fig. 3f) were observed to vary in size from medium to large and round, oval or long strip or angular in shape and may be found near the blood capillaries. They were distributed randomly throughout the pd. The nuclei were round or oval and sometimes irregular in shape with an eccentric position. Their cytoplasm had a lot of secretory granules with variable sizes (Fig. 3e and f). But, with Br-AB-OFG methods or PAS, they were typically similar to TC, so we used immunohistochemical stains to distinguish.
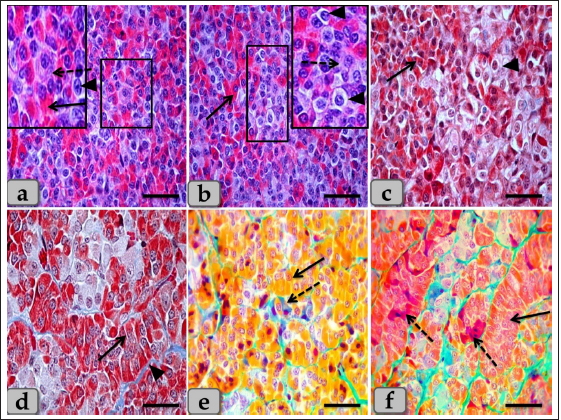
Fig. 2. Illustrative photomicrograph of the pd (a–c) Showing the basophils (dashed arrow), acidophils (arrow), and chromophobes (arrowhead). (d) showing the acidophils (arrow) and intercellular collagen fibers (arrowhead). (e) showing the acidophils or orangeophils; somatotrophs and mammotrophs (arrow), basophils; adrenocorticotrophs (dashed arrow). (f) showing the orangeophils; somatotrophs and mammotrophs (arrow), basophils; thyrotrophs (dashed arrow). Stain: (a,b) H&E, (c,d) Blue Masson’s trichrome, (e,f) Br-Ab- OFG. Scale bars: a–f=40 µm. Some of the glandular epithelial cells appeared, forming a well-defined follicle (Fig. 4a,b and d) whose lumina was filled with large globules and masses of colloidal materials forming colloid filled cyst which reacted positively with alcian blue stain (Fig. 4e and f). In addition, this follicle was observed lined with simple cuboidal epithelium with rounded, central nuclei or columnar epithelium to pseudostratified columnar type in some areas of the strands intermingled with numerous goblet-like cells filled with mucoid substances (Fig. 4b and d). Moreover, a fine reticular network was entwisted around the parenchymal epithelial cells, either arranged in clusters or follicles, and the blood capillaries (Fig. 4c). The pd margin facing pi was covered with simple cuboidal or columnar epithelium to pseudostratified stratified columnar type in some areas (Fig. 5a and b) with interposing goblet-like cells filled with mucoid substances that gave a strongly positive reaction with AB stain (Fig. 5c). The hypophyseal cleft was obvious between pd and pi (Fig. 5b and c), and in some sections, it was filled with amorphous or gelatinous colloid-like substances. Upper to the cone, the pi was a narrow region between the pd and pn, which surrounded the pn, excluding the hypophysis stalk. It was separated partially from the pd by the hypophyseal cleft but closely associated with the pn (Fig. 5a, b and d). The pi was observed housing fewer BVs than the highly vascular pd. It was well-developed in sheep, and with H&E stain, it was filled only with weakly basophilic large cuboidal or polygonal cells with round or oval nuclei. They were arranged in parallel rows, cords (Fig. 5d and e), or in follicles containing a colloid-like substance (colloid-filled cysts) that were frequently seen distributing throughout the pi (Fig. 5f). Furthermore, some follicles appeared to be filled with amorphous substances, while others had only a little, or devoid of any materials in some of the examined sections. Several colloid-filled cysts of variable size and shape were observed within the pi and lined with simple cuboidal to the pseudostratified columnar epithelium (Fig. 5f). With alcian blue and PAS stain; the colloid clarified strongly positive reaction with two stains. Meanwhile, with PFA–AB-PAS- Orange G, these cells stained brightly pink and called melanotropes (Inset box of Fig. 5e).
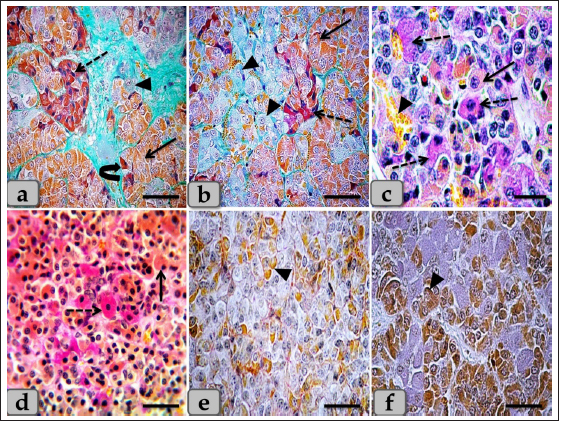
Fig. 3. Illustrative photomicrograph of the pd (a,b) Showing the acidophils or orangeophils (arrow), basophils; adrenocorticotrophs (arrowhead), thyrotrophs (dashed arrow), gonadotrophs (curved arrow). (c,d) Showing the orangeophils (arrow) and basophils (dashed arrow). (e,f) showing positive immunohistochemical reactivity of gonadotrophs against anti-FSH antibody (e) and anti-LH antibody (f). Stain: (a,b) Br-Ab- OFG, (c,d) PAS-orange G, (e,f) immunohistochemical stain against anti-FSH antibody and anti-LH antibody, respectively. Scale bars: a,b,d–f=40 µm & c=20 µm. WC (Cephalic cone of pi) or tongue plate of pi A sagittal section through the anterior part of the pituitary gland of an adult male sheep revealed a cone of glandular epithelial cells protruding from the pi towards the lumen of the hypophyseal cleft in the neighborhood of the pd. This cone looked like a tongue plate of pi or bulb-shaped structure (Figs. 1a & 6a). While in the caudal part of the pituitary gland, the hypophyseal cleft intervenes between the pi and the pd without the glandular cone of pi. WC was elongated and observed free along most of its length. But, posteriorly, the WC was continuous with the posterior region of the pi without any line of demarcations (Fig. 6a). This cone was covered externally with lining epithelium of simple cuboidal to stratified epithelium on the side of the hypophyseal cleft (Fig. 6a–c). Meanwhile, its free border was supported externally by the connective tissue capsule of the pituitary gland (Fig. 6a). By using H&E and specific stains like “Br- Ab- OFG,” Masson’s trichrome, and the PAS-orange G technique, the arrangement and histochemical appearance of the glandular epithelial cells forming this cone parenchyma revealed acidophils that filled the majority of the cone intermingled with chromophobes (Fig. 6b and d). Meanwhile, basophils were mainly localized at the most anterior and posterior or caudal part of the cone (Fig. 6a and b). However, a few individually distributed basophils were also observed in the central part between the acidophils (Figs. 6f & 7a and b). All cone glandular epithelial cells were typically similar to the glandular cells of pd but with different distributions and arrangements. There were clusters and sporadically distributed orangeophilic cells that mainly condensed at the central part of the cone; in between them, unstainable cells, chromophobes, and individual basophils were present (Fig. 6f). Orangeophilic cells, SC & Mammotrophic cells were the most common and predominant cell types in the cone. They were typically similar to each other in structure and staining affinity, but the mammotrophs were relatively smaller in size than the somatotrophs, and their cytoplasmic granules were smaller and relatively coarser. They were distributed either singly or commonly in clusters throughout the cone, especially the central part. They were varied from rounded, oval, or polygonal shaped with single, central, vesicular, round, or oval nuclei with distinct nucleolus. Its cytoplasm had numerous large, spherical secretory granules stained eosinophilic with H&E (Fig. 6d), red with Masson’s trichrome (Fig. 6e), and orange with Br. Ab- OFG (Figs. 6f & 7a and c) failed to stain with PAS.
Fig. 4. A sagittal section of the sheep pituitary gland (a) showing the pd, pi, pn, hypophyseal cleft (arrowhead), and follicles of the glandular epithelial cells (arrow). (b) Higher magnification of (a) showing the glandular epithelial cells lining the follicles (arrow) and the covering epithelium of the pd (arrowhead). (c) showing a fine reticular network that entwisted around & supported the parenchymal epithelial cells of pd (arrow). (d) showing the reticular fibers supporting the follicle (arrow) and the glandular epithelial cells lining the follicles intermingled with goblet-like cells (arrowhead). (e,f) showing the colloid-filled cysts in the pd that were filled with strongly alcian blue positive numerous globules with different sizes (arrow). Stain: (a–d) Gomori’s reticulin, (e,f) PFA-AB-PAS-OG. Scale bars: a,e=300 µm & b–d,f=40 µm. Meanwhile, basophils were arranged in clusters in the most anterior & caudal parts of the cone (Figs. 6b and 7c). In addition, sporadic or individual basophils were also recognized in between acidophils (Figs. 6f & 7a and b). They were less numerous but larger in size than the acidophils and chromophobes. They were rounded, ovoid, or polygonal in shape. Their nuclei were oval or rounded in shape and centrally located with the distinct nucleolus. Its cytoplasm had small-sized secretory granules and was stained slightly basophilic appearance with H&E (Fig. 6b), dark green blue after Br-AB-OFG technique; ACTH (Fig. 6f), and magenta red with Br-AB-OFG methods (Figs. 6f & 7a) or magenta or purple with PAS; Thyrotrophs & gonadotrophs (Fig. 7b), because of the high content of glycoprotein. But, to differentiate between them, TC were more frequently present in clusters. They were larger stellate, pyramidal, polygonal, or rounded in shape. The nuclei were oval or rounded with a small N/C ratio. Their cytoplasm was relatively more voluminous and contained very fine cytoplasmic granules. Meanwhile, GC were observed to be variable in size and shape and individually distributed randomly throughout the cone (Fig. 7a). But, with immunohistochemical stains against anti-FSH antibody (Fig. 8a and b) and anti-LH antibody (Fig. 8c and d), gonadotrophs were observed very few and individually distributed within the cone (Fig. 8a–d), but some areas were observed devoid from any positive immune reactions against FSH and LH antibodies (Fig. 8e and f). With high magnification, they were observed to be variable in size from medium to large and oval or angular, or pyramidal in shape. The nuclei were round or oval in shape. Their cytoplasm had a lot of secretory granules with variable sized that gave strongly positive immune reactions (Fig. 8a).
Fig. 5. A sagittal section of the sheep pituitary gland (a) showing the pd, pi, pn, and hypophyseal cleft (arrowhead). (b) showing the covering epithelium of the pd margin facing the pi at the surface of the pituitary cleft (arrowhead). (c) showing the scattered goblet-like cells intermingled between the pd covering epithelial cells (arrow). (d) showing the pd & pi. (e) Higher magnification of the pi showing parallel rows of weakly basophilic glandular cells (arrow). Meanwhile, the inset box shows brightly pink glandular cells; melanotropes with a PFA-AB-PAS-OG stain. (f) showing the colloid-filled cysts in the pi (arrow) with its lining epithelium (arrowhead). Stain: (a,b) Br-Ab- OFG, (c) PFA-AB-PAS-OG, (d–f) H&E. Scale bars: a,d,f=300 µm & b,c,e=40 µm. Resembling the pd, a number of follicles with different sizes and shapes were scattered within the cone, especially at the periphery. They appeared lined with simple cuboidal, to columnar to pseudostratified columnar ciliated epithelium intermingled with large, distinct mucous secreting cells, goblet-like cells that filled with alcian blue positive mucoid substances. Moreover, the follicle lumen was also filled with Ab-positive colloidal mucoid materials forming a structure like colloid-filled cysts (Fig. 7d–f). Behind the cone, the pn was raised as a ventral outpouching of the floor of the brain and retained its connection with the brain by an infundibular stalk. It was observed as water- drop shape, located in the center of the gland and surrounded by the pi & pd. Unlike the cone, pn was observed to be devoid of any glandular secretory or nerve cells (Fig. 9a and b). It consisted mainly of unmyelinated nerve fibers (Fig. 9c and d), herring bodies (corpusculum neurosecretorium) (Fig. 9e), pituicytes (glia cells, supporting cells), blood capillaries (Fig. 9f), and all the previous were supported by reticular fibers. Unmyelinated nerve fibers were the axons of neurosecretory nerve cell bodies located in the supraoptic and paraventricular nuclei of the hypothalamus. These axons direct the downward forming tract and the hypothalamo-hypophyseal tract transporting the neurosecretory materials. They were oriented in many different directions. They ended with highly branching terminal ends resembling tree roots invaginated at the periphery of pn, forming a palisade zone (Fig. 9c and d), and others ended with herring bodies. Herring bodies were dilated terminal ends of unmyelinated nerve fibers with different shapes and sizes. They were considered as the storage swellings of the neurosecretions. The neurosecretory material of herring bodies was stained deep eosinophilic by H&E. Furthermore, with PFA-AB-PAS-OG, they appeared as a blue-violet mass of hyaline material (Fig. 9e).
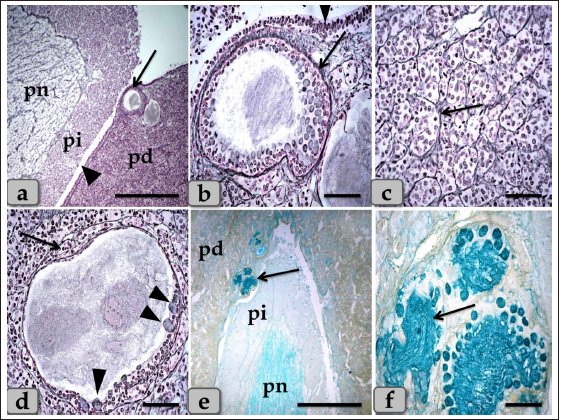
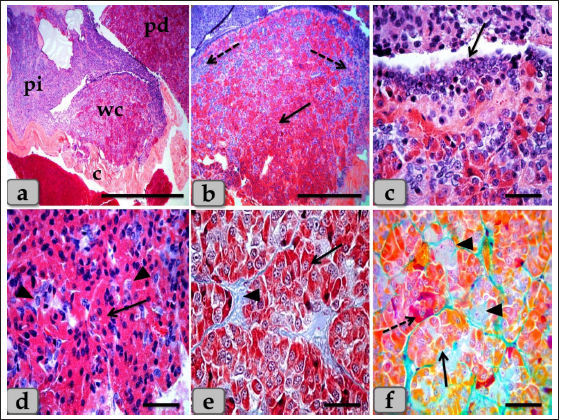
Fig. 6. A photomicrograph of the WC (Cephalic cone of pi), (a) showing the WC, capsule (c), pi, pd. (b) showing the Wulzen’s cone parenchyma; acidophils (arrow) & basophils (dashed arrow). (c) showing the covering epithelium of the WC (arrow). (d) showing the acidophils (arrow) & chromophobes (arrowhead). (e) showing the acidophils (arrow) & intercellular collagen fibers (arrowhead). (f) Showing the acidophils or orangeophils; somatotrophs and mammotrophs (arrow), individual basophils; Adrenocorticotrophs (arrowhead) & thyrotrophs (dashed arrow). Stain: (a–d) H&E, (e) Blue Masson’s trichrome, (f) Br-Ab- OFG. Scale bars: a=400 µm, b=300 µm & c–f=40 µm. Pituicytes were non-secretory supportive neuroglia-like cells with variable shapes and sizes. They are distributed near the blood capillaries between the terminations of nerve fibers. Two types of pituicytes, protoplasmic and fibrous pituicytes (FP), could be distinguished by Br-Ab- OFG and PFA-AB-PAS-OG. Protoplasmic pituicytes (PP) constituted most of the pituicytes. They were elongated oval or ovoid-shaped, larger than fibrous ones, with different protuberances. Their nuclei were long, elliptic, or oval (Fig. 9f). FP, were constituted a handful of pituicytes. They were irregular in shape, smaller in size, with fewer protuberances. Their nuclei were irregularly round or ovoid and stained deep with a larger N/C ratio (Fig. 9f). In some sections, a large wide space or cavity filled with gelatinous material was seen within the center of pn (Fig. 9a and b). This material reacted negatively with both Alcian blue and PAS stains. Furthermore, it appeared as a detached secretory product from the cavity lining cells. The lumen of this cavity was suggested to be connected with the brain ventricle, passing through the infundibulum and lined with simple cuboidal or modified columnar or ependymal-like cells with some basal folds (Fig. 9g–i) that secrete cerebrospinal fluid (CSF). DiscussionThe pituitary gland (hypophysis cerebri) is classified as a main endocrine gland, secreting some hormones which directly affect the vitality of other endocrine organs. It is situated as an appendage of the brain, which refers to its significance as the connection between the nervosa and hormonal mechanisms that control definite functions (Dyce et al., 1987). We described dense plexus of BVs filling the loose connective tissue between the capsule and sphenoid bone. In all the examined sections, this plexus was composed mainly of a rete of medium-sized arteries with well-developed undulating internal elastic lamina and thick muscular walls. Daniel and Prichard (1957) described that the pituitary angioarchitecture remains one of the important aspects of studying the pituitary gland due to the insistent interest in its blood supply and the direction of blood flow. The earliest description of the vasculature of the pituitary gland in sheep showed that it receives its blood supply from two branches on either side. These are the anterior and posterior hypophyseal arteries arising from the internal carotid artery and the carotid rete. These arteries empty in a rich network of BVs surrounding the entire pituitary gland, which shows similarity to humans. Such a network is occasionally known as hypothalamic-pituitary portal circulation or hypothalamo-hypophyseal portal system (Antunes and Muraszko, 1990).
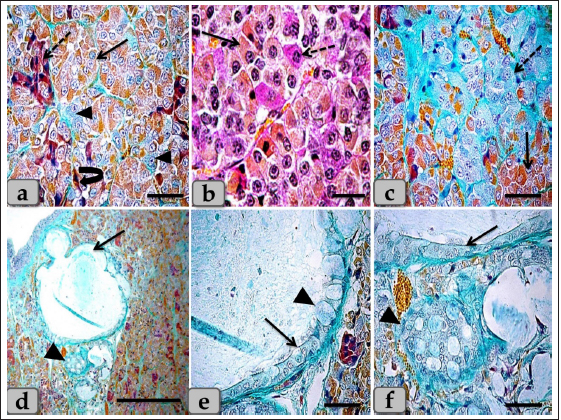
Fig. 7. A photomicrograph of the WC parenchyma (a) showing the acidophils or orangeophils (arrow), basophils; adrenocorticotrophs (arrowhead), thyrotrophs (dashed arrow), gonadotrophs (curved arrow). (b) showing the orangeophils (arrow) and basophils (dashed arrow). (c) showing the orangeophils (arrow), a wide bundle of basophils in the posterior part of the cone (dashed arrow). (d) showing the presence of follicles (arrow) & colloid-filled cysts (arrowhead) with variable sizes in the cone resembling that of pd. (e,f) showing the ciliated glandular epithelial cells lining the follicles (arrow) intermingled with large goblet-like cells that are filled with strongly alcian blue positive materials (arrowhead). Stain: (a,c–f) Br-Ab-OFG, (b) PAS-orange G. Scale bars: a,c,e,f=40 µm, b=20 µm & d=300 µm. Bergland et al. (1977) described that few venous connections were noticed around the hypophyseal gland of sheep and that the adenohypophyseal vascular bed delivers blood (containing adenohypophyseal hormones) to the neurohypophyseal vascular bed, and that these hormones could be delivered to the brain directly after reaching the neurohypophysis. In agreement with Bergland et al. (1977), we did not find venous vessels around the hypophyseal gland. And although not evaluated in the present study, we hypothesize that the hormonal delivery between the adenohypophysis and the hypothalamus to the brain could be through both adenohypophyseal-neurohypophyseal vascular bed (Bergland et al., 1977) and possibly, through the observed cavity, filled with CSF, extending inside the neurohypophysis (seen in the present study, Fig. 9a,b and g). Evidence that this cavity could contribute to the transfer of adenohypophyseal hormones to the brain could be interpreted by earlier findings of diminished plasma LH and FSH after hypothalamo-pituitary disconnection was performed in sheep, as an in vivo model for pituitary isolation that clarified after Clarke et al. (1983). However, future studies are required to confirm this finding. The pd was the most significant part of the gland and was covered with a thin fibrous connective tissue capsule. Its parenchyma consisted mainly of different glandular cells arranged in clusters, nests, and sometimes cords. According to the staining affinities of the secretory granules within the cells, the pd revealed three different cell types; CC, AC, and BC. These findings are very close to those described by Mahmood (2014) in rats and Ye et al. (2018) in Bactrian camels (Camelus bactrianus), and Nasr et al. (2021) in Adult Male Donkey (equas-asinus) Chromophobes were small-sized and polygonal in shape. Its cytoplasm was devoid of any secretory granules, so it appeared lighter or colorless, which was considered the most characteristic feature of these cells. These investigations are in parallelism with those clarified by Jaspal et al. (2011) in camels (Camelus dromedarius) and with Nasr et al. (2021) in Adult Male Donkey (equas-asinus), who clarified that the chromophobes were rounded or polygonal-shaped, negative stained by H&E, their cytoplasm was light blue or colorless, and the nuclei were light blue.
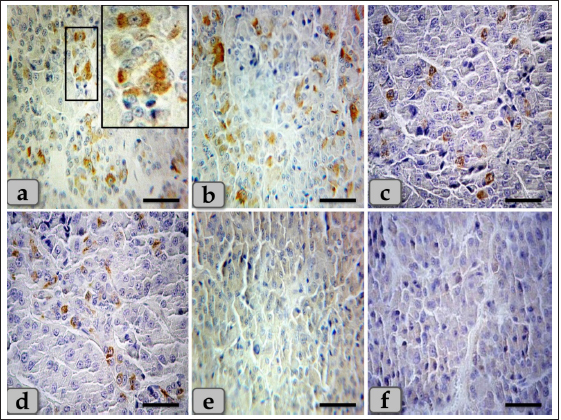
Fig. 8. A photomicrograph of the WC parenchyma, (a–d) showing positive immunohistochemical reactivity of individually distributed gonadotrophs against anti-FSH antibodies (a,b) and anti-LH antibodies (c,d). (e,f) showing the central part of the cone that was observed devoid of any positive immune reactions against anti-FSH and LH antibodies, respectively. Stain: (a,b,e) Immunohistochemical stain against anti-FSH antibody & (c,d,f) immunohistochemical stain against anti-LH antibody. Scale bars: a–f=40 µm. Acidophils were larger than the chromophobes; most were located in the central and lateral parts of pd. They were round or ovoid-shaped, sometimes triangles. Its cytoplasm was stained pink with H&E, but with special stains, the acidophils could differentiate into somatotrophic & LC, and dyed orange with orange G and so were described as orangeophils. However, lactotrophs were smaller and had fewer secretory granules than somatotrophs. These findings are very close and similar to the results described by Jafri (1979) in fish, who clarified that, and with Ye et al. (2018) in Bactrian camels (Camelus bactrianus) who claimed that somatotrophic cytoplasm could be stained orange with orange G and lactotrophic cytoplasm stained nacarat by azocarmine and orange G. BC were less numerous but larger than the acidophils. Their cytoplasm had small-sized and less numerous secretory granules located mainly at the marginal area of the cell cytoplasm. They stained light basophilic with H&E, but with special dyes, the basophil could be differentiated into three different types; corticotrophs, thyrotrophs, and gonadotrophs that stained magenta or purple by PAS. In addition, their cytoplasmic cells had small and less secretory granules located at the periphery of cells. Such diversity is very close to the finding reported by Ye et al. (2018) in Bactrian camels (Camelus bactrianus) and Phyllis Bowie et al. (1974) in rats, who clarified that the secretory granules of the ACTH cell are responsible for the positive PAS reaction. However, compared with another rat anterior pituitary cell types, the corticotroph is relatively poorly granulated, with those granules present being aligned near the plasmalemma. The pi was a narrow region between the pd and pn. It was separated partially from the pd by the hypophyseal cleft but closely associated with the pn. It was filled with weakly basophilic cuboidal or polygonal cells that were arranged in parallel rows or in follicles containing a colloid-like substance that was frequently seen throughout the pi. Furthermore, some follicles appeared to be filled with amorphous substances, while others had only a little or were devoid of any materials in some of the examined sections. These findings were very close and similar to the findings clarified by Perry et al. (1981) in adult sheep.
Fig. 9. A sagittal section of the sheep pituitary gland (a) showing the pd, pi, pn, and lumen of pn (L). (b) showing the water-drop shape of pn. (c) showing the unmyelinated nerve fibers oriented in many different directions in pn (arrow). (d) showing the highly branching terminal ends of the unmyelinated nerve fibers; palisode zone (arrow). (e) showing the dilated terminal ends of unmyelinated nerve fibers; herring bodies (arrowhead). (f) showing the PP (arrow) and FP (arrowhead). (g–i) Higher magnification of (a), (g) showing the large wide cavity filled with gelatinous material in the center of the pn (L). (h,i) showing ependymal-like cells lining the cavity of pn (arrow) and detached secretory products from the cavity lining cells filling the cavity. Stain: (a,g–i) Gomori’s reticulin, (b,e,f) PFA-AB-PAS-OG, (c,d) Bielschowsky technique. Scale bars: a,b=400 µm, g=300 & c–i=40 µm. The WC was reported for the first time by Wulzen (1914) in an ox pituitary gland, who observed a kind of cone that projected from the pi surrounded by hypophyseal cleft and appeared tongue-like. This structure was thereafter stated by Getty (1975). Moreover, Shirasawa et al. (1985), in goats, paid attention to the special topographical correlation of the cone with pd. The present investigation confirmed the presence of a cone-like structure of glandular cells of the pituitary gland of an adult male sheep protruding from the pi towards the lumen of the hypophyseal cleft in the neighborhood of the pd. This finding is similar to Sethi and Singh (2009) on buffalo and Shirasawa et al. (1985) on goats. It was elongated and observed free along the majority of its length. But, posteriorly, the WC was continuous with the posterior region of the pi without any line of demarcations. Moreover, this cone was covered externally with lining epithelium of simple cuboidal to stratified epithelium on the side of the hypophyseal cleft. These investigations coincided with Girod et al. (1982) on the bovine pituitary. In addition, Ganfini (1922) elucidated this cone in fetal ram sections and denominated it "lobule intermedia," which is different from pi, "epithelial cells that invade the entire intermediate lobule" and observed the presence of “clear and dark cells.” Furthermore, Lubberhuizen (1927, 1931) observed this structure in the ovine fetus with the term “eminentia cylindrical.” Moreover, It was described by Legait (1964) in adult sheep and also in the ovine fetus by Racadot (1949), who named it a “chromophilic cone.” Using H&E and specific stains, three different cell types forming the cone parenchyma were clarified; acidophils, chromophobes, and basophils. Acidophils were arranged in clusters at the central part of the cone in between them; unstainable cells and chromophobes were present. Furthermore, basophils were placed in a cluster in the anterior and posterior parts of the cone. However, sporadic or individual basophils were also recognized in between acidophils. This finding is similar to Shirasawa et al. (1985) in goats, who described, through acceptable structural characteristics and immunohistochemical appearances, that the cells forming the cephalic cone typically resembled those of the pd. Furthermore, he reported six types of cells of the cephalic cone-like pd; chromophobes, GH cells, prolactin cells, ACTH cells, TSH cells, and LH cells. He demonstrated that the ultrastructure of GH cells in the cone resembled the prolactin cells. The secretory granules of both types are spherical; the diameter of the former is about 340 nm, whereas that of the latter is about 440 nm. ACTH cells are polygonal-shaped with secretory granules, about 180 nm in diameter, scattered throughout the cytoplasm. TSH cells, spherical in shape, contain the smallest secretory granules with a diameter of about 150 nm. The highly electron-dense LH cells contain numerous secretory granules with a diameter of about 210 nm and irregular nuclei. And also, our findings are supported by Sethi and Singh (2009), who mentioned three types of cells were recognized in the parenchyma of WC of buffalo fetus, i.e., acidophils, basophils, and chromophobes. Moreover, he clarified that the acidophils were predominately located on the periphery. They were oval-shaped with eccentric rounded nuclei. Similarly, observations were recorded by Waage (1953) on sheep. The basophils were round cone-shaped with centrally placed nuclei and were distributed throughout the lobe. Singh and Dhingra (1979) in goat fetuses claimed that basophils were stained with PAS and aniline–blue stains but exhibited no affinity for aldehyde fuchsin and suggested their role in the production of gonadotrophins as an aid to the secretion of pd. Moreover, Girod et al. (1982) of the bovine pituitary described some cells in WC that reacted with anti-oPRL antibodies and were either isolated or clustered. Other cells reacted with anti-hGH, anti-bLH, anti-βoLH, or anti-βhTSH antibodies. Some cells reacted simultaneously with anti-βMSH, anti-1–24ACTH, anti-17–39ACTH, and anti-βLPH, indicating the presence of basophils in the cephalic cone. But, on the contrary, Getty (1975) elucidated that the cells in the cephalic cone were primarily acidophils, somatotrophs, and mammotrophs cells without mentioning the presence of basophils. pn was raised as a ventral outpouching of the brain floor and observed as a water-drop shape. It consisted mainly of unmyelinated nerve fibers, herring bodies, pituicytes, and blood capillaries. Such diversity is similar to the finding stated by Eurell and Frappier (2006), Junqueira et al. (2010), and described by Mahmood (2014) in rats and Ye et al. (2018) in Bactrian camels (Camelus bactrianus). Large wide spaces, cavities, or pn lumen filled with gelatinous material were seen within the center of pn. This material was reacted negatively with both PAS and Alcian blue and suggested to be CSF. It appeared as a secretory product that was secreted and detached from the lumen lining cells. The lumen of this cavity was suggested to be connected with the brain ventricle, passing through the infundibulum and lined with ependymal-like cells. These investigations are in parallel with Jahangirfard et al. (2019) adult turkey, who clarified that a wide infundibular cavity is present inside the pn of the turkey pituitary gland. ConclusionOur investigation concluded that WC is present and well-developed in sheep adenohypophysis. It protruded as a tongue-shaped or bulb-shaped structure from the pi towards the lumen of the hypophyseal cleft in the neighborhood of the pd and behind the pn. Various glandular cells were distinguished, filling the cone, chromophobes, and chromophils of acidophils (somatotroph, mammotroph) & basophils (thyrotroph, corticotroph, gonadotroph) through some histochemical techniques and others by immunoreactivity like gonadotroph. These glandular cells were typically similar to the glandular cells of pd but with different distributions where the cone is formed mainly of acidophils intermingled with chromophobes. Meanwhile, basophils were primarily localized at the anterior and caudal parts of the cone. However, few individual distributions of basophils were also observed in the central region between the acidophils. Author contributionConceptualization: Wael A. M. Ghonimi. Data Curation: Wael A. M. Ghonimi. Methodology: Wael A. M. Ghonimi, Naief Dahran. Histological examination - Images analysis: Wael A. M. Ghonimi. Writing -Original draft: Wael A. M. Ghonimi, Naief Dahran. Review and Editing: Wael A. M. Ghonimi, Naief Dahran. Data availabilityAll data generated during this study are included in this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. ReferencesAntunes, J.L. and Muraszko, K. 1990. The vascular supply of the hypothalamus-pituitary axis. Acta. Neurochir. Suppl. (Wien). 47, 42–47. Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histological techniques, 6th ed. New York, London: Churchill Livingstone, pp: 83–92. Bergland, R., Davis, S. and Page, R. 1977. Pituitary secretes to brain: experiments in sheep. Lancet 310(8032), 276–277. Clarke, I.J., Cummins, J.T. and de Kretser, D.M. 1983. Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology 36(5), 376–384. Daniel, P.M. and Prichard, M.M. 1957. The vascular arrangements of the pituitary gland of the sheep. Q. J. Exp. Physiol. Cogn. Med. Sci. 42(3), 237–248. Dyce, K.M., Sack, W.O. and Wensing, C.J.G. 1987. The endocrine gland. Textbook of veterinary anatomy. Philadelphia, PA: W.B Saunders Company, pp: 205–207. Eurell, J. and Frappier, B.L. 2006. Dellmann’s textbook of veterinary histology, 6th ed. New York, London: Blackwell, pp: 298–306. Ganfini, C. 1922. Su uno speciale lobulo dell’hypophysis cerebri in embrioni di pecora e sul suo significato. Arch. Ital. Anat. Embriol. 19, 95–121. Getty, R. 1975. The anatomy of the domestic animals, Vol. 1. 5th ed. Toronto, Canada: W.B. Saunders Company, pp: 955–956. Girod, C., Dubois, M.P. and Trouillas, J. 1982. Immunocytochemical study of Wulzen’s cone of the bovine pituitary. Cell. Tissue. Res. 227(1), 55–65. Jafri, S.I.H. 1979. Combined stain for fish pituitary. Stain. Technol. 54(2), 93–95. Jahangirfard, R., Shalizar-Jalali, A., Shahrooz, R., Najafi, G. and Minas, A. 2019. Anatomical and cytohistological study of the pituitary gland in adult turkey. Vet. Res. Forum. 10(2), 159–163. Jaspal, S.A.S., Rahman, Z.U. and Cheema, A.M. 2011. Gross morphology and localization of adenohypophyseal cells in camel (Camelus dromedarius) using a new combination of stains. Pak. Vet. J. 31(1), 1–5. Junqueira, L.C., Carneiro, J. and Kelly, I. 2010. Basic histology, 11th ed. New York, Chicago: McGraw- Hill, Medical Publishing Division, pp: 413–422. Legait, H. 1964. Recherches histophysiologiques sur le lobe intermrdiaire de l’hypophyse. Nancy, France: Thrse Mrdecine, pp: 1–118. Lubberhuizen, H.W. 1927. De ontwikkeling van de hypophysis cerebri bij jet schaap (Ovis aries). Leiden, The Netherlands, pp: 1–162. Lubberhuizen, H.W. 1931. Die entwicklung der hypophysis beim Schaf. Z. Anat. Entwickl-Gesch. 96, 1–53. Mahmood, H.B. 2014. Anatomical and histological study of pituitary gland of the rats in Iraq. J. Kerbala. Univ. 12(3), 221–228. Marieb, E.N. and Hoehn, K. 2012. Human anatomy & physiology, 9th ed. Boston, MA: Pearson, pp: 629. Martinez-Lage, M. 2011. The pituitary, 3rd ed. San Diego, CA: Elsevier, pp: 23–25. Nasr, M., Rashed, R., Hassanin, A. and Shogy, K. 2021. Some macroscopic and microscopic observation on the pituitary gland of adult male donkey (equas-asinus). J. Curr. Vet. Res. 3(2), 24–31. Pearse, A.G.E. 1985. Histochemistry: theoretical and applied, 4th ed. Edinburgh, London, Melbourne and New York: Churchill Livingstone. Perry, R.A., Robinson, P.M. and Ryan, G.B. 1981. Ultrastructure of the pars intermedia of the adult sheep hypophysis. Cell. Tissue. Res. 217, 211–223. Phyllis Bowie, E., Glenn Williams, M., Edward, G. and Rennels, E.G. 1974. Evidence for PAS positive reaction of the corticotroph granules of the rat adenohypophysis. Histochemie 38, 281–284. Polak, J.M. and Noorden, V. 1997. Introduction to immunocytochemistry, 2nd ed. Oxford, UK: BIOS, Scientific Publishers. Racadot, J. 1949. Cytogenrse de la glande pituitaire chez quelques mammifères. Nancy, France: Thrse Mrdecine, pp: 1–140. Sethi, R.S. and Singh, O. 2009. Morphogenesis and cytodifferentiation of the Wulzen’s cone during prenatal development of pars distalis adenohypophysis of buffalo. Indian. J. Anim. Sci. 79(8), 798–799. Shirasawa, N., Kihara, H. and Yoshimura, F. 1985. Fine structural and immunohistochemical studies of got adenohypopysial cells. Cell. Tissue. Res. 240(2), 315–321. Singh, Y. and Dhingra, L.D. 1979. Morphogenesis of the hypophysis cerebri in goat. 2. Cone of Wulzen. Indian. J. Anim. Sci. 49, 281–285. Slidders, W. 1961. The OFG and BrAb- OFG methods for staining the adenohypophyis. J. Pathol. Bacteriol. 82, 532–534. Stahnisch, FW. 2015. Max bielschowsky (1869–1940). J. Neurol. 262(3), 792–794. Waage, S. 1953. The morphology of the hypophysis in some artiodactyla. K. Fysiagr. Sallsk. Lound. Forh. 23, 1–5. Wulzen, R. 1914. The morphology and histology of a certain structure connected with the pars intermedia of the pituitary body of the ox. Anat. Rec. 8(8), 403–414. Ye, W., Wang, H., Wang, F. and Wang, J. 2018. Morphology and ultrastructure of the hypophysis in bactrian camels (Camelus bactrianus). Int. J. Morphol. 36(4), 1316–1325. | ||
| How to Cite this Article |
| Pubmed Style Dahran N, Ghonimi WAM. Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Vet J. 2023; 13(3): 307-321. doi:10.5455/OVJ.2023.v13.i3.7 Web Style Dahran N, Ghonimi WAM. Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. https://www.openveterinaryjournal.com/?mno=133922 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i3.7 AMA (American Medical Association) Style Dahran N, Ghonimi WAM. Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Vet J. 2023; 13(3): 307-321. doi:10.5455/OVJ.2023.v13.i3.7 Vancouver/ICMJE Style Dahran N, Ghonimi WAM. Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Vet J. (2023), [cited July 27, 2024]; 13(3): 307-321. doi:10.5455/OVJ.2023.v13.i3.7 Harvard Style Dahran, N. & Ghonimi, . W. A. M. (2023) Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Vet J, 13 (3), 307-321. doi:10.5455/OVJ.2023.v13.i3.7 Turabian Style Dahran, Naief, and Wael A. M. Ghonimi. 2023. Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Veterinary Journal, 13 (3), 307-321. doi:10.5455/OVJ.2023.v13.i3.7 Chicago Style Dahran, Naief, and Wael A. M. Ghonimi. "Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa." Open Veterinary Journal 13 (2023), 307-321. doi:10.5455/OVJ.2023.v13.i3.7 MLA (The Modern Language Association) Style Dahran, Naief, and Wael A. M. Ghonimi. "Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa." Open Veterinary Journal 13.3 (2023), 307-321. Print. doi:10.5455/OVJ.2023.v13.i3.7 APA (American Psychological Association) Style Dahran, N. & Ghonimi, . W. A. M. (2023) Cytodifferentiation of Wulzen's cone of mature male sheep hypophysis cerebri with special emphasis on its parenchymal correlations with the adjacent Pars distalis, Pars intermedia and Pars nervosa. Open Veterinary Journal, 13 (3), 307-321. doi:10.5455/OVJ.2023.v13.i3.7 |