| Research Article | ||
Open Vet J. 2023; 13(7): 846-853 Open Veterinary Journal, (2023), Vol. 13(7): 846-853 Original Research Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in JapanShino Yoshida1, Shingo Maeda2, Tomohiro Yonezawa2 and Tomoki Motegi3*1Faculty of Veterinary Medicine, Hokkaido University Veterinary Teaching Hospital, Hokkaido University, Hokkaido, Japan 2Department of Veterinary Clinical Pathobiology, Veterinary Medical Center, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan 3Section of Computational Biomedicine, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, USA *Corresponding Author: Tomoki Motegi. Section of Computational Biomedicine, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, USA. Email: tmotegi [at] bu.edu Submitted: 26/11/2022 Accepted: 14/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
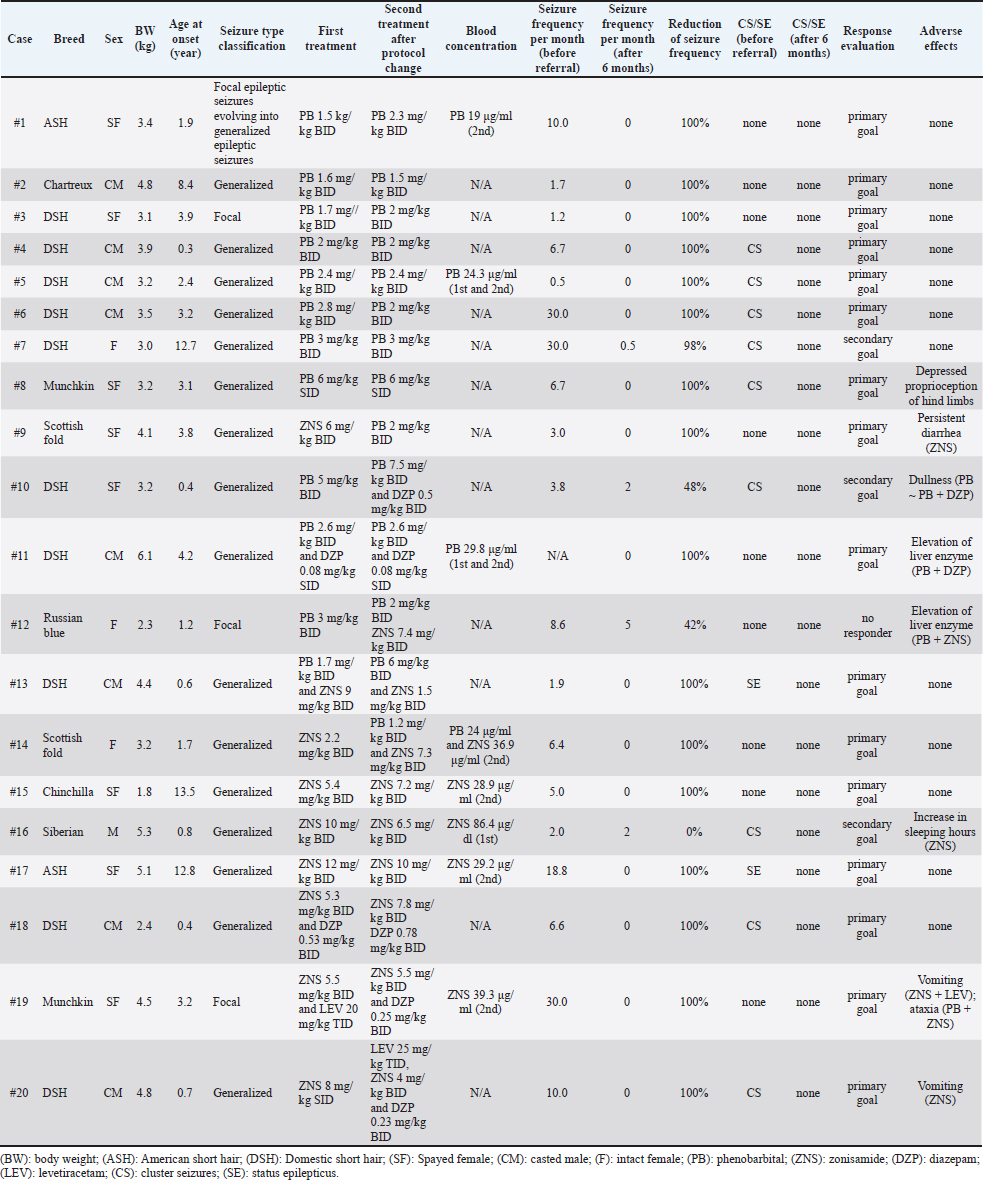
AbstractBackground: Idiopathic epilepsy in cats is a more common disease than previously thought, but little information is available about the medical treatment of feline idiopathic epilepsy. Aim: To assess the therapeutic efficacy and safety of antiseizure medication (ASM) for a minimum of 6 months, including zonisamide (ZNS), in feline idiopathic epilepsy at a referral hospital in Japan. Methods: Twenty cats diagnosed with idiopathic epilepsy treated with ASMs were retrospectively included. Results: Nine cats that were finally treated with phenobarbital (PB) monotherapy reached the primary goal (the seizure frequency after the treatment intervention was less than one seizure every 3 months). Three cats were treated with ZNS monotherapy and two reached the primary goal. Eight cats finally received combination therapy. Two of the three cats receiving PB and ZNS therapy achieved the primary goal, but one was considered no responder. Five cats [PB + diazepam (DZP), ZNS + DZP, and ZNS + levetiracetam + DZP] decreased the seizure frequency and reached the primary goal in all but one cat reached the secondary goal. Adverse events were observed in eight patients, but these were curable. Two patients had vomiting after ZNS monotherapy, one had diarrhea, and another was an increase in sleeping hours. Conclusion: PB was frequently used and seemed effective as both monotherapy and combination therapy. Some cats were treated with ASM protocols containing ZNS. ZNS may be available to treat idiopathic epilepsy in cats. However, ZNS administration may cause adverse events, such as gastrointestinal toxicity, in cats. Keywords: Antiseizure medication, Feline, Idiopathic epilepsy, Zonisamide, Seizure. IntroductionEpileptic seizures are characterized by abnormal excessive or synchronous neuronal activity in the brain (Coates and O’Brien, 2017). In veterinary practice, the incidence of epileptic seizures has been reported to be 2.1% among cats presenting to the hospital for any reason (Schriefl et al., 2008). Idiopathic epilepsy is defined as “recurring seizure syndrome with no detectable underlying abnormalities” (Platt and Risio, 2014). They are generally presumed to be genetic, but the pattern of inheritance is unknown in cats. At present, seizures without detectable cause are also referred to as idiopathic epilepsy in cats. In a study of cats presented to a veterinary neurology referral hospital in Japan, feline idiopathic epilepsy was diagnosed in 29% of the cats visiting the hospital because of brain disorders (Nakamoto et al., 2019). Magnetic resonance imaging (MRI) is now widely used in veterinary practice and is a powerful tool for detecting structural abnormalities that may cause seizures. Thus, the increasing use of MRI helps the differentiation between idiopathic and structural epilepsy. In veterinary medicine, epileptic seizures frequently require treatment with antiseizure medications (ASMs) depending on the frequency and severity of events. In cats, phenobarbital (PB), imepitoin, and levetiracetam (LEV) are often used to treat epilepsy (Charalambous et al., 2018). However, imepitoin is not on the market and is not easily available in Japan. LEV is recommended as a second line ASMs for cats with epilepsy, but it is more expensive than other ASMs and needs administration three times daily, frequent administration of drugs is stressful for cats. Therefore, the other choice for ASMs should be evaluated for feline epilepsy. Zonisamide (ZNS) is licensed to treat canine epilepsy in Japan and is often used to treat dogs with recurrent seizures. In one survey, ZNS is the most frequently held ASMs in Japanese veterinary hospitals, and Japanese clinicians prescribe ZNS second most often, even in feline epilepsy (Mizuno et al., 2022). However, no study has evaluated the efficacy and safety of ZNS in clinical feline cases and ZNS has not been strongly recommended for the treatment of feline epilepsy in a systematic review (Charalambous et al., 2018). Moreover, the follow-up periods of almost all previous studies about ASMs were shorter than 6 months (Charalambous et al., 2018), and the efficacy and safety information has not been clear enough. Therefore, more information on the use of ASMs, including ZNS, is needed to treat feline idiopathic epilepsy. This study used the medical records for at least 6 months in the Veterinary Medical Center (VMC) of the University of Tokyo to evaluate the efficacy and safety of ASMs, including ZNS, for treating feline idiopathic epilepsy. Materials and MethodsInclusion criteria of idiopathic epilepsyCats were included retrospectively if they were referred to the VMC of the University of Tokyo between 2013 and 2019 and were diagnosed with idiopathic epilepsy or presumptive idiopathic epilepsy by the following criteria: two or more unprovoked seizures at least 24 hours apart; no neurological abnormality in the interictal period; neither no complaints indicating intoxication nor blood test abnormalities that may cause reactive seizures (Kwiatkowska et al., 2019); no structural abnormalities which can cause seizures identified on MRI scan (Risio et al., 2015; Stanciu et al., 2017). SignalmentMedical information collected included breed, sex, body weight at the first visit, age at seizure onset, type of seizures, presence of cluster seizures and status epilepticus, and results of physical and neurological examinations, blood test results including complete blood cell count and serum biochemistry profile (sodium, potassium, chloride, calcium, alanine aminotransferase, alkaline phosphatase, total bilirubin, urea, creatinine, glucose, and ammonia) (Kwiatkowska et al., 2019) and MRI findings. In some cases, several examination data, such as urinalysis and cerebrospinal fluid analysis, were lacking but diagnosed as idiopathic epilepsy by clinicians as “presumptive idiopathic epilepsy.” The viral status was examined when viral infection was especially suspected. None of the cats performed electroencephalography. Definitions of terms and efficacy assessmentThe types of seizures were classified based on the International veterinary epilepsy task force consensus statement (Berendt et al., 2015) as generalized epileptic seizures, focal epileptic seizures evolving into generalized epileptic seizures, and focal epileptic seizures. Cluster seizures were defined as two or more seizures within 24 hours. Status epilepticus was clinically defined as (a) greater than 5 minutes of continuous epileptic seizures or (b) two or more discrete epileptic seizures separated by incomplete recovery of consciousness (for generalized convulsive seizures). Each seizure was counted individually and when cluster seizures were noted in a day, the seizure frequency was calculated as one seizure event. To evaluate the efficacy and adverse events of ASMs, information related to the treatment, such as used ASMs, the dosages of these ASMs, adverse events during the treatment, blood concentration of ASMs if available, the frequency of seizures at the diagnosis and before changing ASMs in VMC (before referral), frequency of seizures at 6 months after referral, and presence of cluster seizures and status epilepticus before and after 6 months of treatment, was collected. Seizure frequency was calculated based on the owner’s report. Patients in which the seizure frequency after the treatment intervention was less than one seizure every 3 months were defined as reaching the “primary goal”. Patients showing more than a 50% reduction in the frequency of seizures or preventing cluster seizures or status epilepticus were considered to reach the “secondary goal” (Potschka et al., 2015). Patients that did not reach the primary nor the secondary were considered as “no responders”. Ethical approvalThis work involved using non-experimental animals only (including only owned animals and data from retrospective studies). Established internationally recognized high standards (“best practice”) of individual veterinary clinical patient care were followed. The owner agreed with the informed consent for academic purposes. ResultsAnimals and classification of seizuresSixty-three cats were diagnosed with idiopathic epilepsy (including presumptive) at the VMC of the University of Tokyo between 2013 and 2019. Among those cases, information on follow-up after 6 months was available for 20 cats treated with ASMs, which was included in this study. Exclusions (n=43) were as follows; inadequate records for treatment (n=26), no treatment due to a low frequency of seizures and acceptable for the owner (n=11), withdrawal of treatment (n=2), the presence of sudden behavioral changes such as aggression as the primary symptom, which precluded accurate assessments from determining whether the patient had epilepsy or behavioral problems (n=3), MRI scan was not performed (n=1). Information about the 20 cats suited for treatment evaluation was described in Table 1. Of these 20 cats, nine were males (one sexually intact and eight castrated) and 11 were females (three sexually intact and eight spayed). At the first onset of an epileptic seizure, the patient’s ages ranged from 4.0 months to 13 years (median, 2.8 years). The body weight ranged from 1.8 to 6.1 kg, and the median weight was 3.5 kg. Breeds included were as follows; domestic shorthair (n=10), American short hair, Scottish fold, and Munchkin (n=2, respectively), Russian Blue, Chartreux, Siberian, and Chinchilla (n=1, respectively). The median duration of seizure history of each cat before inclusion in the study was 65 days (range, 8–334 days).**** Table 1. Information about the patients included in the 6-month evaluation.
Among these 20 cats, 16 exhibited generalized epileptic seizures, one exhibited focal epileptic seizures evolving into generalized epileptic seizures, and the remaining three showed focal seizures. Plasma biochemical analysis was conducted in all patients, but some biochemical parameters, such as plasma total protein, albumin, and phosphate levels could not be described for four cats. Twelve cats were tested for retroviral status, and all showed negative findings for both FeLV and FIV. MRI examinations using a 0.3-Tesla unit (AIRIS; Hitachi, Tokyo, Japan) were performed for 16 cats, and MRI examinations using a 3-Tesla unit (Vantage Galan3T SaturnX; Canon, Tokyo, Japan) were conducted for 4 cats, and T1-weighted (T1W), T2-weighted, fluid-attenuated inversion recovery, and postcontrast-T1W images were acquired. Three cats showed structural abnormalities that were not considered the causative lesions: otitis media in the left ear in one cat, ventricular enlargement in one cat, and cranium dysplasia in one cat. CSF was collected from four cats, and there were no remarkable findings. CSF was not collected in the other 16 cats because of the owner’s intention. ASMs and efficacyFor first-line therapy, 10 cats were treated with PB. Among these cats, one subsequently received additional diazepam (DZP) and one received additional ZNS because of the insufficient efficacy. In this study, eight cats, including one changed from ZNS, finally received PB monotherapy with a median dosage of 2 mg/kg q12hr (range, 1.5–3 mg/kg q12 hr), and one was treated with PB 6 mg/kg q24 hr. Eight cats achieved the primary goal in PB monotherapy, and the remaining cat achieved the secondary goal. Blood concentration of PB was available for only two cats (19.0 and 24.3 μg/ml, respectively; reference range 15–30 μg/ml) and both reached the primary goal. Six cats were treated with ZNS as first-line therapy and then three underwent treatment changes subsequently; one was treated with PB because of an adverse event, one cat received additional PB, and one was treated with a combination of ZNS, LEV, and DZP. The remaining three cats continued ZNS monotherapy, with dosages of 6.5, 7.2, and 10 mg/kg q12hr. Finally, two cats achieved the primary goal, and one reached the secondary goal because of the prevention of cluster seizures. Blood concentration of ZNS was available for two cats; 28.9 and 29.2 μg/ml, respectively; reference range 10–30 μg/ml. Both cats reached the primary goal. The blood concentration in the cat did not achieve the primary goal was not available. The other four cats were treated with combination therapy after diagnosis: one was treated with PB and ZNS, one was treated with PB and DZP, one was treated with ZNS and DZP, and one was treated with ZNS and LEV. As mentioned above, two cats were first treated with PB in the primary doctor switched to combination therapies (one PB and DZP, one PB and ZNS). Two cats with ZNS treatment in the primary were added on PB or LEV and DZP, respectively. One cat that was first treated with a combination (ZNS and LEV) was treated with ZNS and DZP because of the owner’s request. Among the eight cats finally receiving combination therapy, two of the three receiving PB and ZNS therapy achieved the primary goal, while the remaining cat was no responder. Among two cats receiving PB and DZP therapy, one achieved the primary goal, and the other achieved the secondary goal. Two cats treated with a combination of ZNS and DZP achieved the primary goal. Finally, one cat treated with ZNS, LEV, and DZP reached the primary goal. Adverse eventsAdverse events were assessed in 20 cats. Case #11 (PB + DZP) and case #12 (PB + ZNS) showed elevated liver enzyme levels without clinical signs. Transient vomiting was shown in case #19 (ZNS + LEV) and case #20 (ZNS). Plasma ZNS concentrations in these two cats were 10.1 and 33.5 μg/ml, respectively. The former cat also showed ataxia when DZP was added at 0.5 mg/kg per day, which was resolved by dose reduction (DZP 0.25 mg/kg per day). Persistent diarrhea was shown in case#9 (ZNS), resulting in a switch to PB treatment. Case#8 (PB) had transient delayed proprioception of the hind limbs. Case#16 (ZNS) showed an increase in sleeping time when ZNS was administered at 20 mg/kg per day, which was resolved with a dosage reduction of ZNS. Blood concentration at 20 mg/kg per day was 86.4 μg/ml and the concentration after dose reduction was not available. Case#10 (PB) had shown chronic dullness since PB monotherapy, and no treatment had been given. DiscussionPB is a commonly used ASM to treat feline epilepsy. In a previous study, among cats treated using a protocol that included PB, a 50% reduction in seizure frequency was achieved in 93% of epileptic cats (Finnerty et al., 2014). In the present study, nine cats were treated with PB monotherapy, of which eight were seizure-free and did not experience any adverse events. Thus, PB effectively reduced seizure frequency and was used safely to treat feline idiopathic epilepsy in this study. In this study, ZNS monotherapy was continued in three-sixths of the cats; two reached the primary goal, and the remaining one achieved the secondary goal. In addition, among the seven cats treated with combination therapy containing ZNS, six were responders during the 6-month assessment period. ZNS is often used to treat canine epilepsy, especially in Japan, because it is licensed for treating canine epilepsy and is inexpensive. ZNS monotherapy has been reported to show an efficacy of 60% in canine idiopathic epilepsy (Chung et al., 2012), and the effectiveness of ZNS combination therapy in canine idiopathic seizures has been reported to range from 50% to 82% (Klopmann et al., 2007; Dewey et al., 2014). Our research was the first report to use ZNS in spontaneous feline idiopathic epilepsy and show 6-month efficacy in controlling epileptic seizures. The ZNS treatment could become yet another option for feline idiopathic epilepsy. This study observed adverse events associated with ZNS in4 out of 11 cats. Two cats experienced vomiting, one showed diarrhea, and one showed excessive sleeping behavior. In an experimental study that investigated ZNS toxicity in cats, 50% of the cats were administered 20 mg/kg ZNS per day and showed high blood concentration suffered from adverse reactions, such as anorexia, diarrhea, and vomiting (Hasegawa et al., 2008). However, in our study, most cats treated with ZNS received lower dosages than those used in the previous study and the concentration of ZNS was within the treatment level in dogs (reference range, 10–40 μg/ml). In another cat, ZNS was changed to PB because of persistent diarrhea, even though the ZNS dosage was much lower than 20 mg/kg daily. Thus, our findings indicate that ZNS treatment, even at doses less than 20 mg/kg per day, may cause adverse events, especially gastrointestinal signs, in cats. The combination of PB and ZNS controlled the frequency of seizures in three cats. From the point of view of pharmacokinetics, PB has been proven to induce cytochrome P-450 (CYP) 3A4 expression in the human liver (Martin et al., 2003), and ZNS can be metabolized and inactivated by CYP3A4 in human medicine. Furthermore, CYP1A, CYP2B, CYP2C, and CYP3A activities are elevated in dogs after 35 consecutive days of PB administration (Hojo et al., 2002). In addition, repeated PB administration has been shown to enhance the clearance of ZNS in dogs, resulting in reduced serum concentration (Orito et al., 2008). However, it is not clear whether continuous PB administration induces CYP activity in cats. The findings of experiments in healthy cats suggest that CYP2C activity may be pretty low in cats compared to that of dogs (Shah et al., 2007) and that PB metabolism did not show significant differences after 21 days of continuous administration (Cochrane et al., 1990). Therefore, it is assumed that cats’ CYP-induced auto metabolism and other ASM metabolism may not occur (Risio, 2014). Based on this hypothesis, even if ZNS is used concurrently with PB, alteration of pharmacokinetics may not be considered, and this combination may be a reasonable choice for combination therapy. Elevation of liver enzyme activity (not otherwise specified) was observed in two cats, of which one received PB and ZNS and the other received PB and DZP. PB sometimes causes elevated alanine transferase levels in cats (Finnerty et al., 2014; Hermans et al., 2022), therefore elevation of liver enzyme observed in this study possibly is explained by the use of PB. ZNS has also been reported to induce elevation of the levels of liver enzymes, especially alkaline phosphatase, in dogs (Klopmann et al., 2007; Boothe and Perkins, 2008; Dewey et al., 2014; Smith et al., 2022) but the exact mechanism of why this elevation of liver enzyme occurs is still undetermined. Experimental administration of ZNS in cats did not induce liver enzyme level elevation (Hasegawa et al., 2008), and cats that received ZNS monotherapy did not experience increased liver enzyme activity in the present study. However, it is premature to conclude that ZNS does not contribute to the elevation of liver enzyme activity in cats. DZP has been reported to potentially cause idiosyncratic fulminant and lethal liver failure in cats (Center et al., 1996; Hughes et al., 1996). It is hypothesized that it may be due to the lower glucuronidation capacity of cats compared to dogs (Beusekom et al., 2015). Although DZP is occasionally used for feline epilepsy in Japan (Mizuno et al., 2022), the prevalence of this idiosyncratic adverse effect is unknown, and there is only one report after the 2000s from Canada (Park, 2011). Our study also revealed using DZP as an adjunctive ASM in five cats from the medical records. Still, it did not show lethal liver failure, and just one cat that received concurrent ZNS showed elevated liver enzyme levels. A previous report indicated that the level of liver enzymes should be measured before and after starting DZP treatment. Discontinuation of DZP is recommended if any adverse events are observed, especially in the first week (Center et al., 1996). Of course, enough consideration and caution are needed, if no significant adverse events are observed, DZP can be one of the choices for the add-on ASMs of feline idiopathic epilepsy by measuring the concentration of hepatic enzymes frequently. The main limitation of this study was the small number of cases for each drug, especially ZNS, and the lack of ASM concentration in some cases. Although this seems to be the first report to use ZNS in clinical cases of feline epilepsy, a more extensive study is needed to validate the efficacy of ZNS and identify possible adverse events of ZNS. In this study, ZNS could be used as monotherapy and combination therapy to treat feline idiopathic epilepsy in a total of nine cats. However, ZNS administration may cause adverse events, such as gastrointestinal adverse effects, in cats. AcknowledgmentsThis work was funded by the JSPS KAKENHI Grant-in-Aid for Young Scientists (grant JP20K15675). Conflict of interestThe authors declared no potential conflicts of interest for this research, authorship, and/or publication of this article. FundingThis investigation received auspices from the JSPS KAKENHI Grant-in-Aid for Young Scientists (grant JP20K15675). Data availabilityThe entire data substantiating the conclusions of our study is encompassed within this manuscript. Author contributionsDr. Yoshida and Dr. Motegi planned this research and collected information and wrote the manuscript. Dr. Maeda and Dr. Yonezawa approved this research. This research also includes the patients that were diagnosed by Dr. Maeda, Dr. Yonezawa, and Dr. Motegi. ReferencesBerendt, M., Farquhar, R.G., Mandigers, P.J.J., Pakozdy, A., Bhatti, S.F.M., Risio, L.D., Fischer, A., Long, S., Matiasek, K., Muñana, K., Patterson, E.E., Penderis, J., Platt, S., Podell, M., Potschka, H., Pumarola, M.B., Rusbridge, C., Stein, V.M., Tipold, A. and Volk, H.A. 2015. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC. Vet. Res. 11, 182. Beusekom, C.D., van Heuvel, J.J.M.W., van den Koenderink, J.B., Russel, F.G.M. and Schrickx, J.A. 2015. Feline hepatic biotransformation of diazepam: differences between cats and dogs. Res. Vet. Sci. 103, 119–125. Boothe, D.M. and Perkins, J. 2008. Disposition and safety of zonisamide after intravenous and oral single dose and oral multiple dosing in normal hound dogs. J. Vet. Pharmacol. Ther. 31, 544–553. Center, S.A., Elston, T.H., Rowland, P.H., Rosen, D.K., Reitz, B.L., Brunt, J.E., Rodan, I., House, J., Bank, S., Lynch, L.R., Dring, L.A. and Levy, J.K. 1996. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J. Am. Vet. Med. Assoc. 209, 618–625. Charalambous, M., Pakozdy, A., Bhatti, S.F.M. and Volk, H.A. 2018. Systematic review of antiepileptic drugs’ safety and effectiveness in feline epilepsy. BMC. Vet. Res. 14, 64. Chung, J., Hwang, C., Chae, J., Ahn, J., Kim, T., Seo, K., Lee, S. and Youn, H. 2012. Zonisamide monotherapy for idiopathic epilepsy in dogs. New. Zeal. Vet. J. 60, 357–359. Coates, J.R. and O’Brien, D.P. 2017. Brain diseases. In Textbook of veterinary internal medicine expert consult. Eds., Ettinger, S.J., Feldman, E.C. and Cote, E. Amsterdam, The Netherlands: Elsevier, pp: 3309–3384. Cochrane, S.M., Parent, J.M., Black, W.D., Allen, D.G. and Lumsden, J.H. 1990. Pharmacokinetics of phenobarbital in the cat following multiple oral administration. Can. J. Vet. Res. 54, 309–312. Dewey, C.W., Guiliano, R., Boothe, D.M., Berg, J.M., Kortz, G.D., Joseph, R.J. and Budsberg, S.C. 2014. Zonisamide therapy for refractory idiopathic epilepsy in dogs. J. Am. Anim. Hosp. Assoc. 40, 285–291. Finnerty, K.E., Heller, H.L.B., Mercier, M.N., Giovanella, C.J., Lau, V.W. and Rylander, H. 2014. Evaluation of therapeutic phenobarbital concentrations and application of a classification system for seizures in cats: 30 cases (2004–2013). J. Am. Vet. Med. Assoc. 244, 195–199. Hasegawa, D., Kobayashi, M., Kuwabara, T., Ohmura, T., Fujita, M. and Orima, H. 2008. Pharmacokinetics and toxicity of zonisamide in cats. J. Feline. Med. Surg. 10, 418–421. Hermans, M., Charalambous, M., Pakozdy, A., Eisl-Glantschnigg, U., Neßler, J., Meervenne, S.A.V., Serrano, G., Cornelis, I., Ham, L.V., Paepe, D., Broeckx, B.J. and Bhatti, S.F. 2022. Evaluation of the effect of phenobarbital administration on the biochemistry profile, with a focus on serum liver values, in epileptic cats. J. Feline. Med. Surg. 24, 530–538. Hojo, T., Ohno, R., Shimoda, M. and Kokue, E. 2002. Enzyme and plasma protein induction by multiple oral administrations of phenobarbital at a therapeutic dosage regimen in dogs. J. Vet. Pharmacol. Ther. 25, 121–127. Hughes, D., Moreau, R.E., Overall, K.L. and Winkle, T.J.V. 1996. Acute hepatic necrosis and liver failure associated with benzodiazepine therapy in six cats, 1986–1995. J. Vet. Emerg. Crit. Care. 6, 13–20. Klopmann, T.V., Rambeck, B. and Tipold, A. 2007. Prospective study of zonisamide therapy for refractory idiopathic epilepsy in dogs. J. Small. Anim. Pract. 48, 134–138. Kwiatkowska, M., Hoppe, S., Pomianowski, A. and Tipold, A. 2019. Reactive seizures in cats: a retrospective study of 64 cases. Vet. J. 244, 1–6. Martin, H., Sarsat, J.P., de Waziers, I., Housset, C., Balladur, P., Beaune, P., Albaladejo, V. and Lerche-Langrand, C. 2003. Induction of cytochrome P450 2B6 and 3A4 expression by phenobarbital and cyclophosphamide in cultured human liver slices. Pharmaceut. Res. 20, 557–568. Mizuno, S., Asada, R. and Hasegawa, D. 2022. Questionnaire survey on the usage of antiseizure drugs for dogs and cats in Japanese veterinary hospitals (2020). Vet. Med. Sci. 8, 1466–1471. Nakamoto, Y., Uemura, T., Hasegawa, H., Nakamoto, M. and Ozawa, T. 2019. Feline neurological diseases in a veterinary neurology referral hospital population in Japan. J. Vet. Med. Sci. 81, 879–885. Orito, K., Saito, M., Fukunaga, K., Matsuo, E., Takikawa, S., Muto, M., Mishima, K., Egashira, N. and Fujiwara, M. 2008. Pharmacokinetics of zonisamide and drug interaction with phenobarbital in dogs. J. Vet. Pharmacol. Ther. 31, 259–264. Park, F.M. 2011. Successful treatment of hepatic failure secondary to diazepam administration in a cat. J. Feline. Med. Surg. 14, 158–160. Platt, S. and Risio, L.D. 2014. Idiopathic epilepsy and genetics. In Canine and feline epilepsy: diagnosis and management. Eds., Risio, L.D. and Platt, S. Wallingford, UK: CABI, pp: 207–218. Potschka, H., Fischer, A., Löscher, W., Patterson, N., Bhatti, S., Berendt, M., Risio, L.D., Farquhar, R., Long, S., Mandigers, P., Matiasek, K., Muñana, K., Pakozdy, A., Penderis, J., Platt, S., Podell, M., Rusbridge, C., Stein, V., Tipold, A. and Volk, H.A. 2015. International veterinary epilepsy task force consensus proposal: outcome of therapeutic interventions in canine and feline epilepsy. BMC. Vet. Res. 11, 177. Risio, L.D. 2014. Zonisamide. In Canine and feline epilepsy: diagnosis and management. Eds., Risio, L.D. and Platt, S. Wallingford, UK: CABI, pp: 414–424. Risio, L.D., Bhatti, S., Muñana, K., Penderis, J., Stein, V., Tipold, A., Berendt, M., Farqhuar, R., Fischer, A., Long, S., Mandigers, P.J.J., Matiasek, K., Packer, R.M., Pakozdy, A., Patterson, N., Platt, S., Podell, M., Potschka, H., Batlle, M.P., Rusbridge, C. and Volk, H.A. 2015. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC. Vet. Res. 11, 148. Schriefl, S., Steinberg, T.A., Matiasek, K., Ossig, A., Fenske, N. and Fischer, A. 2008. Etiologic classification of seizures, signalment, clinical signs, and outcome in cats with seizure disorders: 91 cases (2000–2004). J. Am. Vet. Med. Assoc. 233, 1591–1597. Shah, S.S., Sanda, S., Regmi, N.L., Sasaki, K. and Shimoda, M. 2007. Characterization of cytochrome P450-mediated drug metabolism in cats. J. Vet. Pharmacol. Ther. 30, 422–428. Smith, T.K., Cameron, S. and Trepanier, L.A. 2022. Incidence of hepatopathies in dogs administered zonisamide orally: a retrospective study of 384 cases. J. Vet. Intern. Med. 36, 576–579. Stanciu, G.D., Packer, R.M.A., Pakozdy, A., Solcan, G. and Volk, H.A. 2017. Clinical reasoning in feline epilepsy: which combination of clinical information is useful? Vet. J. 225, 9–12. | ||
| How to Cite this Article |
| Pubmed Style SY, Maeda S, Yonezawa T, Motegi T. Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Vet J. 2023; 13(7): 846-853. doi:10.5455/OVJ.2023.v13.i7.6 Web Style SY, Maeda S, Yonezawa T, Motegi T. Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. https://www.openveterinaryjournal.com/?mno=131930 [Access: May 17, 2024]. doi:10.5455/OVJ.2023.v13.i7.6 AMA (American Medical Association) Style SY, Maeda S, Yonezawa T, Motegi T. Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Vet J. 2023; 13(7): 846-853. doi:10.5455/OVJ.2023.v13.i7.6 Vancouver/ICMJE Style SY, Maeda S, Yonezawa T, Motegi T. Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Vet J. (2023), [cited May 17, 2024]; 13(7): 846-853. doi:10.5455/OVJ.2023.v13.i7.6 Harvard Style , S. Y., Maeda, . S., Yonezawa, . T. & Motegi, . T. (2023) Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Vet J, 13 (7), 846-853. doi:10.5455/OVJ.2023.v13.i7.6 Turabian Style , Shino Yoshida, Shingo Maeda, Tomohiro Yonezawa, and Tomoki Motegi. 2023. Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Veterinary Journal, 13 (7), 846-853. doi:10.5455/OVJ.2023.v13.i7.6 Chicago Style , Shino Yoshida, Shingo Maeda, Tomohiro Yonezawa, and Tomoki Motegi. "Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan." Open Veterinary Journal 13 (2023), 846-853. doi:10.5455/OVJ.2023.v13.i7.6 MLA (The Modern Language Association) Style , Shino Yoshida, Shingo Maeda, Tomohiro Yonezawa, and Tomoki Motegi. "Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan." Open Veterinary Journal 13.7 (2023), 846-853. Print. doi:10.5455/OVJ.2023.v13.i7.6 APA (American Psychological Association) Style , S. Y., Maeda, . S., Yonezawa, . T. & Motegi, . T. (2023) Evaluation of antiseizure medications including zonisamide in feline idiopathic epilepsy at a referral hospital in Japan. Open Veterinary Journal, 13 (7), 846-853. doi:10.5455/OVJ.2023.v13.i7.6 |