| Original Article | ||
Open Vet J. 2023; 13(2): 188-192 Open Veterinary Journal, (2023), Vol. 13(2): 188–192 Original Research Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in 10 dogsCurtis Wells Dewey1,2*, Mark Rishniw3, Kasie Sakovitch1 and Jordan Hollenbeck11Elemental Pet Vets, PLLC, Freeville, New York, USA 2Chi University, Reddick, Florida, USA 3Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA *Corresponding Author: Curtis Wells Dewey. Elemental Pet Vets, PLLC, Freeville, New York, USA. Email: elementalpetvets [at] outlook.com Submitted: 08/11/2022 Accepted: 14/01/2023 Published: 11/02/2023 © 2023 Open Veterinary Journal
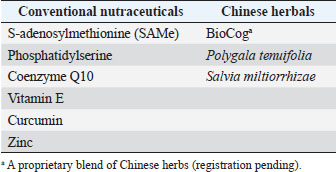
AbstractBackground: Canine cognitive dysfunction (CCD), the dog analog of human Alzheimer’s disease (AD), is a progressive neurodegenerative condition that presents many treatment challenges. There are few effective drugs with acceptable side effects for AD/CCD, which has prompted investigation into non-drug options, collectively termed nutraceuticals. Nutraceutical supplements are conceptually divided into conventional (Western) and non-conventional (Eastern) ingredients. Many of these individual supplements have shown in vitro and/or in vivo efficacy in ameliorating neuronal damage in rodent models, and some have demonstrated positive effects on cognition in rodent models and clinical trials in dogs and humans with cognitive impairment. Aim: The purpose of this open-label clinical trial was to investigate the effect of an oral integrative (combination of conventional nutraceuticals and Chinese herbals) supplement (CogniCaps®) on cognitive scores when administered to aging dogs with CCD over a 2-month period. Methods: Ten aging (>9-year-old) dogs with moderate (16–33) cognitive scores were recruited and administered oral CogniCaps® for two months. No additional drugs or nutraceuticals directed at improving cognitive function were allowed during the study period. Baseline cognitive scores were compared with those procured at 30 and 60 days. Cognitive scores for baseline, 30- and 60-days post-treatment were compared. Results: Cognitive scores improved at 30 days (38% reduction) and 60 days (41% reduction) post-treatment (p=0.002). Scores did not differ between 30- and 60-day assessments (p=0.7). Conclusion: The results of this small preliminary study suggest that the integrative supplement CogniCaps® might improve cognitive scores in dogs with CCD within the first 30 days of administration and that this improvement is sustained at 60-day follow up. Keywords: Canine, Cognitive, Alzheimer’s, Integrative, Supplement. IntroductionCanine cognitive dysfunction (CCD) is the canine analog of human Alzheimer’s disease (AD); both disorders are characterized by progressive cognitive decline and a host of pathophysiological aberrations that cause dysfunction of brain neurons and supporting cells. These aberrations are multifactorial and interrelated and include the following: oxidative and inflammatory neuronal damage; excitotoxic neuronal damage; brain vascular compromise; neuronal mitochondrial dysfunction; deposition of toxic beta-amyloid (Aβ) and tau protein around neurons and blood vessels; premature neuronal death (apoptosis); decline of brain cholinergic activity; and impaired neurogenesis (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019). The lack of safe and effective drugs for AD/CCD has prompted investigation into non-drug therapies to ameliorate cognitive decline, including nutraceuticals. Nutraceuticals comprise a wide variety of non-drug supplements and contain conventional (Western) and non-conventional (Eastern) ingredients. This conceptual division between conventional and non-conventional nutraceuticals is somewhat arbitrary and vague, with some previously considered non-conventional supplements having become standard (conventional) therapy as proof of efficacy has accumulated (Libro et al., 2016; Tewari et al., 2018; Makkar et al., 2020). There are numerous conventional and non-conventional (mainly Chinese herbal) individual nutraceutical supplements that have demonstrated beneficial effects against AD/CCD pathologic processes in vitro and in vivo; some of these supplements have also exhibited efficacy in rodent AD models and clinical trials in humans with AD (Yang et al., 2009; Chang et al., 2016; Libro et al., 2016; Jiang et al., 2017; Tewari et al., 2018; Makkar et al., 2020; Bednarikova et al., 2021; Zhou et al., 2022). For many of the Chinese herbals used for cognitive impairment, the active molecular compounds and their biochemical pathways of action have been elucidated (Yang et al., 2009; Chang et al., 2016; Jiang et al., 2017; Tewari et al., 2018; Bednarikova et al., 2021; Zhou et al., 2022). Cognicaps® is an integrative (combination of conventional nutraceuticals and Chinese herbals) supplement for CCD formulated by one of the authors (CD). Each individual ingredient was selected based on evidence of efficacy (research model and/or clinical trial) and tolerability (low incidence of adverse effects). The active ingredients are listed in Table 1. The purpose of this preliminary open-label clinical trial was to evaluate CogniCaps® in aging dogs with moderate cognitive impairment by comparing baseline cognitive questionnaire scores at 30- and 60-days post-supplement institution with baseline scores. We hypothesized that dogs would experience significant (p < 0.05) improvement in cognitive scores after treatment with the integrative supplement. Table 1. Active ingredients in CogniCaps® supplement.
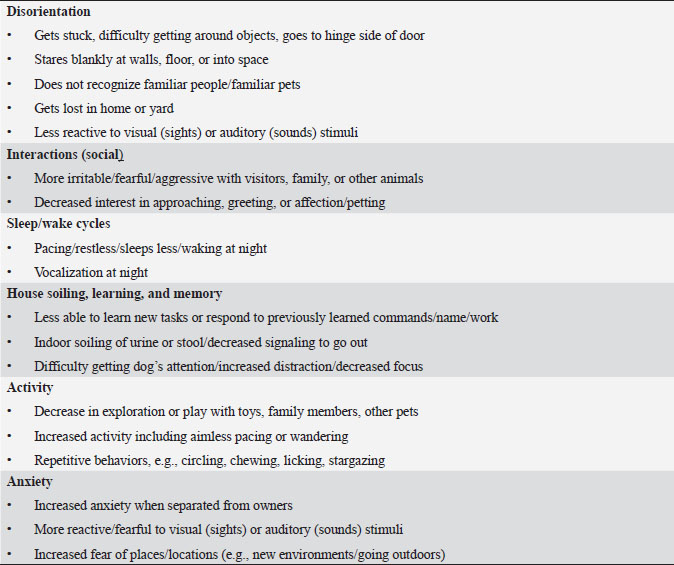
Table 2. DISHAA (Disorientation; Interactions-social; Sleep/wake cycle; House soiling, learning and memory; Activity; and Anxiety) scoring system used to assess cognitive ability in dogs. Each subcategory (bullet point) is scored as: 0=none, 1=mild, 2=moderate, and 3=severe. Total scores are assessed as: 4–15=mild impairment, 16–33=moderate impairment, >33=severe impairment.
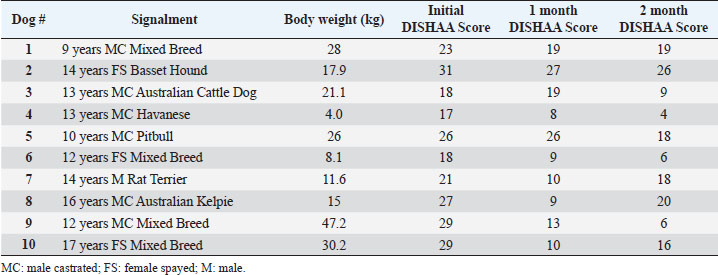
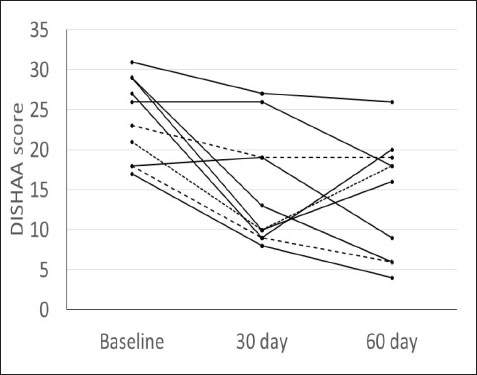
Materials and MethodsWe conducted a prospective open-label, non-comparative clinical trial. We recruited aging dogs (>9 years old) with a clinical diagnosis of CCD. Recent (within 3 months prior to the study) bloodwork (CBC, serum biochemistry) with absence of any metabolic cause for cognitive dysfunction (e.g., anemia, renal failure, etc.) was required for case inclusion. We based the diagnosis of cognitive dysfunction on historical complaints and neurologic examination findings. A board-certified neurologist (CD) conducted all neurological examinations. Clients consented to administering the integrative supplement (1 capsule for dogs 30 lbs and under, 2 capsules for dogs 30–60 lbs, etc.) twice daily for 60 days. No additional drugs or supplements (including dietary changes) directed at improving cognitive function were allowed during the study period. Each client filled out a cognitive dysfunction questionnaire used in a previously published research investigation (Table 2) (Pan et al., 2018) at time 0 (baseline) and 30- and 60-days after instituting the supplement. Briefly, this short questionnaire examines six variables: Disorientation, Interactions-social, Sleep/wake cycles, House soiling/learning/memory, Activity, and Anxiety (DISHAA) to produce a cumulative score that determines the degree of cognitive impairment or dysfunction. Case inclusion was restricted to dogs with cognitive scores in the moderate (16–33) or severe (>33) category. Statistical analysesBecause of the small sample population, we compared the DISHAA scores using a Friedman test, followed by pairwise Wilcoxon Signed Ranks tests (three tests). The alpha value was set at 0.05 for all these comparisons. Ethical approvalPermission from the animals’ owners was granted to participate in the clinical trial, and a clinical investigation client consent form was signed by the animal’s owner. ResultsWe recruited 13 aging dogs; however, three dogs were excluded from analysis: two because of non-compliance (not administering the supplement as directed, lack of follow-up), and one because of inappetence associated with the supplement (the supplement was discontinued prior to 30-day follow up). Therefore, we analyzed data from 10 dogs. Data from the 10 study dogs are summarized in Table 3. Breeds included mixed breed (4), and one each of Bassett Hound, Australian Cattle dog, Havanese, Pitbull, Rat Terrier, and Australian Kelpie. Ages ranged from 9 to 17 years (median=13 years). There were six male castrated, one male intact, and three female spayed dogs. No evidence of concurrent disease conditions was reflected in bloodwork results from any of the study participants. Median DISHAA scores for baseline and 30 and 60 day follow up were 24, 15, and 14.2, respectively. All dogs included in the study had DISHAA scores in the “moderate” category (range: 17–31). Cognitive scores decreased by 30 days (38%) and remained lower than baseline scores at 60 days (41%; p=0.002). We found no difference between 30- and 60-day cognitive scores (p =0.70) (Fig. 1). DiscussionOur small, open-label, uncontrolled study suggests that the integrative supplement CogniCaps® improves cognitive scores in aging dogs with moderate CCD. Based on the small sample size, the lack of a control group and the absence of more rigorous cognitive testing, these results should be viewed cautiously. However, because cognitive scores improved within 30 days in most dogs, clients might be more willing to enroll their pets in a placebo-controlled study. One dog experienced inappetence (and was excluded). Table 3. Signalment, body weights, and DISHAA scores for 10 dogs receiving CogniCaps®.
Fig. 1. DISHAA scores (y-axis) plotted against time (x-axis) for 10 dogs. Encouragingly, only one dog had a one-point higher DISHAA score at 30 days than at baseline, and another dog had the same score at 30 days as at baseline. However, DISHAA scores in both dogs decreased at 60 days. It is possible that these two dogs were simply not as responsive to the supplement as others in the study. It is also possible that the delayed response could reflect variability in metabolism of the supplement ingredients among dogs. Somewhat concerning was the rise in DISHAA scores in three dogs at 60 days (compared to the 30-day scores). Nevertheless, in all three dogs, these scores remained lower at 60 days than at baseline. Considering that CCD, like human AD, is a progressive disorder, the rise in DISHAA scores during the second half of the evaluation period may reflect disease progression in these patients. As with all uncontrolled clinical trials that use subjective measures of effect, the risk of client bias could have resulted in a more favorable outcome than might be real. It is possible that clients were more attentive to their dogs during the study period than usual, which could have influenced their answers to the questionnaire forms. More objective and non-biased methods of cognitive testing are available for evaluating dogs with CCD, including delayed nonmatching to positioning memory tasks, attention tasks, food searching tasks, and problem-solving tasks (Landsberg et al., 2012; Chapagain et al., 2018; Gonzalez-Martinez et al., 2013). Despite the limitations of this preliminary study, our observations warrant further investigation into the efficacy and tolerability of CogniCaps® in a larger cohort of dogs with CCD with a placebo control and more stringent behavioral testing. In addition to investigating this supplement via a placebo-controlled study, long-term follow-up would be useful information, considering the progressive nature of CCD. Conflict of interestDr. Dewey formulated CogniCaps® and receives a percentage of sales. ReferencesBednarikova, Z., Gancar, M., Wang, R., Zheng, L., Tang, Y., Luo, Y., Huang, Y., Spodniakova, B., Ma, L. and Gazova, Z. 2021. Extracts from Chinese herbs with anti-amyloid and neuroprotective activities. Int. J. Biol. Macromol. 179, 475–484. Chang, D., Liu, J., Bilinski, K., Xu, L., Steiner, G.Z., Seto, S.W. and Bensoussan, A. 2016. Herbal medicine for the treatment of vascular dementia: an overview of scientific evidence. Evid. Based Complement Alternat. Med. 2016, 7293626. Chapagain, D., Range, F., Huber, L. and Viranyi, Z. 2018. Cognitive aging in dogs. Gerontology 64(2), 165–171. Dewey, C.W., Davies, E.S., Xie, H. and Wakshlag, J.J. 2019. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. North Am. Small Anim. Pract. 49, 477–499. Gonzalez-Martinez, A., Rosado, B., Pesini, P., Garcia-Belenguer, S., Palacio, J., Villegas, A., Suarez, M.L., Santamarina, G. and Sarasa, M. 2013. Effect of age and severity of cognitive dysfunction on two simple tasks in pet dogs. Vet. J. 198(1), 176–181. Jiang, Y., Gao, H. and Turdu, G. 2017. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorg. Chem. 75, 50–61. Landsberg, G.M., Nichol, J. and Araujo, J.A. 2012. Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet. Clin. North Am. Small Anim. Pract. 42, 749–768. Libro, R., Giacoppo, S., Soundara Rajan, T., Bramanti, P. and Mazzon, E. 2016. Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview. Molecules 21(4), 518. Makkar, R., Behl, T., Bungau, S., Zengin, G., Mehta, V., Kumar, A., Uddin, M.S., Ashraf, G.M., Abdel-DAim, M.M., Arora, S. and Oancea, R. 2020. Nutraceuticals in neurological disorders. Int. J. Mol. Sci. 21(12), 4424. Pan, Y., Landsberg, G., Mougeot, I., Kelly, S., Xu, H., Bhatnagar, S., Gardner, C.L. and Milgram, N.W. 2018. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): a prospective double blinded placebo controlled clinical study. Front. Nutr. 12(5), 1–10. Tewari, D., Stankiewicz, A.M., Mocan, A., Sah, A.N., Tzvetkov, N.T., Huminiecki, L., Horbańczuk, J.O. and Atanasov, A.G. 2018. Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front. Aging Neurosci. 10, 3. Yang, X., Dai, G., Li, G. and Yang, E.S. 2009. Coenzyme Q10 Reduces β-amyloid plaque in an APP/PS1 transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 41(1), 110–113. Zhou, X., Venigalla, M., Raju, R. and Münch, G. 2022. Pharmacological considerations for treating neuroinflammation with curcumin in Alzheimer’s disease. J. Neural. Transm. 129(5–6), 755–771. | ||
| How to Cite this Article |
| Pubmed Style Dewey CW, Rishniw M, Sakovitch K, Hollenbeck JE. Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Vet J. 2023; 13(2): 188-192. doi:10.5455/OVJ.2023.v13.i2.6 Web Style Dewey CW, Rishniw M, Sakovitch K, Hollenbeck JE. Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. https://www.openveterinaryjournal.com/?mno=127893 [Access: July 16, 2025]. doi:10.5455/OVJ.2023.v13.i2.6 AMA (American Medical Association) Style Dewey CW, Rishniw M, Sakovitch K, Hollenbeck JE. Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Vet J. 2023; 13(2): 188-192. doi:10.5455/OVJ.2023.v13.i2.6 Vancouver/ICMJE Style Dewey CW, Rishniw M, Sakovitch K, Hollenbeck JE. Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Vet J. (2023), [cited July 16, 2025]; 13(2): 188-192. doi:10.5455/OVJ.2023.v13.i2.6 Harvard Style Dewey, C. W., Rishniw, . M., Sakovitch, . K. & Hollenbeck, . J. E. (2023) Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Vet J, 13 (2), 188-192. doi:10.5455/OVJ.2023.v13.i2.6 Turabian Style Dewey, Curtis Wells, Mark Rishniw, Kasie Sakovitch, and Jordan Elizabeth Hollenbeck. 2023. Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Veterinary Journal, 13 (2), 188-192. doi:10.5455/OVJ.2023.v13.i2.6 Chicago Style Dewey, Curtis Wells, Mark Rishniw, Kasie Sakovitch, and Jordan Elizabeth Hollenbeck. "Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.." Open Veterinary Journal 13 (2023), 188-192. doi:10.5455/OVJ.2023.v13.i2.6 MLA (The Modern Language Association) Style Dewey, Curtis Wells, Mark Rishniw, Kasie Sakovitch, and Jordan Elizabeth Hollenbeck. "Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.." Open Veterinary Journal 13.2 (2023), 188-192. Print. doi:10.5455/OVJ.2023.v13.i2.6 APA (American Psychological Association) Style Dewey, C. W., Rishniw, . M., Sakovitch, . K. & Hollenbeck, . J. E. (2023) Oral administration of an integrative supplement (CogniCaps®) improves cognitive scores in aging dogs with canine cognitive dysfunction for at least two months: An open-label investigation in ten dogs.. Open Veterinary Journal, 13 (2), 188-192. doi:10.5455/OVJ.2023.v13.i2.6 |