| Original Article | ||
Open Vet J. 2023; 13(3): 288-296 Open Veterinary Journal, (2023), Vol. 13(3): 288–296 Original Research Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovary syndromeAchmad Rheza1, Budi Santoso1 and Widjiati Widjiati2*1Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Widjiati Widjiati. Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: widjiati [at] fkh.unair.ac.id. Submitted: 31/10/2022 Accepted: 10/02/2023 Published: 07/03/2023 © 2023 Open Veterinary Journal
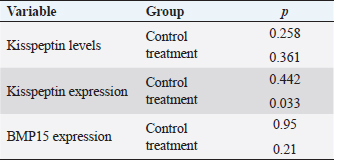
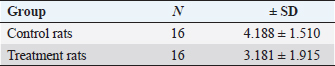
AbstractBackground: Kisspeptin is a neuropeptide that has an important role in the female reproductive cycle which is indicated by its role in regulating the hypothalamic-pituitary-gonadal axis. Aims: To analyze the correlation between serum kisspeptin levels, ovarian kisspeptin expression, and ovarian Bone Morphogenic Protein-15 (BMP15) expression in polycystic ovary syndrome (PCOS) model rats. Methods: The research was accurate experimental research with a post-test design-only control group and was carried out from August to October 2022 at the Faculty of Veterinary Medicine Universitas Airlangga. 32 Rattus novergicus rats were divided into a control group and a PCOS model group. Blood serum and ovaries were obtained from all groups. In addition, blood serum was examined for kisspeptin levels by ELISA technique, and kisspeptin expression and BMP15 Ovaries were examined immunohistochemically. Results: Serum kisspeptin levels and ovarian kisspeptin expression of the PCOS model group were not significantly higher than those of the control group (p > 0.05, p > 0.05). The ovarian BMP15 expression of the PCOS model group was not significantly lower (p > 0.05) than that of the control group. Ovarian kisspeptin expression and ovarian BMP15 expression did not significantly correlate with serum kisspeptin levels (p > 0.05). In contrast, there was a significant correlation (p < 0.05) between ovarian kisspeptin expression and ovarian BMP15 expression. Conclusion: Serum kisspeptin levels and ovarian kisspeptin expression of the PCOS model group were not higher than those of the control group, and the ovarian BMP15 expression of the PCOS model group was not lower than that of the control group. There was no correlation between serum kisspeptin levels with ovarian kisspeptin expression and ovarian BMP15 expression. However, a significant correlation was found between ovarian kisspeptin expression and ovarian BMP15 expression. Keywords: BMP15, Kisspeptin, Oocyte quality, PCOS, Reproductive health. IntroductionThe polycystic ovary syndrome (PCOS) is defined by the presence of a hyperandrogenic state associated with chronic anovulation in women without adrenal or pituitary disorders (Azziz et al., 2016; Speroff et al., 2020) until now; the main cause is still evident. The World Health Organization estimates that more than 116 million women (3.4%) have PCOS worldwide (Azziz et al., 2016). PCOS is widely considered to be the leading cause of infertility in women of childbearing age, with an incidence of around 6%–21%, with causes of infertility including ovulatory factors (20%–40%), tubal and peritoneal factors (30%–40%), male factor (30%–40%), and the rest are mainly unexplained (Naqvi et al., 2020; Bulsara et al., 2021). Abnormalities of Gonadotropin-Releasing Hormone (GnRH) pulsations that occur in PCOS patients will increase the Luteinizing Hormone/Follicle Stimulating Hormone (LH/FSH) ratio, resulting in disruption of folliculogenesis, steroidogenesis, and increased intra-ovarian androgen production which ultimately results in impaired oocyte maturation (Dumesic et al., 2015; Speroff et al., 2020). The quality of the oocyte is determined by factors such as the ability of meiotic maturation, fertilization, and good embryonic development, and it ends in a healthy pregnancy. Conversely, disruption of follicular growth in PCOS patients leads to a decrease in the number of good oocytes/embryos, which ultimately increases the rate of infertility (Li et al., 2021). A kisspeptin is a group of neuropeptides that play an essential role in the function of the hypothalamic-pituitary-gonadal axis (HPG axis), where its expression and receptors can be found in various body organs, from central to peripheral tissues such as the ovaries, especially in granulosa cells, theca cells, cumulus-oocyte complex, and corpus luteum (Harter et al., 2018). Kisspeptin can directly or indirectly influence reproduction and the central nervous system. Kisspeptin can stimulate the release of gonadotropins by two mechanisms; indirectly by, kisspeptin neurons that bind to their receptors will stimulate GnRH neurons in the hypothalamus, which will then secrete LH and FSH through stimulation in the pituitary. Second, Kisspeptin neurons will directly bind to their receptors in the pituitary, which will then stimulate the secretion of LH and FSH (Hameed et al., 2011; Basini et al., 2018; Cao et al., 2019). Disruption in the HPG feedback pathway, which increases the LH/FSH ratio, is thought to be associated with the onset of PCOS in approximately 35%–90% of patients (Hameed et al., 2011). Increased levels of kisspeptin will lead to disruption of the hypothalamic-pituitary-ovarian axis, disrupting the pulsation of GnRH, resulting in an increase in the LH/FSH ratio, causing hyperandrogenic and hyperinsulinemic conditions and, ultimately, insulin resistance. Bone Morphogenic Protein-15 (BMP15) is an oocyte-derived growth factor member of the transforming growth factor-b. The ovary’s mRNA protein was primarily found in oocytes and granulosa cells. BMP15 is initially found in primordial follicular oocytes and is progressively expressed by oocytes in growing follicles during folliculogenesis up to 12-hour postovulatory oocytes (Wei et al., 2014). BMP15 has an essential function as a fertility marker in humans. It plays an important role in granulosa cells, follicular development, and embryo quality, so the impaired expression of these factors leads to infertility (Sanfins et al., 2019). BMP15 promotes follicular growth and maturation, regulates the sensitivity of follicular granulosa cells to the action of FSH and determines ovulation, prevents granulosa cell apoptosis, and increases oocyte developmental competence (Persani et al., 2014). Therefore, disrupting the BMP15 signaling pathway can affect the oocyte maturation process and reduce developmental potential. In addition, there is increased apoptosis of granulosa cells and changes in the expression level of apoptotic proteins in PCO, which may be related to abnormal changes in the BMP15 signaling pathway. Oocytes taken from follicles with high levels of BMP15 have a higher fertilization rate, better division, and the potential for better embryonic development and quality than oocytes from follicles with relatively low levels of BMP15, so it can be concluded that oocyte quality is a crucial factor to determine embryonic development (Wu et al., 2007). Impaired folliculogenesis and steroidogenesis processes in PCOS lead to impaired follicular development, as indicated by low BMP15 expression in PCOS patients. In this study, we used rats as a PCOS model using an androgen in the form of testosterone propionate (TP). Experimental animals were used as the research method that we performed was contrary to ethical problems where the inspection measures carried out were too invasive on humans. TP was used in this study based on studies that previously have been carried out, which obtained model rats having similar conditions to PCOS in humans. Rats, used as PCOS model in several previous studies, have similarities to human PCOS conditions according to the Rotterdam criteria, such as the presence of anoestrous conditions (not ovulation/anovulation) as evidenced by vaginal swabs and by histopathological examination, which showed a low level of Graffian follicles and corpus luteum, hyperthecosis, high level of cystic follicles, thick ovarian walls, and insulin resistance conditions (Beloosesky et al., 2004; Singh, 2005; Manneras et al., 2007; Santoso, 2009). This study aimed to examine the effect of kisspeptin on oocyte quality in PCOS model rats and whether serum kisspeptin levels can represent ovarian kisspeptin concerning oocyte quality as indicated by BMP15 parameters. Materials and MethodsAnimalsThis study used Rattus novergicus strain Wistar rats as a model for PCOS. R. Novergicus is an animal with a weight of 160–180 grams, 3 months old, and a length of about 40 cm from nose to tail with a shorter tail than its body. MethodsThere were 32 rats divided into two groups (16 rats in each group), namely the control group (P0) and the PCOS model group (P1). Treatment was conducted for 28 days for each group. The rats in the control group were injected with propylene glycol for 28 days, and those in the model group were injected with TP for 28 days. Propylene glycol was used as one of the TP solvents, so to make the same treatment as the model group, we used propylene glycol in the control group rats. The use of TP, doses, and length of treatment was based on a previous study that would produce similar conditions/characteristics to PCOS in humans. Before being injected, all rats in both groups confirmed that they were in the proestrus phase. Likewise, when they were about to be terminated, they were in the metestrous/diestrous stage. In the P1 group, we created a rat model of PCOS by administering TP injections at a dose of 1 g/100 g body weight (BW) rats for 28 days. Based on previous studies, injections of TP in PCOS model rats for 28 days would produce conditions/characteristics similar to PCOS in humans, with the anestrous phase obtained through vaginal swabs and the appearance of polycystic ovaries, hyperthecosis, luteinized stroma, and insulin resistance conditions (Beloosesky et al., 2004; Singh, 2005; Manneras et al., 2007; Santoso, 2009). In the control group, propylene glycol was injected for 28 days. After 28 days of injection, the rats in both groups were terminated. To check the kisspeptin levels in each rat, blood samples were taken intracardially and then examined by ELISA method using the rat kisspeptin 1 ELISA kit (Cat. No E0905Ra, Bioassay Technology Laboratory). The expression of ovarian kisspeptin and ovarian BMP15 samples for the term kisspeptin and BMP15 were taken from the ovaries of each rat. The rats were terminated after 28 days of treatment by os cervical dislocation. Expression of kisspeptin and BMP15 in the ovary was examined by immunohistochemical method. Ovarian samples were assessed semi-quantitatively according to the modified Remmele method (Novak et al., 2007), where the Remmele scale index (Immuno Reactive Score/IRS) is the result of multiplying the percentage score of immunoreactive cells with the color intensity score. The data for each sample was the average value of IRS observed in 10 different fields of view at 400× magnification. This examination was carried out using a Nikon E100 light microscope equipped with a 12-megapixel Optilab advance plus digital camera and image raster image processing software. Statistical analysisThe research data were tested for normality using the Kolmogorov-Smirnov test. If the data has a distribution of p < 0.05, the analysis will be continued using the 2-sample-test if it is normally distributed and using the Mann-Whitney U test if it is not normally distributed. The relationship between a set of variables using intermediate variables was subjected to regression tests; the regression test shows significant results if p < 0.05. Ethical approvalThis study has received ethical clearance from the Animal Care and Use Committee Faculty of Veterinary Medicine Universitas Airlangga, Surabaya, Indonesia (number 2.KE.108.8.2022). All procedures carried out in this study were by the United Kingdom Animal Act 1986. All surgeries were conducted under anesthesia, and all efforts were made to minimize the suffering. ResultsThe treatment results to each group, where the rats in the control group were injected with propylene glycol, while the PCOS model group was given an injection of TP at a dose of 1 mg/100 g of BW. Each group was treated for 28 days, then the rats in both groups were terminated, and there was an increase in the rats’ BW compared to that before treatment. This characteristic shows an increase in average values of the rats’ BW after treatment compared to those before treatment. From the results of the Kolmogorov-Smirnoff normality test with a significance number of p < 0.05, the three parameters obtained different results, in which the effects on serum kisspeptin levels and ovarian BMP15 expression were normally distributed, while the results on the test on ovarian kisspeptin expression parameters were not normally distributed. From the results of the normality test shown in Table 1, for the parameters of serum kisspeptin levels, a difference analysis test was carried out using an independent t-test because the two data were normally distributed, and this test was to compare the control group and the PCOS model group. Table 1. The results of the Shapiro-Wilk normality test for serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression.
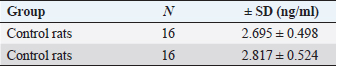
Table 2. The results of the independent t-test difference in serum kisspeptin levels.
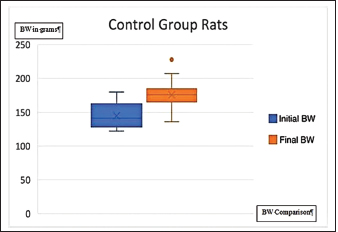
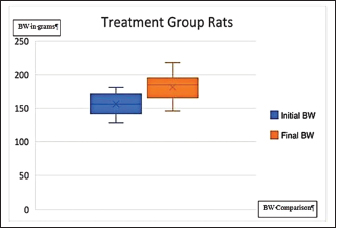
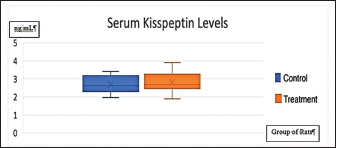
Fig. 1. Graph of control group rat BW before and after administration of propylene glycol (BW=body weight). Table 2 shows the results of the analysis of the f difference in serum kisspeptin levels p < 0.502, statistically showing no significant difference between the two groups, although the average value of the kisspeptin level of the treatment group is higher than that of the control group. Several studies indicate that kisspeptin levels of PCOS patients are higher than that of healthy women. Figure 1 shows a graph of the rats’ BW in the control group before and after the administration of propylene glycol. Figure 2 shows a diagram of the rats’ BW in the treatment group before and after administering TP. Figure 3 shows a graph of the difference in serum kisspeptin levels between the control and treatment groups. From the results of the normality test, the ovarian kisspeptin expression parameter is shown in Table 3. In addition, a Mann-Whitney difference analysis test was performed because one of the data in the treatment group was not normally distributed, and the purpose of the test was to compare the control group and the PCOS model group.
Fig. 2. The graph on the rats’ BW of the treatment group before and after administration of TP (BW=body weight).
Fig. 3. Kisspeptin serum levels in control and treatment groups. Table 3. The results of the Mann-Whitney difference test of ovarian kisspeptin expression.
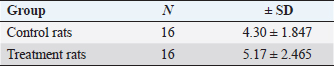
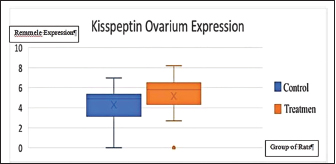
The analysis results on the difference in ovarian kisspeptin expression were obtained with p < 0.502. This statistically indicated no significant difference between the two groups, although the average value of ovarian kisspeptin expression of the treatment group was higher than that of the control group. Figure 4 shows a graph of the difference in ovarian kisspeptin expression between the control and treatment groups. Figure 5 shows images of ovarian kisspeptin expression seen using the immunohistochemical method, where kisspeptin expression is demonstrated by the expression obtained in ovarian Graafian follicles of PCOS model rats with an image that shows the expression of immunoreactive cells presented in brown color, which is the binding of antigen, antibody, and chromogen. The image shows that the expression of kisspeptin in the treatment group is higher than that in the control group. From the results of the normality test, a difference analysis test was carried out using an independent t-test for the ovarian BMP15 expression parameter because the two data were normally distributed, and the test was intended to compare the control group and the PCOS model group.
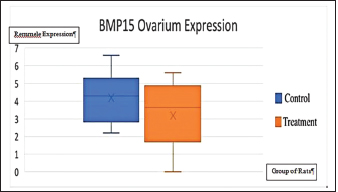
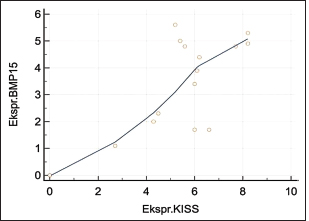
Fig. 4. Expression of kisspeptin serum in the control and treatment groups. Table 4 shows the analysis results on the difference in ovarian BMP15 expression with p < 0.109. This statistically indicates no significant difference between the two groups, although the average value of ovarian BMP15 expression of the treatment group is lower than that of the control group. Figure 6 illustrates the differences in ovarian BMP15 expression in the two groups. Figure 7 shows images of ovarian BMP15 expression seen using the immunohistochemistry method, where BMP15 expression is demonstrated by the expression obtained in ovarian Graafian follicles of PCOS model rats with an image that shows the expression of immunoreactive cells presented in brown color, which is a binding of antigen, antibody, and chromogen. This image shows that the BMP15 expression of the treatment group is slightly lower than that of the control group. After the differential test was performed, the correlation test was carried out to find the correlation between parameters. Table 5 shows the correlation test results between serum kisspeptin levels and ovarian kisspeptin expression, with p: 0.675 (p < 0.05). This statistically indicates no significant correlation between serum kisspeptin levels and ovarian kisspeptin expression in this study. Secondly, we tested the correlation between serum kisspeptin levels and ovarian BMP15 expression, as shown in Table 6, where p: 0.239 (p < 0.05) was obtained. This also showed no significant correlation between serum kisspeptin levels and ovarian BMP15 expression. Next, we tested the correlation between ovarian kisspeptin expression and ovarian BMP15 expression. Table 7 shows the correlation test with p: 0.021 (p < 0.05). This shows a significant result, so it can be concluded that there is a significant correlation between ovarian kisspeptin expression and ovarian BMP15 expression. Figure 8 shows a graph of the significant correlation between ovarian kisspeptin expression and ovarian BMP15 expression.
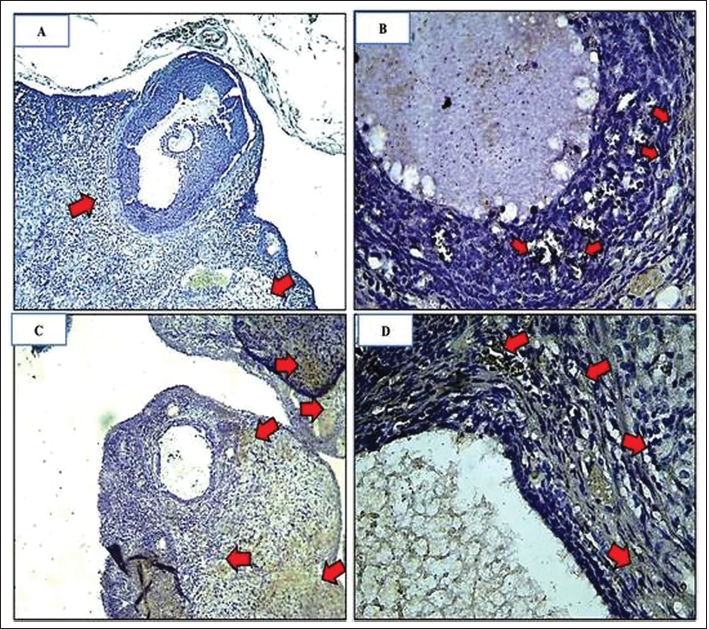
Fig. 5. Characteristics of ovarian kisspeptin expression. Comparison of kisspeptin expression in granulosa cells in rat ovaries. Arrows indicate kisspeptin expression, which is indicated by the presence of chromogen brown color (arrows). (A): group C(−). IHC, Bar=110 µm; (B): group C(−) IHC, Bar=50 µm; (C): group C(+) IHC Bar=110 µm; (D): group C(+) IHC Bar=50 µm. Table 4. Results of independent t-test differences in ovarian BMP15 expression.
Table 5. Correlation of serum kisspeptin levels with ovarian kisspeptin expression.
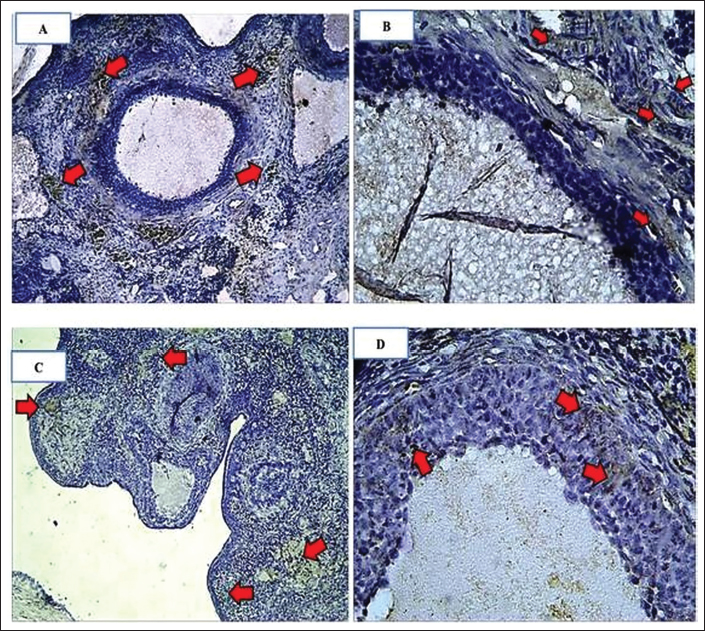
Fig. 6. Expression of ovarian BMP15 in control and treatment groups.
Fig. 7. Characteristics of ovarian BMP15 expression. Comparison of BMP15 expression in granulosa cells in mouse ovaries. The arrow indicates the expression of BMP15, which is indicated by the presence of a chromogen brown color (arrow). (A): group C(−). IHC, Bar=110 µm; (B): group C(−) IHC, Bar=50 µm; (C): group C(+) IHC Bar=110 µm; (D): group C(+) IHC Bar=50 µm. Table 6. Correlation of serum kisspeptin levels with ovarian BMP15 expression.
Table 7. Correlation of ovarian kisspeptin expression with ovarian BMP15 expression.
Fig. 8. Graph of the relationship between ovarian kisspeptin expression and expression of BMP15. DiscussionSeveral studies on kisspeptin in PCOS show an increase in kisspeptin levels in PCOS patients compared to that in healthy women. Kisspeptin levels also differ in a woman, depending on her ovulation cycle. The levels are the lowest during the follicular phase, increase during pre-ovulation, and are at the highest level during the luteal phase. Increased kisspeptin levels in the preovulatory phase are thought to be responsible for the LH surge. The kisspeptin secretion varies endogenously within an ovulatory cycle and is unrelated to estradiol. In addition, there are differences in kisspeptin levels influenced by age. Kisspeptin levels will be disturbed if a woman suffers from PCOS (Latif and Rafique, 2015). In our study, the serum kisspeptin levels of the PCOS model group were not significantly higher (p > 0.05) than that of the control group. However, the average values of the serum kisspeptin levels in the PCOS model group were higher than in the control group. The experimental rats in this study were treated with TP for 28 days so that PCOS model rats were obtained with similar characteristics and conditions to PCOS in humans as well as metabolic disorders such as insulin resistance; this was carried out based on previous studies (Beloosesky et al., 2004; Singh, 2005; Manneras et al., 2007; Santoso et al., 2009). The results of our study are in line with the previous study by Ozay et al. (2016), in which his research revealed that there was a slight increase in kisspeptin levels in the PCOS group compared to that of the regular group, but statistically, no significant difference was found, and it was also concluded that kisspeptin was directly proportional to LH and leptin levels. However, it did not significantly correlate with insulin levels, HOMA- IR, and glucose/insulin ratio (Ozay et al., 2016). Chen et al. (2010) in his research stated that there was an increase in kisspeptin levels in PCOS patients compared to the control group, and it was positively correlated with LH and testosterone levels. On the contrary, it was concluded that there was no correlation between kisspeptin and insulin resistance, which is probably due to the small number of samples in the research. Yilmaz et al. (2014) in study concluded that there was an increase in kisspeptin levels in PCOS patients compared to that of the control group. It was positively correlated with LH, T, DHEAS, mFG scores, and FAI and is negatively correlated with SHBG. In this study, there was no correlation between increased levels of kisspeptin and insulin resistance in PCOS patients. The research on the variation of kisspeptin in PCOS patients stated that PCOS patients were significantly associated with increased kisspeptin levels, increased LH, and decreased FSH compared to healthy people. There was no relationship between PCOS, prolactin, estradiol, and insulin conditions (Akad et al., 2022). Emecky et al. stated that although there was an increase in kisspeptin levels in PCOS patients compared to normal women, there was no significant difference in kisspeptin levels in regular PCOS patients, and there was a positive correlation between kisspeptin levels and LH, Testosterone, and DHEA levels (Gorkem et al., 2018). We consider that kisspeptin’s working mechanisms cannot control several hormonal parameters, which can affect the significance of the results in this study. As a result, this research was carried out on experimental animals, which cannot be said to be 100% similar to PCOS in humans. However, the PCOS model rats have similar characteristics to PCOS patients. Therefore, although the results obtained are similar to the human conditions, it can affect the study results. Ovarian kisspeptin expression of PCOS model rats was higher than that of the control group. The expression of kisspeptin and kisspeptin receptors in the ovary indicates that kisspeptin also acts locally in the ovary. The granulosa cells are the main synthesis site of kisspeptin. (Cao et al., 2018). Kisspeptin directly affects granulosa cells due to a large number of kisspeptin mRNA in the ovaries of PCOS patients and the increased expression of KISS and KISS1R in ovarian hyperstimulation (Araujo et al., 2020; Risvanli et al., 2020). From the study results, we analyzed that there may be different working mechanisms that affect the significance of the effects on kisspeptin expression in the ovaries, as we know that kisspeptin can directly or indirectly affect the ovaries, where kisspeptin is found not only in the hypothalamus but also the kisspeptin gene or its receptor in the ovary so that it can work directly locally. So, in the beginning, an increase in kisspeptin will disrupt the hypothalamic-pituitary axis, and in the end, it will the aromatization process. Calculating the ovarian BMP15 expression in the control and treatment groups had results that were not significantly lower (p > 0.05). However, the average values of BMP15 expression of the treatment group were lower than those of the control group. This shows that the PCOS model rats have poor oocyte quality. The insignificant results of the analysis test may be caused by the use of experimental animals in the study. However, the experimental animals have similar characteristics and conditions to PCOS in humans. The correlation test showed no significant correlation between serum kisspeptin levels with ovarian kisspeptin expression and ovarian BMP15 expression. The possibility was that there was a difference in central and peripheral working mechanisms, where kisspeptin can be influenced by several hormones such as Leptin, Estradiol, LH, Testosterone, HOMA-IR, and GDF9 so that it can affect the test results. Another possibility is that there are still differences between studies conducted on PCOS model rats and those directly on humans with PCOS; of course, it will give significant results if undertaken directly on humans. Consequently, the clinical use of serum kisspeptin levels cannot represent kisspeptin expression in the ovaries. The last correlation test between ovarian kisspeptin expression and ovarian BMP15 expression showed a significant correlation with p: 0.021 (p < 0.05), so it can be said that there is a significant correlation between ovarian kisspeptin expression and ovarian BMP15 expression. These results are in line with several studies showing that kisspeptin receptors are also found in the ovaries, especially in granulosa cells, which increases kisspeptin levels in PCOS and decreases the BMP15 expression (Persani et al., 2014; Sanfins et al., 2018). Furthermore, the reduced expression of BMP15 in the ovary is in accordance with the theory that shows that oocytes with low levels of BMP15 will have poorer oocyte quality than normal ones (Wei et al., 2014). The study conducted has not researched the effect of various types of PCOS, therefore, it is expected that the following research will investigate that. ConclusionSerum kisspeptin levels and ovarian kisspeptin expression of the PCOS model group were not higher than those of the control group, and the ovarian BMP15 expression of the PCOS model group was not lower than that of the control group. There was no correlation between serum kisspeptin levels with ovarian kisspeptin expression and ovarian BMP15 expression. However, a significant correlation was found between ovarian kisspeptin expression and ovarian BMP15 expression. It can be concluded that an increase in kisspeptin did not affect oocyte quality as indicated by BMP15. However, another hormonal parameter not examined in this study may affect oocyte quality, such as Leptin, Estradiol, LH, FSH, Testosterone, HOMA-IR, and GDF9, and the various types of PCOS may also affect the oocyte quality in PCOS, therefore it requires further research. Authors’ contributionAR, WW, and BS designed this research. AR, WW, and BS conducted a survey and took samples at the samples field. All authors examined samples in the research laboratory. All authors compiled, read, revised, and approved the final manuscript. Conflict of interestAll authors declare that there is no conflict of interest. FundingThe authors received financial support for implementing Internal Research Universitas Airlangga Number 978/UN3/2022 and publishing this article. ReferencesAkad, M., Socolov, R., Furnică, C., Covali, R., Stan, C.D., Crauciuc, E. and Pavaleanu, I. 2022. Article: kisspeptin variations in patients with polycystic ovary syndrome—a prospective case-control study. Medicina 58, 776. Araujo, B.S., Baracat, M.C.P., Dos Santos, S.R., de Oliveira, N.C., Maciel, G.A.R., Lobo, R.A., Soares-Jr, J.M. and Baracat, E.C. 2020. Kisspeptin influence on polycystic ovary syndrome-a mini review. Reprod. Sci. 27(2), 455–460. Azziz, R., Carmina, E., Chen, Z., Dunaif, A., Laven, J.S., Legro, R.S., Lizneva, D., Natterson-Horowtiz, B., Teede, H.J. and Yildiz, B.O. 2016. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2, 16057. Basini, G., Grasselli, F. and Bussolati, S. 2018. Presence and function of kisspeptin/KISS1R system in swine ovarian follicles. Theriogenology 115, 1–8. Beloosesky, R., Gold, R., Almog, B., Sasson, R., Dantes, A., Land-Bracha, A., Hirsh, L., Itskovitz-Eldor, J., Lessing, J.B., Homburg, R. and Amsterdam, A. 2004. Induction of polycystic ovary by testosterone in immature female rats: modulation of apoptosis and attenuation of glucose/insulin ratio. Int. J. Mol. Med. 14(2), 207–215. Bulsara, J., Patel, P., Soni, A. and Acharya, S. 2021. A review: brief insight into polycystic ovarian syndrome. Endocr. Metab. Sci. 3, 100085. Cao, Y., Li, Z., Jiang, W., Ling, Y. and Kuang, H. 2019. Reproduction function of kisspeptin/KISS1R system in the periphery. Reprod. Biol. Endocrinol. 17, 65–84. Chen, X., Mo, Y., Li, L., Chen, Y., Li, Y. and Yang, D. 2010. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 149, 72–76. Dumesic, D.A., Oberfield, S.E., Stener-Victorin, E., Marshall, J.C., Laven, J.S. and Legro, R.S. 2015. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr. Rev. 36(5), 487–525. Gorkem, U., Togrul, C., Arslan, E., Oruc, A.S. and Duman, N.B. 2018. PCOS pathogenesis: kisspeptin role. Is there a role for kisspeptin in pathogenesis po polycystic ovary syndrome? Gynecol. Endocrinol. 34(2), 157–160. Hameed, S., Jayasena, C.N. and Dhillo, W.S. 2011. Review: kisspeptin and fertility. J. Endocrinol. 208(2), 97–105. Harter, C.J.L., Kavanagh, G.S. and Smith, J.T. 2018. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 238(3), R173–R183. Latif, R. and Rafique, N. 2015. Serum kisspeptin levels across different phases of the menstrual cycle and their correlation with serum oestradiol. Neth. J. Med. 73(4), 175–178. Li, J., Chen, H., Gou, M., Tian, C., Wang, H., Song, X., Keefe, D.L., Bai, X. and Liu, L. 2021. Molecular features of polycystic ovary syndrome revealed by transcriptome analysis of oocytes and cumulus cells. Front. Cell. Dev. Biol. 6(9), 735684. Manneras, L., Cajander, S., Holmang, A., Seleskovic, Z. and Lystic, T. 2007. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148(8), 3781–3791. Naqvi, S.M.A.S., Bhattarai, J.B., Li, H. and Wang, X.W. 2020. Polycystic ovary syndrome and infertility. Int. J. Adolesc. Med. Health. 34(2), 1–9. Ozay, O.E., Ozay, A.C., Acar, B., Cagliyan, E., Secil, M. and Kume, T. 2016. Original article. Role of kisspeptin in polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 32(9), 718–722. Persani, L., Rosssetti, R., Di Pasquale, E., Cacciatore, C. and Fabre, S. 2014. The fundamental role of bone morphogenic protein 15 in ovarian function and its involvement in female fertility disorders. Hum. Reprod. Update. 20(6), 869–883. Risvanli, A., Ocal, H., Timurkaan, N., Ipek, P., Seker, I. and Karabulut, B. 2020. Expression of the anti-mullerian hormone, kisspeptin 1, and kisspeptin 1 receptor in polycystic ovary syndrome and controlled ovarian stimulation rat models. Bosn. J. Basic. Med. Sci. 20(1), 37–43. Sanfins, A., Rodrigues, P. and Albertini, D.F. 2018. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 35(10), 1741–1750. Santoso, B. 2009. Follicular wall thickening in the case of polycystic ovary syndrome (A laboratory experimental study using Rattus novergicus as an experimental animal in the case of polycystic ovary syndrome). Dissertation, Program Studi S3 Ilmu Kedokteran, Program Pasca Sarjana, Universitas Airlangga, Surabaya, Indonesia. Singh, K.B. 2005. Persistent estrus rat model of polycystic ovary disease: an update. Fertil. Steril. 84(2), 1228–1234. Speroff, L., Taylor, H.S., Pal, L. and Deli, E. 2020. Chronic anovulation and polycystic ovary syndrome. Clinical gynecologic endocrinology and infertility. 9th ed. Baltimore, MD: William Lippincot William and Wilkins, pp: 940–1049. Tang, R., Ding, X. and Zhu, J. 2019. Kisspeptin and polycystic ovary syndrome. Front. Endocrinol. (Lausanne). 10, 298. Wei, L.N., Huang, R., Li, L.L., Yang, C. and Li, Y. 2014. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 31, 1483–1490. Wu, Y.T., Tang, L., Cai, J., Ly, X.E. and Xu, J. 2007. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum. Reprod. 22(6), 1526–1531. Yilmaz, S.A., Kerimoglu, O.S., Pekin, A.T., Incesu, F., Dogan, N.U., Celik, C. and Unlu, A. 2014. Metastin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 180, 56–60. | ||
| How to Cite this Article |
| Pubmed Style Rheza A, Santoso B, Widjiati W. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Vet J. 2023; 13(3): 288-296. doi:10.5455/OVJ.2023.v13.i3.5 Web Style Rheza A, Santoso B, Widjiati W. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. https://www.openveterinaryjournal.com/?mno=125648 [Access: July 12, 2025]. doi:10.5455/OVJ.2023.v13.i3.5 AMA (American Medical Association) Style Rheza A, Santoso B, Widjiati W. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Vet J. 2023; 13(3): 288-296. doi:10.5455/OVJ.2023.v13.i3.5 Vancouver/ICMJE Style Rheza A, Santoso B, Widjiati W. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Vet J. (2023), [cited July 12, 2025]; 13(3): 288-296. doi:10.5455/OVJ.2023.v13.i3.5 Harvard Style Rheza, A., Santoso, . B. & Widjiati, . W. (2023) Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Vet J, 13 (3), 288-296. doi:10.5455/OVJ.2023.v13.i3.5 Turabian Style Rheza, Achmad, Budi Santoso, and Widjiati Widjiati. 2023. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Veterinary Journal, 13 (3), 288-296. doi:10.5455/OVJ.2023.v13.i3.5 Chicago Style Rheza, Achmad, Budi Santoso, and Widjiati Widjiati. "Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome." Open Veterinary Journal 13 (2023), 288-296. doi:10.5455/OVJ.2023.v13.i3.5 MLA (The Modern Language Association) Style Rheza, Achmad, Budi Santoso, and Widjiati Widjiati. "Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome." Open Veterinary Journal 13.3 (2023), 288-296. Print. doi:10.5455/OVJ.2023.v13.i3.5 APA (American Psychological Association) Style Rheza, A., Santoso, . B. & Widjiati, . W. (2023) Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open Veterinary Journal, 13 (3), 288-296. doi:10.5455/OVJ.2023.v13.i3.5 |