| Original Article | ||
Open Vet J. 2023; 13(3): 253-261 Open Veterinary Journal, (2023), Vol. 13(3): 253–261 Original Research Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleenAli Ahmed Hasan, Omar Younis Altaey* and Ghada Abdulrahman SultanDepartment of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Omar Younis Altaey. Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: omar.younes [at] uomosul.edu.iq. Submitted: 01/11/2022 Accepted: 05/02/2023 Published: 01/03/2023 © 2023 Open Veterinary Journal
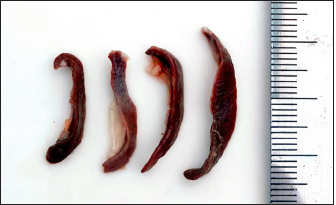
AbstractBackground: The golden hamster is a choice model for investigating many visceral and splenic infections and neoplastic and retrospective lesions. Aim: To study hamsters' spleen's morphological, histological, and histochemical structure. Methods: Samples were collected from eight healthy adult golden hamsters and then fixed with 10% buffered formalin. Later, samples were processed, sectioned, and stained with Hematoxylin and Eosin as well as Masson’s Trichrome stain. Other slides were further stained with Periodic Acid Schiff and Alcian blue 2.5 stain (PAS) for histochemical evolution; the gross measurement was performed for the splenic length, width, and thickness, while the histological measures included the splenic capsular and trabecula thickness, diameter of white pulp follicles, splenic sinusoids and central arteries and proportion of white and red pulps. Results: The macroscopic findings revealed that the spleen was red-brown lanciform on the left side of the dorsolateral abdominal wall. The morphological measurements for splenic length, width, and thickness were 26.6 ± 7.67, 4.17 ± 1.65, and 1.70 ± 0.01 mm, respectively. The histological observations showed that the splenic capsule was composed of two layers (serosal and subserosal). The inner layer sends trabeculae dividing the splenic parenchyma irregularly, and the splenic parenchyma comprises the white and red pulp. The white pulp follicles included the mantle, marginal zones, and the PALS (periarterial lymphatic sheath), while the red pulp constituted splenic cords and sinuses. The histomorphological findings showed that white pulp follicles and the central artery mean diameter were 252.62 ± 8.07 µm and 54.45 ± 0.36 µm respectively, the proportion of white to a red pulp was 0.49 ± 0.01, the splenic capsule, trabecula and the wall of splenic arteries showed an intense positive activity to PAS stain and negative or weak in other splenic structures. Conclusion: The similarities and differences in the spleen between the laboratory animals and hamsters were apparent in this article, so understanding the morphological and histological structure of the spleen presents significant assistance with species identification to select the appropriate experimental animal model in future medical research Keywords: Golden hamster, Histology, Laboratory animals, Morphology, Spleen. IntroductionThe spleen is a remarkable lymphoid organ that integrates innate and adaptive immunity in a coordinated way leading to the decisive removal of blood-borne microorganisms and old erythrocytes from circulation. Moreover, it is vital in the metabolism of iron and lipid (Mebius and Kraal, 2005). The spleen of rodents, in histological sections, is encased by a capsule composed of collagen and elastic fibers that extend into the splenic parenchyma through trabeculae. The splenic parenchyma primarily comprises two morphological compartments, the red and white pulp (Maynard and Downes, 2019). The red pulp is characterized by a network of reticular cells, reticular fibers, and venous sinuses that are organized to form splenic cords. On the other hand, the white pulp comprises nodular and diffused lymphocytes arranged around arteries, in addition to many macrophages and dendritic cells (Cesta, 2006). The golden hamster Mesocricetus auratus is widely used as an experimental model for the study of splenic and cutaneous form of human leishmaniosis as well as its intense need to understand leishmania infection since other murine and laboratory animal has either no effect or show different immune response which is not clear in human compared to hamster immune response (Saini and Rai, 2020). On the other hand, the lymphatic system was studied to understand the impact of hibernation on the microstructure of the spleen, as animals in hibernation have been observed to have smaller lymphoid follicles and a disorganized cells arrangement (Bouma et al., 2010), furthermore hamster’ spleen was considered to study many pathological and neoplastic retrospective lesions (Tuan et al., 2018). Recent studies found that splenic pathological changes are characteristic signs of various infectious diseases, and hamsters are used as a model for studying many of them, like SARS-CoV2 (Rizvi et al., 2022), Ebola (Ebihara et al., 2013) and Yellow fever (McArthur et al., 2020), However, many studies documented the standard structural, and histological composition of the spleen in different laboratory animals like white rats Rattus norvegicus (Maynard and Downes, 2019), albino mice Mus musculus (Cesta, 2006), guinea pigs Cavia porcellus, and rabbits Oryctolagus cuniculus (Qasem et al., 2015). But the scarcity of existing information about the anatomical, histological, and histochemical characteristics of the spleen in golden hamsters. This represents a significant gap in our understanding of the organ structure and its function in this species. Examining these morphological parameters of golden hamsters’ spleen is important in clinical, pathological, and functional studies, potentially providing valuable insight for ongoing medical and biological research. Materials and MethodsSamples preparation and anatomical studyRegardless of the sex, eight adult clinically healthy golden hamsters (M. auratus) of 12 weeks of age and (110.4 ± 6.17 g) weight were used in this study obtained from the local animals Market, the study was carried out during April 2022 at the anatomy department of the veterinary college of Mosul university – Iraq. First, the animals were sacrificed after an intraperitoneal injection of (120 mg/kg) of pentobarbital (Nembutal®). Later, the abdominal cavity was opened, and the spleen was collected and placed into physiological saline to clean them from blood and tissue debris. Then samples were examined and weighed individually, and the length, width, and thickness were measured using a Digital Vernier caliper (LOUISWARE, China). At the same time, the volume was calculated using the mathematical formula V=4/3 * π * A * B * C where V, A, B, C represent the volume, length, width, and thickness since the body of spleen typically 3- dimensional elliptical Figure (Tanaka, 2009). Then samples were immersed into 10% buffered formalin and kept for 48 hours. Histological and histochemical analysisAfterward, samples were washed with distilled water, cut, and dehydrated in ascending series of graded alcohol concentrations 70%, 80%, 90%, and 100%, later they were cleared with xylene for 30 minutes, then were infiltrated with melted paraffin 58°C and cast into blocks, 5 micrometers thick sections were performed using a rotary microtome (OS-315, UK). They were mounted with DPX mounting media on glass slides, cells stained with Harries hematoxylin and eosin stain as well as Masson’s trichrome stain according to the protocol of Bancroft (Suvarna et al., 2019). They were examined under the light microscope (Olympus-Dm-CBAD, Japan) for routine histological assessments. And for histochemical analysis. Some other sections were stained with Periodic Acid Schiff and Alcian blue 2.5 stain (PAS/AB) for the detection of glycoprotein and acidic mucopolysaccharides (Jones, 2020) Histomorphometric evaluationHistomorphometric measurements for the splenic parameters were performed using a 3. 0 USB digital camera (OMAX 18.0 MP, China) provided with image processing software, the parameters measured in 20 microscopic fields of the splenic sections chosen randomly, included the thickness of capsule, trabecula thickness, white pulp number per field, white pulp width and length, white pulp to red pulp ratio (percentage) and splenic sinusoids and the central arteries diameter. Statistical analysisDescriptive statistical analysis was performed on the data generated from gross and histomorphometric studies to obtain the Mean (M), standard error of the mean (SEM), and the percentages (%) using IBM SPSS version 25 software at p ≤ 0.05. Ethical approvalanimal handled according to AVMA guidelines (Underwood and Anthony, 2020), and approved by the IACU committee of the college. ResultsThe macroscopic observations revealed that the spleen of the golden hamster was an elongated lanciform reddish-brown structure composed of a body and two ends arched cranially and straight caudally and two surfaces: visceral and parietal. The visceral surface, characterized by the splenic hilus, extends along the spleen. It divides it into two unequal parts the spleen fixed from the dorsal end with gastrosplenic ligament attached between the hilus and the forestomach and fixed ventrally with the left kidney by a splenorenal ligament with an average weight of (0.219 ± 0.05 g), located adjacent to the left side of dorsolateral abdominal wall between the 12th and 13th intercostal space, against the dorsal and lateral surface of the stomach (Fig. 1). The morphological measurements for the total length of the spleen were (26.6 ± 7.67 mm). and the mean width was (4.17 ± 1.65 mm). The average thickness was (1.70 ± 0.01 mm). The spleen obtains blood supply from many short vessels branching from the splenic artery, and the left gastric artery passes through the gastro-splenic ligament to the hilus of the spleen; the volume of the spleen was calculated as an elliptical geometrical figure. It was (7.39 ± 7.36 cm3) (Table 1).
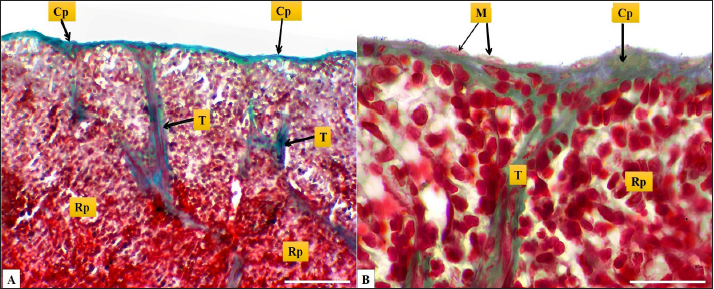
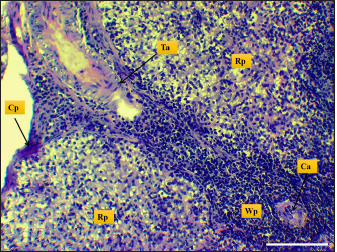
Fig. 1. Photograph illustrates the spleen of a hamster which’s an elongated lanciform-like shape. The microscopic observations found that a capsule of two layers surrounds the spleen: the outer serosal layer comprised of peritoneal mesothelium (mesothelial cells) and the inner subserosal layer composed of irregular dense connective tissue interfused with elastic and smooth muscle fibers. the thickness means of the capsule was (22.41 ± 0.51 µm) (Table 2). The inner layer of the capsule extends deep to form the trabecula, which irregularly subdivides the splenic parenchyma; the trabeculae are constituted of; collagen fibers bundles of elastic and smooth muscle fibers (Fig. 2). The large trabecula provides lanes of pathways for arteries and veins to pass into the spleen (Fig. 3). The mean thickness of trabeculae was (26.58 ± 1.20 µm) (Table 2). The splenic arteries and veins were primarily noticeable and situated within the vascular sheath of the splenic hilus, surrounded with connective and adipose tissue and filled with erythrocytes (Fig. 4). The splenic parenchyma was composed of; red pulp and white pulp. The red pulp was the predominant portion of the parenchyma, which was a branched network of reticular tissue, lymphocytes, neutrophils, macrophages, mast cells, and hemosiderin deposition (Fig. 5); the different types of splenic cells identified according to the cell structure, cell size, nucleus shape, and the presence of cytoplasmic granules and cytoplasmic extensions, the cells arranged to form channel-like cellular cords (splenic cords), these cords surrounding large venous sinusoids (splenic sinusoids). The parenchyma demonstrates a considerable level of mitotic activity, as evidenced by the significant number of mitotic figures present upon examination (Fig. 6); the average diameter of the splenic sinusoids was (13.89 ± 0.82 µm) (Table 2). While the white pulp was composed of small, spherical lymphatic follicles of about 4–5 in each (×4) microscopic field, comprised mainly of intensive small B-lymphocyte accumulation around the central artery (mantle zone) followed by circular fibrous tissue (marginal zone) blended with red pulp cell cords, these follicles showed no germinal centers (Fig. 7) the central splenic artery appeared as a cylindrical structure composed of lining endothelial cells, an elastic tunica media consisting of smooth muscle cells, and an outer tunica adventitia composed of connective tissue (Fig. 8). The mean diameter of white pulp follicles was (252.62 ± 8.07 µm) and the mean diameter of the central artery was 54.45 ± 0.36 µm (Table 2). The white pulp also had diffuse lymphatic tissue bridges called periarterial lymphatic sheath (PALS) attached to the edges of follicles and surrounding the white pulp artery that passes through the large trabecula to the parenchyma. The PALS area is composed of two lymphatic tissue zones: the inner PALS zone, which their cells intensely stained, representing the large T-lymphocytes, and the outer PALS zone which is slightly faint, representing the small B-lymphocytes (Fig. 9). The proportion means of white pulp in hamster’s spleen were (33.30% ± 0.048%) while the proportion of red pulp was 66.70% ± 0.048% (Fig. 10) (Table 3). The histochemical observations of the hamster’s spleen showed an intense reaction of the splenic capsule, trabecula, and splenic connective tissue to the periodic acid-Schiff reaction (Fig. 11), as well as the wall of the splenic arteries and the central arteries (Fig. 12), while the activity of the stain was moderate to weak in red pulp parenchymal cells mainly in the reticular and macrophage, reflect the presence of glycoproteins components within these structures. The white pulp showed an intense to moderate response to the AB 2.5 stain and was weak with other red pulp tissue components (Fig. 13). DiscussionThe spleen of a golden hamster was an elongated lanciform reddish-brown structure located adjacent to the left side of the dorsolateral abdominal wall against the dorsal and lateral surface of the stomach, Qasem et al. (2015) reported that the spleen of rabbits is a light red finger-like structure located to the left of the dorsal and parietal surface of the stomach, and found that the spleen in Guinea pigs is blackish-red quadrilateral present in the left hypochondriac subregion, as well as Kaufman and Bard (2000), mentioned that the spleen of mice elongated ribbon-like suspended between the left abdominal wall and the greater curvature of the stomach, these findings were parallel to what found in the spleen of hamster. Table 1. The gross morphometric measurements of the spleen in the adult golden hamster.
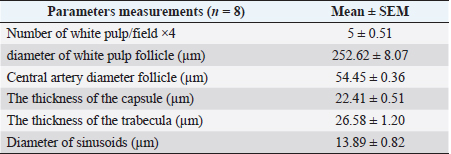
Table 2. The histomorphometric measurements of the spleen in the adult golden hamster.
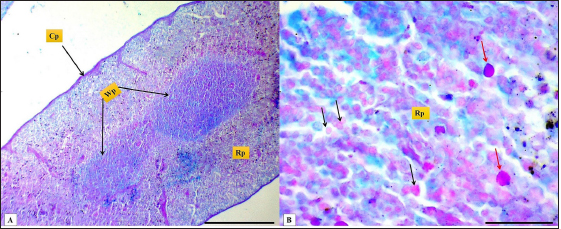
Fig. 2. (A): splenic capsule (Cp), splenic trabeculae (T), the splenic red pulp (Rp). (B): splenic mesothelial cells (serosal layer) (M), sub serosal layer, Masson’s Trichrome stain (A: scale bar=100 µm; B: scale bar=30).
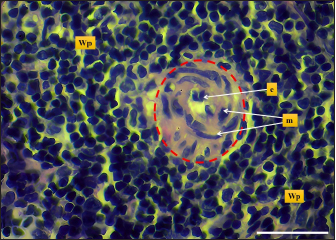
Fig. 3. Microphotograph shows trabecular artery (Ta), central artery (Ca), splenic capsule (Cp), splenic red pulp (Rp), splenic white pulp (Wp) (H&E, Scale bar=100 µm).
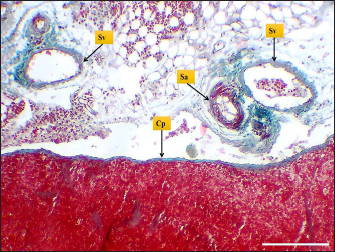
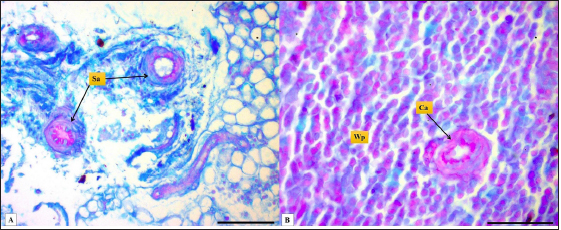
Fig. 4. Microphotograph shows splenic vein (Sv), splenic artery (Sa), and capsule (Cp). Masson’s Trichrome stain (Scale bar=300 µm). The present study found that the spleen of the hamster fixed to the neighboring organs with two ligaments: the gastro-splenic and splenorenal ligaments; in mice, Kaufman and Bard (2000) mentioned that the spleen set with two ligaments: gastro-splenic ligament, which joins the spleen with the greater curvature of the stomach, and splenorenal ligament joins the spleen with left kidney, which is similar to our observations, In compare to other laboratory animals, Qasem et al. (2015) recorded that rabbits’ spleen fixed with gastro-splenic ligament only, while the guinea pigs’ spleen set with three broad ligaments (gastrosplenic, splenophrenic and splenorenal ligaments) connect the spleen with the stomach, diaphragm and left kidney. The differences in ligaments depend on the size of the digestive system and how far it extends inside the abdominal cavity. The morphological measurements of the spleen in hamsters differed from the authors' observations, attributing those differences to the animals’ size, relative weight, and shape of the spleen. The spleen of hamster is shorter compared to the spleen of rabbits and rats, Dimitrov (2012) and Voloshin et al. (2014) mentioned that the length of the spleen in rabbits and adult albino rats was 33.3 and 49.8 mm, respectively, while it was longer than mice and guinea pig spleen. Uwah et al. (2021) and Qasem et al. (2015) noted that the length of guinea pigs' spleen was 24.5 cm, and mice's spleen length was 18.1 mm.
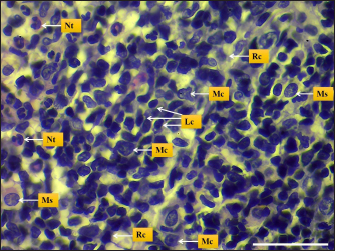
Fig. 5. Microphotograph shows different spleen cells, Lymphocytes (Lc), macrophages (Mc), neutrophils (Nt), reticular cells (Rc), and Mast cells (Ms) (H&E; Scale bar=30 µm).
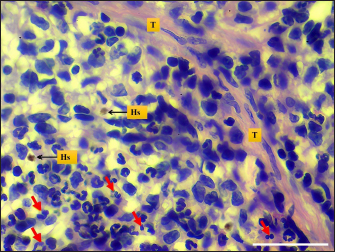
Fig. 6. Microphotograph shows hemosiderin deposition (Hs), trabeculae (T), and the mitotic figures (red arrows) (H&E; Scale bar=30 µm).
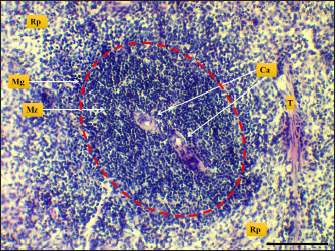
Fig. 7. Microphotograph shows white pulp (dashed circle), mantle zone (Mz), marginal zone (Mg), red pulp (Rp), central artery (Ca), capsule (Cp), and trabeculae (T) (H&E; Scale bar=100 µm).
Fig. 8. Microphotograph shows central artery (dashed circle), endothelial cells (e), tunica media smooth muscle fibers (m), white pulp (Wp) (H&E; Scale bar=30 µm). The width of hamsters’ spleen is narrower in contrast to other animals, the authors (Dimitrov, 2012; Voloshin et al., 2014; Qasem et al., 2015; Uwah et al., 2021) reported that spleen widths in rats, guinea pigs, rabbits, and mice were 14, 12.2, 5.44, and 8,1 mm respectively. The weight of the spleen in hamster was nearly close to that of mice which were (0.240 ± 21.49 g) according to (Uwah et al., 2021), whereas the weight of the spleen in rats was 0.249 ± 0.61 g) (Voloshin et al., 2014), rabbits (0.547 ± 0.03 g) and in guinea pigs (0.641 ± 0.88 g) (Qasem et al., 2015). The microscopic architecture of the hamster’s spleen was similar to some laboratory animals, with minimal differences noticed in the spleen of other species, the spleen of the hamster is composed of two subserosal layers and from fibrous and muscular components, Cesta (2006) and Rahmoun et al. (2019) found that spleen in mice, rats and rabbits surrounded with two layers capsule, the inner composed of connective tissue and thin, smooth muscular lamina, and the outer consisting of mesothelial cells, these findings identical to the tablet of hamster spleen. On the other hand, Haley (2017) commented that the spleen in minipigs, cat, and dogs have a distinct thick capsule composed of interwoven layers of elastic and smooth muscle fibers, which differs from the hamster spleen Haley (2017) believe that the spleen in the mentioned animals is contractile and can change its size, expand to maintain blood storage while rodents’ spleen does not show contractile, rapidly empty and has a defense role.
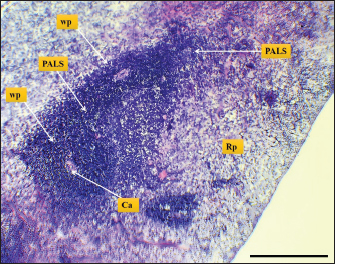
Fig. 9. Microphotograph shows white pulp follicles (Wp), red pulp (Rp), PALS, central artery (Ca) (H&E; Scale bar=300 µm).
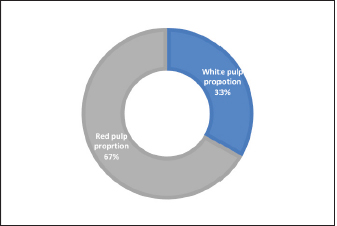
Fig. 10. Chart shows the proportions of white and red pulp in the spleen of an adult golden hamster. In the current study, the thickness measurement of the splenic capsule in hamsters was close to the laboratory animals, Rahman et al. (2016) reported that the mean thickness of the rabbit capsule was 33.3 µm, while in mice was less than (25 µm) according to Mubaraki et al. (2016). Moreover, the splenic trabeculae of hamsters were long extended deep into the parenchyma composed of fibrous tissue, smooth muscle fibers, arteries, and veins, equivalent descriptions presented by (Cesta, 2006) in mice and rats. Rahman et al. (2016) also mentioned similar details in rabbits and found that the thickness of trabeculae was 30 µm. The red pulp of splenic parenchyma constituted a network of splenic cords and splenic sinusoids in hamsters, Haley (2017) reported that the spleen in minipigs and mice was non-sinusoidal, the sinusoids very small and lacked lining endothelial cells, attribute these differences to the poor development of sinuses and the functional hemopoietic activity of spleen that extend throughout life in compare to rats and rabbits. While Cesta (2006), Rahmuon et al. (2019), and Maynard and Downes (2019) adverted that the spleen had large true sinusoids lined with elongated endothelial cells and loose connective tissue in laboratory animals and humans, which is like what is seen in hamster’ spleen in our line of works. The white pulp in the spleen of hamsters was composed of the lymphoid follicles and the PALS, the strands comprised lymphocytes aggregations arranged into the mantle and marginal zones; Haley (2017) reported that follicles in minipigs were small, ellipsoid, and less distinct compared to rodents but the PALS in minipigs were well developed surrounding the capillaries that arise from white pulp artery. The follicle edges attached or separated from PALS in rats while it’s studded within follicles in mice (Steiniger, 2015), which agrees with our observations in hamster, Steiniger (2015) also found that white pulp follicles area per tissue in mice were higher than in human. Still, the number of follicles among rodents does not differ. Ward (1999) also found that the marginal zone and PALS in rat and mice were delineated, like those of hamster’ spleen, but it is less distinct in human; it is believed that the arrangement and the number of lymphoid follicles has a relationship with splenic size and the ratio of white to the red pulp. In this paper, the white pulp follicle in golden hamster was a bit smaller than in rats and rabbits, Rahman et al. (2016) noticed that the diameter of splenic white pulp in rabbits and rats were (342.6 µm) and (295 µm), and Ward (1999) found that white pulp proportion in mice tends to be greater than in rats. Still, the follicle was smaller, rats’ white pulp to red pulp proportion was (28%) to (72%), which is closer to the balance in the spleen of the hamster. The examination of the spleens’ histochemistry revealed an intense reaction to PAS stain in capsules, trabecula. At the wall of splenic blood vessels, these findings were parallel to those (Gautam and Mishra, 2015, Kaur et al., 2022) mentioned that glycoprotein components in these structures were high. It’s necessary for the physiological function and development of the spleen. Gautam and Mishra (2015) reported that red pulp in the spleen of goats showed weak or moderate activity to AB stain, similar to our findings in hamsters, and mentioned that intensity increases with age advancement. This study has a limitation in its scope of examination, as it only covers a subset of morphological parameters, particularly at the cellular level, and does not incorporate the use of other histochemical markers. In addition, the study lacks information on the experimental manipulation's developmental and morphological impact. These may limit the generalizability of the results and conclusions to other developmental age stages. Therefore, it is recommended that future studies include a more comprehensive range of morphological parameters and additional biochemical markers, as well as examination across multiple animal models and different age groups, to enhance the understanding of the overall splenic structure. Table 3. The proportions components of the splenic parenchyma in the adult golden hamster.
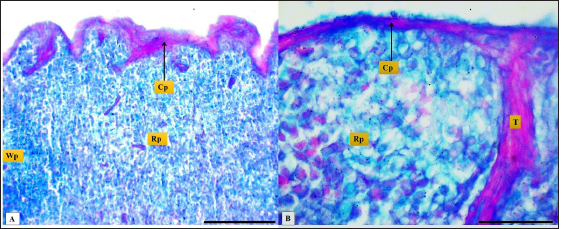
Fig. 11. Microphotograph (A&B) shows white pulp follicles (Wp), the red pulp (Rp), splenic capsule and trabeculum (T), PAS/AB 2.5 stain (A: scale bar=100 µm; B: scale bar=30 µm).
Fig. 12. Microphotograph (A&B) shows splenic arteries (Sa), central artery (Ca), white pulp (Wp) PAS/AB 2.5 stain, (A: scale bar=100 µm; B: scale bar=30 µm).
Fig. 13. Microphotograph (A&B) shows white pulp (Wp), red pulp (Rp), splenic capsule (Cp), the reticular cells (black arrows), and the macrophages (red arrows), PAS/AB2.5 stain, (A: scale bar=300 µm; B: scale bar=30 µm). ConclusionThe golden hamster is widely used as a model of choice for various splenic infectious studies like visceral Leishmaniasis in humans and animals; the similarities and differences in the spleen between the laboratory animals and hamster were evident in this article, including the splenic size volume and weight also in the thickness of capsule, white pulp measurements and the ratio of white pulp to the red pulp. Therefore, the understanding of the morphological and histological structure of the spleen presents significant assistance with species identification to select the appropriate experimental animal model in future medical research. AcknowledgmentsThe authors are thankful to the College of Veterinary Medicine, University of Mosul, for the encouragement and assistance. Conflict of interestThe authors have no conflicts of interest to declare Authors contributionThe research was conceptualized by Ali Hasan, who also conducted the animals’ anatomy; Omar Younis contributed to writing the initial draft and performed the histological measurements and data analysis. Ghada Abdulrahman conducted the histological and histochemical examination and the appropriate demonstration for the histological sections. All authors contributed to the manuscript's reading, reviewing, revising, and approving the final version. ReferencesBouma, H.R., Carey, H.V. and Kroese, F.G. 2010. Hibernation: the immune system at rest? J. Leukoc. Biol. 4, 619–624. Cesta, M.F. 2006. Normal structure, function, and histology of the spleen. J. Toxicol. Pathol. 34, 455–465. Dimitrov, R.S. 2012. Comparative ultrasonographic, anatomotopographic and macromorphometric study of the spleen and pancreas in rabbit (Oryctolagus cuniculus). Not. Sci. Biol. 4, 14–20. Ebihara, H., Zivcec, M., Gardner, D., Falzarano, D., Lacasse, R., Rosenke, R., Long, D., Haddock, E., Fischer, E., Kawaoka, Y. and Feldmann, H. 2013. A Syrian golden hamster model is recapitulating ebola hemorrhagic fever. J. Infect. Dis. 207, 306–318. Gautam, A. and Mishra, U. 2015. Histochemical and transmission electron microscopic studies of the spleen in prenatal stages of goat. Indian. J. Anim. Res. 49, 59–62. Haley, P.J. 2017. The lymphoid system: a review of species differences. J. Toxicol. Pathol. 30, 111–123. Jones, M.L. 2020. Histotechnology a self instructional text. In Eds., Carson, F.L. and Cappellano, C. Chicago, IL: ASCP Press, pp: 400. Kaufman, M.H. and Bard, J.B. 2000. The anatomical basis of mouse development. San Diego, CA: Gulf Professional Publishing. Kaur, H., Singh, O. and Pathak, D. 2022. Histochemical and immunohistochemical studies on pig spleen (Sus scrofa). Indian. J. Vet. Sci. Biotechnol. 18, 24. Maynard, R.L. and Downes, N. 2019. Anatomy and histology of the laboratory rat in toxicology and biomedical research. Cambridge, MA, Academic Press. McArthur, M.A., Zhang, S.L., Li, L., Tesh, R.B. and Barrett, A.D. 2020. Molecular characterization of hamster-adapted yellow fever virus. J. Vector. Borne. Dis. 3, 222–227. Mebius, R.E. and Kraal, G. 2005. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616. Mubaraki, M.A., Hafiz, T.A., Dkhil, M.A. and Al-Quraishy, S. 2016. Beneficial effect of Punica granatum peel extract on murine malaria-induced spleen injury. BMC. Complement. Altern. Med. 16, 221. Qasem, H., Hiba, O., Farhan, S. and Ahmed, A. 2015. A comparative anatomical and morphological study of spleen in rabbit (Oryctologus Cuniculus) and Guinea pig (Caviaporcellus). J. Kerbala. Uni. 11, 147–155. Rahman, N., Tandon, R., Ghaus, F., Moinuddin, A., Akram, W. and Faruqi, N.A. 2016. Comparative anatomy of spleen: histomorphometric study in human, goat, buffalo, rabbit and rat. Acad. Anat. Int. 2(1), 28–32. Rahmoun, D.E., Fares, M.A., Bouzebda-Afri, F. and Driss, K.B. 2019. An anatomical and histological study of the rabbit spleen development in the postnatal period in Algeria. Online. J. Ani. Fed. Res. 9(2), 44–50. Rizvi, Z.A., Dalal, R., Sadhu, S., Binayke, A., Dandotiya, J., Kumar, Y., Shrivastava, T., Gupta, S.K., Aggarwal, S. and Tripathy, M.R. 2022. Golden Syrian hamster as a model to study cardiovascular complications associated with SARS-CoV-2 infection. Elife 11, e73522. Saini, S. and Rai, A.K. 2020. Hamster, a close model for visceral leishmaniasis: opportunities and challenges. Parasite. Immunol. 42, e12768. Steiniger, B.S. 2015. Human spleen microanatomy: why mice do not suffice. Immunology 145, 334–346. Suvarna, S.K., Layton, C. and Bancroft, J.D. 2019. Tissue processing. In Bancroft's theory and practice of histological techniques. Eds., Suvarna, S.K., Layton, C. and Bancroft, J.D. Amsterdam, The Netherlands, Elsevier. Tanaka, E.Y. 2009. Análise de fatores preditivos de ressecção visceral no tratamento operatório de doentes portadores de hérnia incisional gigante com perda de domicílio submetidos a pneumoperitônio progressivo pré-operatório. Doctoral thesis, Universidade de São Paulo, São Paulo, Brazil. Tuan, Y.C., Wan, R.C., Kao, J.P., Chiou, H.Y., Takahashi, K. and Liao, J.W. 2018. Retrospective pathological studies of splenic lesions in domestic hamsters (Phodopus spp.). J. Comp. Pathol. 164, 37–43. Underwood, W. and Anthony, R. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. Available via https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf Uwah, A.F., Effiong, B.O., Obot, J.S., Henry, E.U. and Umoh, E.U. 2021. Cardiovascular and hepatic functions of Plasmodium Berghei infected mice treated with artemether-lumefantrine and Eremomastax Speciosa. World. J. Pharm. Pharm. Sci. 10(7), 1431–1447. Voloshin, V.N., Koveshnikov, V.G. and Voloshina, I.S. 2014. Morphology of the spleen in adult albino rats after whole-body exposure to low-level of toluene. Int. J. Anat. Res. 2, 421–430. Ward, J. 1999. Thymus, spleen, and lymph nodes. In Pathology of the mouse. Ed., Maronpot, R.R. Vienna, IL: Cache River Press. | ||
| How to Cite this Article |
| Pubmed Style Hasan AA, Altaey OY, Sultan GA. Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Vet J. 2023; 13(3): 253-261. doi:10.5455/OVJ.2023.v13.i3.1 Web Style Hasan AA, Altaey OY, Sultan GA. Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. https://www.openveterinaryjournal.com/?mno=125117 [Access: July 11, 2025]. doi:10.5455/OVJ.2023.v13.i3.1 AMA (American Medical Association) Style Hasan AA, Altaey OY, Sultan GA. Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Vet J. 2023; 13(3): 253-261. doi:10.5455/OVJ.2023.v13.i3.1 Vancouver/ICMJE Style Hasan AA, Altaey OY, Sultan GA. Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Vet J. (2023), [cited July 11, 2025]; 13(3): 253-261. doi:10.5455/OVJ.2023.v13.i3.1 Harvard Style Hasan, A. A., Altaey, . O. Y. & Sultan, . G. A. (2023) Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Vet J, 13 (3), 253-261. doi:10.5455/OVJ.2023.v13.i3.1 Turabian Style Hasan, Ali Ahmed, Omar Younis Altaey, and Ghada Abdulrahman Sultan. 2023. Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Veterinary Journal, 13 (3), 253-261. doi:10.5455/OVJ.2023.v13.i3.1 Chicago Style Hasan, Ali Ahmed, Omar Younis Altaey, and Ghada Abdulrahman Sultan. "Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen." Open Veterinary Journal 13 (2023), 253-261. doi:10.5455/OVJ.2023.v13.i3.1 MLA (The Modern Language Association) Style Hasan, Ali Ahmed, Omar Younis Altaey, and Ghada Abdulrahman Sultan. "Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen." Open Veterinary Journal 13.3 (2023), 253-261. Print. doi:10.5455/OVJ.2023.v13.i3.1 APA (American Psychological Association) Style Hasan, A. A., Altaey, . O. Y. & Sultan, . G. A. (2023) Morphological, histological, and histochemical study of the adult golden hamster (Mesocricetus auratus) spleen. Open Veterinary Journal, 13 (3), 253-261. doi:10.5455/OVJ.2023.v13.i3.1 |