| Original Article | ||
Open Vet J. 2023; 13(2): 233-240 Open Veterinary Journal, (2023), Vol. 13(2): 233–240 Original Research Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: description, possible interpretation, and review of the principal physiological hematopoietic mechanismsVanessa Turinelli1*, Giacomo Rossi2 and Alessandra Gavazza21IDEXX Laboratories-Italia S.r.l, Milan, Italy 2School of Biosciences and Veterinary Medicine, University of Camerino, Matelica, Italy *Corresponding Author: Vanessa Turinelli. IDEXX Laboratories-Italia S.r.l, Milan, Italy. Email: Vanessa-Turinelli [at] idexx.com Submitted: 12/09/2022 Accepted: 31/01/2023 Published: 21/02/2023 © 2023 Open Veterinary Journal
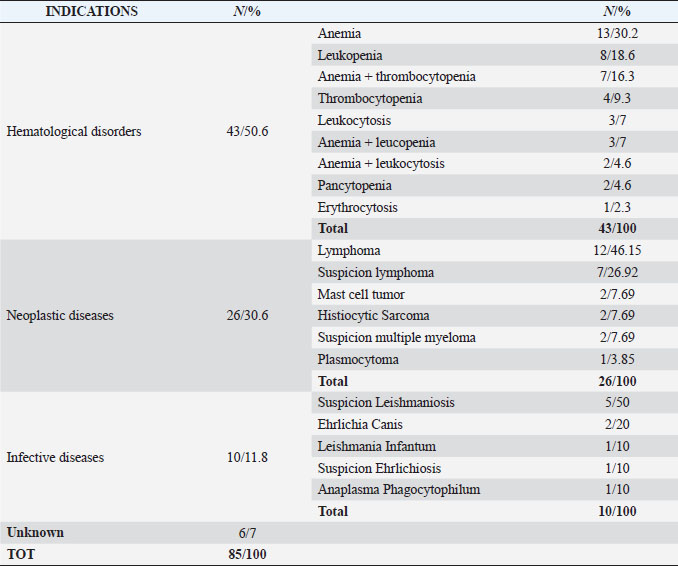
AbstractBackground: In clinical routine, it can happen that to an abnormal hemogram corresponds an unexpected cytological normal bone marrow examination that can be difficult to interpret and to manage. Aim: This cytologically retrospective study wants to evaluate a consistent number of qualitative and quantitative normal bone marrow exams according to the hematological and clinical-pathological data to judge if this normality is by itself a pathologic state. Methods: Six hundred and thirteen bone marrow samples were examined. The bone marrow cytological examinations were performed using morphological and numerical criteria together with a complete hemogram, after the identification of clinical or hematological alterations such as multiple lymph nodes enlarged, positive leishmania serological result, staging of neoplasia, cytopenia, increased number of cells, or suspicion of malignant blood disorders. Results: Of the 613 bone marrow samples evaluated, 85 (14%) were classified as normal or without cytological abnormalities; however, only 28 (33%) of those cases had a normal hemogram associated, whereas 55 (65%) had one or more cytopenia and 2 (2%) had increased blood cells count. Conclusion: From this study emerges that cytological bone marrow examinations without any morphological or numerical abnormalities are often associated with altered hematological exams and for this reason, they should not be considered normal and should lead to other deepened investigations. Keywords: Canine, Bone marrow, Cytological examination, Hemogram data. IntroductionBlood and bone marrow cytological examination represents the unique tool to explore the hematopoietic tissue that can be altered by several intrinsic and extrinsic conditions. As a rule, a significant hemogram alteration, like for example non-regenerative anemia, persistent neutropenia or thrombocytopenia, abnormal blood cell morphology, unexplained elevations in blood cell numbers or presence of immature cells in peripheral blood leads to a bone marrow cytological plus or menus histological examination, depending on the case. Very often the bone marrow appears modified, and, for example, it can be stimulated in response to the peripheral cytopenia, or it can appear depressed if it has been damaged by infectious agents or toxin or it can be altered by a neoplastic transformation of the hematopoietic cells. Anyway, to an abnormal hemogram usually corresponds an altered bone marrow that can help to understand the underlying disease or sometimes lead to a definitive diagnosis (malignant hemopathies or leishmania infection, for example). However, it can happen that to an abnormal hemogram corresponds an unexpected cytological normal bone marrow examination that can be difficult to interpret and to manage. Other important indications of bone marrow cytological examination, that are not always related to hemogram alterations and that can be associated to normal cytology-histology are hypercalcemia, hyperproteinemia, fever of unexplained origin, staging for malignancies, and monitoring of chemotherapy administration (Harvey, 2012; Raskin and Messick, 2012; Turinelli et al., 2015). However, in those cases, a normal bone marrow cytology or histology should not pose real interpretative problems. So far, few retrospective studies of bone marrow examination have been published and only one study provided bone marrow differential cell counting for different diseases (Weiss, 2006; Turinelli et al., 2015). The aim of this study was to provide a general overview regarding the prevalence of canine normal bone marrow cytological exam in correlation with the hemogram and clinical-pathological data and to investigate if this normality can be inappropriate and can be considered by itself a pathologic state. Materials and MethodsCanine bone marrow samples examined over a 5-year period were retrospectively reviewed. Six hundred and thirteen bone marrow aspirate samples were classified using cytological and numerical criteria. The bone marrow cytological examinations were performed after the identification of clinical and/or hematological alterations such as multiple lymph nodes enlarged, positive leishmania serological test, staging of neoplasia, cytopenia, increased number of cells, or suspicion of malignant blood disorders. A complete blood count (CBC) including blood film examination performed within 24 hours of bone marrow collection was carried out for all patients. The instrumental CBC data were obtained with the hematology analyzer Advia 120 Hematology System®, Siemens (Germany). Anemia, thrombocytopenia, and neutropenia were diagnosed when the hemoglobin concentration was less than 13.4 g/dl, platelets less than 143,000/μl, and neutrophils less than 2,940/μl, respectively. Erythrocytosis, thrombocytosis, and leukocytosis were diagnosed when the total erythrocyte count was greater than 8,700,000/μl, platelet count greater than 448,000/μl and leukocyte count greater than 17,600/μl (with lymphocytosis considered when the total lymphocytes exceeded 4,950/μl), respectively. Less than 10% of cases had concurrent bone marrow core biopsy collected. Bone marrow sampling was performed from various sites including proximal humerus, iliac crest, or trochanteric fossa of the proximal femur, cost-chondral junction of the 7th, 8th, and 9th ribs and the 3rd, 4th, 5th sternebra. Bone marrow aspirate smears were prepared through a squash technique, immediately after sample collection of live animals. Aspirates were stained with May Grünwald Giemsa. All the bone marrow samples were reviewed by a single-boarded clinical pathologist using a standardized method. The first step of the analysis was assessment of sample quality. Only samples with at least 8–10 unit particles (even if these particles were on multiple different slides) were included in the study (Fig. 1). All samples of inadequate quality (i.e. poor cellularity or crushed or formalin-fixed samples) were excluded from the study. The cellularity of bone marrow samples was estimated by examining the proportion of cells versus fat presence in particles using a 200× magnification. Normal cellularity varies between 75% and 25% cells (young and old animals, respectively) of the unit particles (Harvey, 2012; Raskin and Messick, 2012; Turinelli et al., 2015). A differential cell count was performed by evaluating 500 nucleated cells in a monolayer within the preparations using a 1,000× magnification. For the erythroid lineage, the cells were separated into rubriblasts, prorubricytes, rubricytes, and metarubricytes. For the myeloid-granulocytic lineage the cells were separated into myeloblasts together with promyelocytes, neutrophil myelocytes, neutrophil metamyelocytes, band/segmented neutrophils, total eosinophilic cells, and total basophilic cells. Lymphocytes, plasma cells, monocytes, macrophages, and blasts of lymphoid and uncertain lineage were counted separately. Megakaryocytes and their precursors were not included in the differential cell count. The megakaryocytic lineage was evaluated at low magnification using a 100× magnification and counting the number of megakaryocytes per particle. The degree of maturation and morphology were also assessed (Fig. 2). The myeloid-to-erythroid ratio (M:E) was calculated as previously described (Harvey, 2001, 2012; Grindem et al., 2002; Turinelli et al., 2015). Bone marrow was considered unremarkable when any morphological and maturation abnormalities were found, and when the cellularity was within normal limits compared to literature and laboratory-established reference limits. The number of megakaryocytes per unit particles was 3–7, the M:E was 0.41–2.14, the rubriblasts plus prorubricytes were less than 8% of all nucleated cells and myeloblasts plus promyelocytes were less than 4% of all nucleated cells (Fig. 3). Total lymphocytes counts did not exceed the 6% of total nucleated cells, whereas plasma cells, monocytic phagocytes, and undifferentiated blasts were less than 1% (Turinelli et al., 2015). The cut off used for lymphoma bone marrow metastasis was 3% (Marconato et al., 2013). Ethical approvalNot needed for this study as it is a retrospective study. ResultsThis study included 613 dogs, of which 331 (54%) were male and 282 (46%) were female. The medium age was 8 years. Of the 613 bone marrow samples cytologically examined, a relevant proportion, 85 cases (14%), were considered normal based on morphological and numerical results (Turinelli et al., 2015). For those cases, the main indications that lead to the cytological bone marrow exam are listed in Table 1. The most representative indications were hematological disorders that was subdivided in several subgroups corresponding to the principal blood alteration. Anemia was the most important hematological disorder and could be associated with others cytopenia. Neoplastic diseases, already diagnosed or suspected, represented the second most important indications, and inside of this broad group, lymphoma represented the majority of the cases. Infective diseases were also well represented with a majority of Leishmania Infantum infection (diagnosed or suspected). Six cases were submitted for a bone marrow cytological examination without any indications and without any hematological alteration.
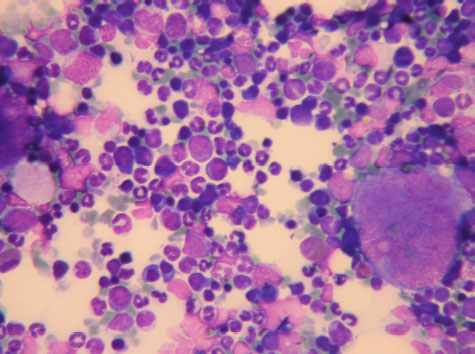
Fig. 1. Bone Marrow Particle consisting of all the lineage precursors and mature cells. May Grunwald Giemsa ×40 objective.
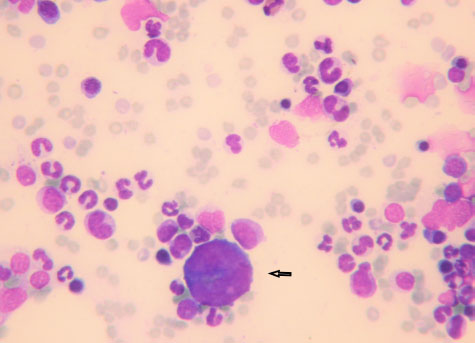
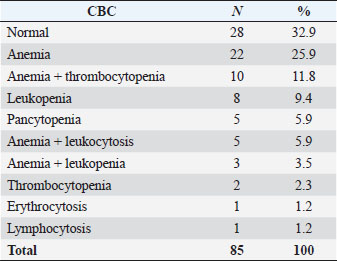
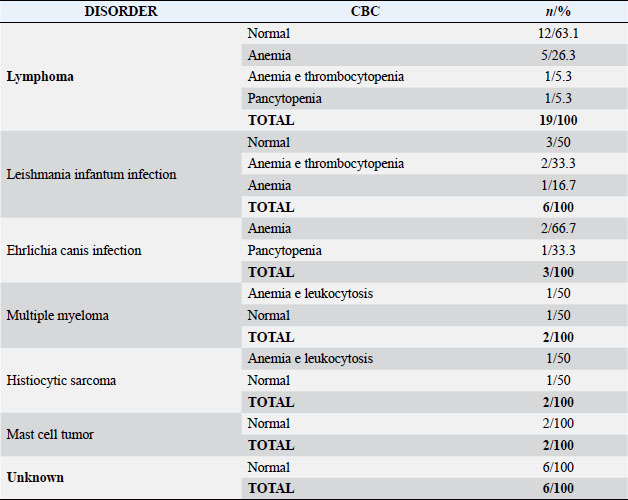
Fig. 2. Promegakariocyte (arrow) and several erythroid and myeloid precursors. May Grunwald Giemsa ×40 objective. All the normal bone marrow samples were analyzed together with a CBC. The results of the hemogram were listed in Table 2. Anemia, alone or associated with other cytopenia, was the most important hematological alteration. However, a normal CBC was found in a significant proportion of cases, more often in the lymphoma and unknown indication subgroups (Table 3). DiscussionThe hematopoietic system function can be assessed only by the simultaneous examinations of the blood and bone marrow. The data coming from those quantitative and qualitative exams should be interpreted all together. A normally functioning hematopoietic tissue is characterized by a normal CBC associated to a bone marrow without any numerical or morphological abnormalities (Harvey, 2012; Turinelli et al., 2015). However, it is important to outline that the bone marrow is a very dynamic tissue that can change quite rapidly, depending on the stimulus to which is exposed and to the alteration in its microenvironment. The bone marrow changes are related to the blood ones, but they can move up or follow the CBC alteration with a certain degree of time (Harvey, 2012; Raskin and Messick, 2012; Turinelli et al., 2015). Both tissues are under the control of a plethora of cytokines that have effect on different cell lineages at different stages and that can be related to precise clinical state, like for example erythropoietin that is secreted by renal cells under hypoxic condition.
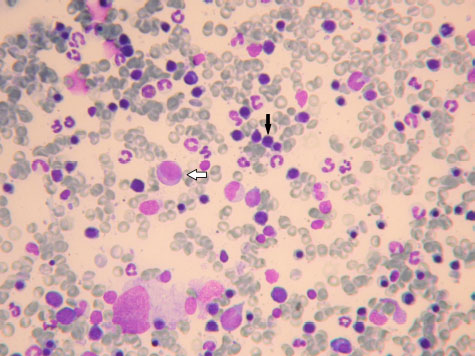
Fig. 3. Promyelocyte (white arrow) and several rubricytes (black arrow). May Grunwald Giemsa ×40 objective. Bone marrow cytological exams is often a second step exam that is requested only after the evidence of a hematological alteration, such as a cytopenia or particular clinical symptoms, like multiple enlarged lymph nodes, or after a suspicion of an infective or neoplastic disease that is known to have a particular tropism for the bone marrow tissue, like, for example, Leishmania Infantum protozoa (Harvey, 2012; Turinelli et al., 2015). In this retrospective study of 613 myelograms, we found 85 (14%) normal bone marrow cytological exams that were performed because of several reasons. A previous study (Weiss, 2006) reported a higher percentage of 21.8%. The most important indication was an already underlined hematological alteration (50.6% of the total cases), followed by a suspicion of bone marrow neoplastic infiltration (30.6% of the total cases). Only 11.8% of the bone marrows were performed because of a suspicion of infective disease, more often Leishmania Infantum, followed by Ehrlichia Canis. A small proportion (7%) of bone marrow cytological exams had an unknown indication and clinical history. For all the bone marrow exams a new CBC was performed, no matter if there was already a hemogram done. Of the 85 normal bone marrow samples, only 28 (32.9%) were associated to a normal CBC and could be considered, with a high grade of certainty, representative of a good functioning hematopoietic tissue. The other normal cytological bone marrow exams (67.1%) were associated to several hematological abnormalities, which the most important one was anemia alone (25.9%), or associated to other cytopenia, like for example, thrombocytopenia (11.8%). Only few cases (4.6%) had a pancytopenia. A bone marrow cytological exam without any morphological or numerical abnormalities but associated to one or more peripheral cytopenia should not be considered truly normal; the cytopenia should be considered, at least partially, of central origin, more often if there is bicytopenia or pancytopenia. A well-functioning bone marrow tissue should respond to a peripheral hypoxia following an anemia and the releasing of erythropoietin principally by the kidney, as well as the releasing of other cytokine that are produced in response to a particular cytopenia like, for example, thrombopoietin or granulocytic stimulating factor. If this do not happen, a bone marrow listlessness or a cytokine deficiency or inadequate production should be suspected. In these cases, a dosage of the relevant cytokine and a second myelogram, associated to a core biopsy for a simultaneous histological exam, should be performed in the next days, because, probably, some hypoplastic condition will appear. Table 1. Cytological bone marrow examination’s indications.
Table 2. Hemogram data.
In the altered hemogram group few cases of increased number of cells were found: 5 (5.9%) were characterized by an association of anemia and neutrophilic leukocytosis, 1 (1.2%) was an erythrocytosis and 1 (1.2%) a lymphocytosis. The number of neutrophils within the blood of dogs depends on several factors, including rate of release from the bone marrow, shifting of neutrophils between the marginal pool and the circulating pool in the blood and the emigration rate of neutrophils from blood into tissue. For this reason, it is possible that a neutrophilia could be associated to a normal myelogram (Shultze, 2000). Moreover, in the dog, the marrow neutrophils reserve is very important and contains approximately a 5-day supply of cells. This means that in those cases a recent stimulus could have called back the marrow storage neutrophils in the peripheral blood without evidence of a myeloid-granulocytic stimulation (Graham, 2000). Probably a second bone marrow exam performed some days after, could have been altered and a myeloid hyperplasia could have been found. Table 3. Hemogram data of the principal bone marrow disorders/indications.
The erythrocytosis associated to a normal myelogram could be an artefact or the consequence of splenic contraction (Watson, 2000) whereas the lymphocytosis could not be related to a bone marrow alteration because lymphocytes can come from other sites. They in fact are unique among leukocytes in their ability to recirculate (Shultze, 2000). The staging or the suspicion of lymphoma bone marrow metastasis represented the second most important indication for the bone marrow cytological exam (19 cases, 22.3% of all the cases and 46.1% of the neoplastic diseases). The majority of them has a normal CBC (63.1%). This finding is completely in agreement with the literature because it is already well known that stage V lymphoma, which is classically defined as involvement of the blood, bone marrow, and/or extranodal sites, is not as frequently identified as other stages of lymphoma when routine diagnostic tests are applied (Graff et al., 2014). Moreover, the incidence of lymphoma bone marrow metastasis seems to be low as it is demonstrated by two important retrospective study on bone marrow disorders, that show only 2.4% and 4% of incidences, respectively (Turinelli et al., 2015; Weiss, 2006). Inside the neoplastic disease indications, already diagnosed histiocytic sarcoma and suspected multiple myeloma were poorly represented (2.3% each one) and both were associated to a normal CBC (50%) or to and altered hemogram consisting of anemia plus leukocytosis (50%). This is in line with the literature because it is already known that both histiocytic sarcoma and multiple myeloma are characterized by bone marrow neoplastic cells infiltration/proliferation even if sometimes the neoplastic cells could be heterogeneously disseminated over the bone marrow sites; for this reason, it is suggested to sample multiple sites (Vail, 2000, 2013; Moore et al., 2006; Moore, 2017) or to perform core biopsy. This could explain the normal myelogram in those few cases found, otherwise, it is possible that the histiocytic sarcoma did not have already infiltrated the bone marrow and the multiple myeloma was not the reason of the clinical illness. Two cases of mast cell tumor (MCT) staging were included in the group and both have a normal CBC. This finding is in agreement with the literature, in fact, it has been already reported that buffy coat smears are not useful for the diagnosis or staging of canine MCTs and higher numbers of mast cells can be seen on the buffy coat smears of dogs with non-MCT diseases compared to dogs with MCT (Kiuppel, 2017). Moreover, the incidence of bone marrow MCT metastasis seems to be very rare with an incidence of 2.8% of cases in a previous study evaluating a series of 157 dogs presented for MCT in which a CBC and a myelogram were performed (Poggiani et al., 2013). Indeed, although systemic mastocytosis has been reported in dogs, this is a rare condition, and the vast majority of dogs with cutaneous MCTs do not have circulating mast cells or evidence of bone marrow infiltration (Dobson and Scase, 2007). Suspected or diagnosed infective diseases were the third most important indication for the myelogram. Inside this group leishmaniosis was the principal represented infection (5 cases suspected and 1 already diagnosed through serological exam; 7% of the total exams). Normal CBC was detected in half of cases (50%) whereas anemia, alone or associated to thrombocytopenia, was found in the other 50%. Bone marrow cytology of canine leishmaniosis is often characterized by erythroid hypoplasia, myeloid, plasma cells, and macrophages hyperplasia. Amastigotes can be found inside the cytoplasm of the macrophages or spared in the background but if they are few they cannot be found or, maybe, difficult to see. The most important CBC alteration is the presence of non-regenerative anemia (Paltrinieri et al., 2016). In this study, any bone marrow alterations were found, neither in the diagnosed case, nor in the suspected ones, maybe because the number of parasites was low or maybe because the dogs were not infected at all. A polymerase chain reaction test, PCR, on bone marrow samples, was suggested in all the cases. Two diagnosed and one suspected ehrlichiosis were included in this study (3.5%). All the cases presented an altered CBC, characterized by anemia alone or pancytopenia that is in line with the literature (Harrus and Waner, 2011). The normal myelogram could be explained because of an early stage of the disease in which the bone marrow is not already compromised, even if mild cytopenia are detected in the blood. With the progression of the disease the bone marrow is almost always affected, and the pancytopenia become more severe. This is the hallmark of the chronic and severe form (Harrus and Waner, 2011). Serial CBC and a second cytological bone marrow exam, together with a core biopsy for histological control were strongly recommended, for all the cases. Finally, only six normal myelograms (7%) were performed without any clinical or hematological indication; all those bone marrows were associated to a normal CBC. Those findings suggested that, probably, the clinical problem/suspicion did not involve the hematopoietic tissue. In conclusion, this study showed that a normal myelogram, if not associated to a normal CBC, rarely indicates a well-functioning hematopoietic system. If the hemogram is altered a normal myelogram should be considered as not representative of a well-functioning bone marrow tissue and the cause could be intrinsic or extrinsic (deficit of stimulating factors, alteration in the bone marrow cells receptors, stroma, etc.) and should lead to a delayed second investigation because bone marrow and blood are dynamic tissues that can change rapidly and not simultaneously. When possible, a core biopsy should be associated in order to better asses the cellularity and eventually the neoplastic infiltration. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesDobson, J.M. and Scase, T.J. 2007. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J. Small Anim. Pract. 48, 424–431. Graff, E.C., Spangler, E.A., Smith, Q., Denhere, M. and Brauss, M. 2014. Hematologic findings predictive of bone marrow disease in dogs with multicentric large-cell lymphoma. Vet. Clin. Pathol. 43(4), 505–512. Graham, S.S. 2000. Neutrophils. In Schalm’s veterinary hematology. 5th ed. Eds., Feldman, B.F., Zinkl, J.G., Jain, N.C. Philadelphia, PA: Lippincott Williams and Wilkins, pp: 281–294. Grindem, C.A., Neel, J.A. and Juopperi, T.A. 2002. Cytology of bone marrow. Vet. Clin. N. Am. Small Anim. Pract. 32, 1313–1374. Harrus, S. and Waner, T. 2011. Diagnosis of canine monocytropic ehrlichiosis (Ehrlichia canis): An overview. Vet. J. 187(3), 292–296. Harvey, J.W. 2001. Bone marrow examination. In Atlas of veterinary hematology. Eds., Harvey, J.W. Philadelphia, PA: W.B. Saunders Company, pp: 102–161. Harvey, J.W. 2012. Bone marrow examination, In: Veterinary hematology. A diagnostic guide and color atlas. Eds., Harvey, J.W. Veterinary Hematology.St Louis Missouri,LA: Elsevier Saunders, pp: 240–258. Kiuppel, M. 2017. Mast cell tumors. In Tumors in domestic animals. 5th ed. Eds., Meuten, D.J. Iowa, IA: Wiley Blackwell, pp: 176–194. Marconato, L., Martini, V., Aresu, L., Sampaolo, M., Valentini F., Rinaldi, V. and Comazzi, S. 2013. Assessment of bone marrow infiltration diagnosed by flow cytometry in canine large B cell lymphoma: Prognostic significance and proposal of a cut-off value. Vet. J. 197(3), 776781. Moore, P.F. 2017. Histiocytic diseases. In Tumors in domestic animals, 5th ed. Eds., Meuten, D.J. Iowa, IA: Wiley Blackwell, pp: 322–333. Moore, P.F., Affolter, V.K. and Vernau, W. 2006. Canine hemophagocytic histiocytic sarcoma: A proliferative disorder of CD11d+ macrophages. Vet. Clin. Pathol. 43, 632–645. Paltrinieri, S., Gradoni, L., Roura, X., Zatelli, A. and Zini, E. 2016. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 45(4), 552–578. Poggiani, S., Viana, F.F., Branquinho, R.P. and Gama, F.G. 2013. Bone marrow involvement in a dog with mast cell tumor. Braz. J. Vet. Pathol. 6(2), 69–72. Raskin, E.R. and Messick, J.B. 2012. Bone marrow cytologic and histologic biopsies: indications, techniques, and evaluation. Vet. Clin. North. Am. Small. Anim. 42, 23–42. Shultze, A.E. 2000. Interpretation of canine leukocytes response, in Feldman. In Schalm’s veterinary hematology, 5th ed. Eds., Zinkl, B.F., Jain, J.G. Philadelphia, PA: Lippincott Williams and Wilkins, pp: 366–379. Turinelli, V., Gavazza, A., Stock, G. and Fournel-Fleury, C. 2015. Canine bone marrow cytological examination, classification and reference values: a retrospective study of 295 cases. Res. Vet. Sci. 103, 224–230. Vail, D.M. 2000. Plasma cell tumors and macroglobulinemia. In Schalm’s veterinary hematology, 5th ed. Eds., Feldman, B.F., Zinkl, J.G., Jain, N.C. Philadelphia, PA: Lippincott Williams and Wilkins, pp: 654–659. Vail, D.M. 2013. Myeloma-related disorders. In Small animal clinical oncology, 5th ed. Eds., Withrow, J.S., Vail, D.M., Rodney, L.P. St Louis, LA: Elsevier, pp: 665–673. Watson, A.D.J. 2000. Erythrocytosis and polycythemia. In Schalm’s veterinary hematology. 5th ed. Eds., Feldman, B.F., Zinkl, J.G., Jain, N.C. PhiladelphiaPA: Lippincott Williams and Wilkins, pp: 216–221. Weiss, D.J. 2006. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004). J. Vet. Intern. Med. 20, 955–961. | ||
| How to Cite this Article |
| Pubmed Style Turinelli V, Rossi G, Gavazza A. Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Vet J. 2023; 13(2): 233-240. doi:10.5455/OVJ.2023.v13.i2.12 Web Style Turinelli V, Rossi G, Gavazza A. Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. https://www.openveterinaryjournal.com/?mno=113543 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i2.12 AMA (American Medical Association) Style Turinelli V, Rossi G, Gavazza A. Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Vet J. 2023; 13(2): 233-240. doi:10.5455/OVJ.2023.v13.i2.12 Vancouver/ICMJE Style Turinelli V, Rossi G, Gavazza A. Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Vet J. (2023), [cited July 01, 2025]; 13(2): 233-240. doi:10.5455/OVJ.2023.v13.i2.12 Harvard Style Turinelli, V., Rossi, . G. & Gavazza, . A. (2023) Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Vet J, 13 (2), 233-240. doi:10.5455/OVJ.2023.v13.i2.12 Turabian Style Turinelli, Vanessa, Giacomo Rossi, and Alessandra Gavazza. 2023. Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Veterinary Journal, 13 (2), 233-240. doi:10.5455/OVJ.2023.v13.i2.12 Chicago Style Turinelli, Vanessa, Giacomo Rossi, and Alessandra Gavazza. "Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms." Open Veterinary Journal 13 (2023), 233-240. doi:10.5455/OVJ.2023.v13.i2.12 MLA (The Modern Language Association) Style Turinelli, Vanessa, Giacomo Rossi, and Alessandra Gavazza. "Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms." Open Veterinary Journal 13.2 (2023), 233-240. Print. doi:10.5455/OVJ.2023.v13.i2.12 APA (American Psychological Association) Style Turinelli, V., Rossi, . G. & Gavazza, . A. (2023) Retrospective study of 613 canine cytologically normal bone marrows associated to altered hemograms: Description, possible interpretation and review of the principal physiological hematopoietic mechanisms. Open Veterinary Journal, 13 (2), 233-240. doi:10.5455/OVJ.2023.v13.i2.12 |