| Research Article | ||
Open Vet. J.. 2025; 15(7): 2993-3005 Open Veterinary Journal, (2025), Vol. 15(7): 2993-3005 Research Article Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbitsDalia M. A. Elmasry1*, Nayera M. Alatfeehy2, Mai M. Morsy2, Shaimaa H. Shaltot2, Heba Farouk2 and Aalaa S. A. Saad31Nanomaterial Research and synthesis Unit, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Giza, Egypt 2Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Giza, Egypt 3Biotechnology Research Department, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Giza, Egypt *Corresponding Author: Dalia M. A. Elmasry, Nanomaterial Research and synthesis Unit, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Giza, Egypt Email: dr_daliaelmasry [at] yahoo.com Submitted: 22/06/2025 Revised: 23/06/2025 Accepted: 23/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Listeria monocytogenes is a serious foodborne pathogen affecting public health and animal production. In rabbits, it causes systemic infections, leading to economic and welfare concerns. Rising antibiotic resistance highlights the need for natural alternatives to conventional antimicrobials. Aim: This study evaluated the antibacterial efficacy of propolis-pollen nanoemulsions (PP/NEs) against L. monocytogenes infection in rabbits, comparing its effect with that of ciprofloxacin and assessing its ability to modulate bacterial virulence gene expression. Methods: A total of 120 rabbits (10 weeks old) were randomly assigned to six groups (n=20/group). Group 1 served as a negative control. Group 2 (positive control) was orally infected with L. monocytogenes (1.3 × 109 CFU/ml). Group 3 received only PP/NE as prophylaxis before L. monocytogenes (1.3 × 109 CFU/ml) infection. Group 4 was infected and treated with the ciprofloxacin antibiotic (30 ml/kg). Group 5 was infected and treated with PP/NE. Group 6 infected and treated with a combination of ciprofloxacin (30 ml/kg) and PP/NE. Bacterial loads in liver and brain tissue samples were measured, and expression levels of the hlyA, inlA, and actA genes were evaluated using RT-PCR. Results: Treatment with PP/NE, either alone or combined with ciprofloxacin, significantly reduced bacterial counts compared with the positive controls. The liver and brain counts in the PP/NE+ciprofloxacin group were the lowest (5.67E+00 CFU, respectively). Virulence gene expression was significantly downregulated by day 21, particularly in the combination group, with hlyA, inlA, and actA levels of 0.097, 0.11, and 0.046, respectively. Conclusion: PP/NE exhibited strong antibacterial activity against L. monocytogenes and significantly reduced the expression of key virulence genes. It has potential as a natural therapeutic or prophylactic agent in rabbit farming and as a food safety enhancer, potentially reducing antibiotic reliance. Keywords: Listeria monocytogenes, Toxin genes, Propolis-pollen nano-emulsion, Antibiotics, Rabbits. IntroductionThe potential of the rabbit industry to boost Egyptian revenues and address the meat shortage is considerable. Egypt ranks among the world’s top five rabbit meat producers, with a significant contribution from small to medium-sized rabbit farms, resulting in high production levels (El-Samadony et al., 2019). Listeriosis caused by Listeria monocytogenes represents a serious concern for rabbit farming operations worldwide, with significant implications for animal health, farm productivity, and food safety. Clinical infections in rabbits typically manifest as encephalitis (circling disease), septicemia, or reproductive failure, often leading to high mortality rates, particularly among young and pregnant (OIE, 2022). The bacterium spreads primarily through contaminated feed, especially improperly fermented silage, and can persist in farm environments, making eradication challenging (Rodriguez et al., 2021). Subclinical infections may go undetected while still causing substantial economic losses through reduced growth rates, decreased reproductive performance, and increased susceptibility to secondary infections (Rodrigues et al., 2024). Beyond animal health impacts, L. monocytogenes contamination of rabbit meat poses food safety risks, as the pathogen can survive refrigeration and potentially cause severe human listeriosis (EFSA, 2022). Current control strategies emphasize strict biosecurity measures, proper feed storage, and emerging alternatives, such as probiotic supplementation and phage therapy (Pownall et al., 2021, Wang et al., 2024). Current practices for managing L. monocytogenes infections in rabbits are inadequate for several reasons: limited documentation results in underreporting, which hinders the development of effective prevention and treatment strategies. A study conducted in Portugal found that many L. monocytogenes isolates were resistant to sulfamethoxazole-trimethoprim, making treatment more complicated and underscoring the need for alternative therapies. Furthermore, the bacterium’s persistence in various environmental samples on farms poses significant challenges for biosecurity. Stress and poor husbandry practices also contribute to listeriosis by weakening the immune responses of rabbits (Rodrigues et al., 2024). Upon ingestion, L. monocytogenes breach the oral or gastrointestinal barrier and spreads to specific organs, evading detection by the body’s defenses in mammals. This invasion was facilitated by InlA and InlB (Wiktorczyk-Kapischke et al., 2023). Subsequently, L. monocytogenes is enclosed by a vacuole, which is then ruptured via a variety of enzymes, among them the pore-forming toxin listeriolysin (LLO) (produced by the hly gene), phosphatidyl-inositol phospholipase C (PI-PLC), and phosphatidylcholine phospholipase C (PC-PLC) produced by plcA and plcB, respectively (Elsayed et al., 2023). After entering the cytosol, L. monocytogenes uses the actin assembly-inducing protein (actA) produced by the actA gene to polymerize actin. This facilitates intracellular migration and the development of bacteria-containing protrusions (Rupp et al., 2015). PrfA is a crucial transcriptional regulator in L. monocytogenes, functioning as a dimeric protein within the Crp-Fnr superfamily. It enhances transcription by binding to a specific DNA sequence known as the PrfA box, which is an inverted repeat motif. This motif overlaps with the −35 region of various promoters and plays a significant role in the regulation of virulence-associated genes. By facilitating this interaction, PrfA effectively promotes the transcription of genes critical for pathogen virulence, as highlighted in the research by Gaballa et al. (2021). This process emphasizes how crucial PrfA is to L. monocytogenes’ pathogenicity. Propolis, a sticky and resinous substance, is created by honeybees using tree and plant materials. Its composition varies depending on factors such as season, location, and surrounding flora. Additionally, propolis contains waxes, resins, pollen, water, and impurities, along with acids, polyphenols, terpenes, esters, and minerals. Polyphenols, particularly flavones and flavonols, are its key components (Rendueles et al., 2023). Pollen is a natural antioxidant source rich in phenolic acids and flavonoids, which have health benefits such as anti-inflammatory, antibacterial, and immunostimulant effects (Rzepecka-Stojko et al., 2015). The practices for controlling L. monocytogenes infections remain inadequate because of limitations in the detection, treatment, and prevention of infections. Traditional diagnostic methods often lack sensitivity and can result in delayed or missed diagnoses, particularly in subclinical or early-stage infections (Ferreira et al., 2014). Moreover, L. monocytogenes exhibits remarkable environmental resilience, enabling it to persist in farm settings, food-processing environments, and within biofilms on equipment surfaces, where standard sanitation protocols may be ineffective (Carpentier and Cerf, 2011). Treatment is further complicated by the emergence of antibiotic-resistant strains, including those resistant to commonly used antibiotics such as sulfamethoxazole, trimethoprim, and tetracycline, as reported in both clinical and animal isolates. Additionally, underreporting and limited surveillance—especially in non-human species such as rabbits—impede the development of targeted control strategies and contribute to gaps in epidemiological data (Rodrigues et al., 2024). Finally, stress factors and inadequate husbandry practices in animal production settings compromise immune function, increasing susceptibility to infection and undermining the effectiveness of current management protocols (Asres and Amha, 2024). Collectively, these challenges underscore the need for novel therapeutic approaches, improved biosecurity, and more robust monitoring systems to effectively control Listeria infections. Propolis-pollen nanoemulsion (PP/NE) were formulated and studied for their antibacterial activity, effects on L. monocytogenes survival, and influence on toxin gene expression (hlyA, actA, and inlA), exploring their potential as an alternative to antibiotics or in combination with ciprofloxacin. Materials and MethodsIn our study, we used a field isolate of L. monocytogenes isolated from a rabbit farm. Strain confirmationStandard biochemical tests, including Gram staining, motility at 25 °C and 37 °C, catalase, oxidase, and acid generation from mannitol, rhamnose, and xylose, were then performed. β-hemolytic activity, and the Christine-Atkins Munch Petersen (CAMP) analyses of Listeria spp. isolates were performed (ISO, 2017). Then the confirmed isolates were kept at −80oC. The strain was confirmed to be positive for the 16s mRNA and virulence genes (hlyA, actA, inlA) (Gou, and Ahn, 2010, Swetha et al., 2015, Pospo et al., 2023). Preparation and characterization of PP/NEThe phase titration approach was used in the Nanotechnology Research Unit to prepare PP/NE, according to the changes described by Sorour et al. (2021) (patient registering with the Egyptian Academy of Scientific Research under number EG/P/2024/653). PP/NE was characterized using LC-MS/MS) by Nawha Company and the JEOL JSM-1400 transmission electron microscopy (TEM) model. The bactericidal activity of PP/NE on L.monostogenes strain by the JEOL JSM-1400. African green monkey kidney cells (Vero cells) were used to test the cytotoxicity of PP/NE. These cells were acquired from the source and cytotoxicity test evaluation by using Sulforhodamine B (SRB) stain (Vichai and Kirtikara, 2006). The minimum inhibitory concentration (MIC) of PP/NE against the L monostogenes ATCC 35152 strain (obtained from the Animal Health Research Institute). The efficacy of PP/NE was assessed using the thiazolyl blue tetrazolium bromide (MTT) technique, as per the instructions provided by Requena et al. (2019). 100 μl of Mueller–Hinton Broth (MHB) was added to each well of a 96-well plate. Following two-fold dilutions throughout the rows, 100 μl of the stock solution was added to the first well in each row. Therefore, the concentrations of the tested substances (PP/NE) ranged from 100% to 0.049%. Then, each well received a 100-μl inoculum of bacteria, equal to a 0.5 McFarland standard. The plates were incubated at 37 °C for 24 hours. Following incubation, each well received 10 μl of MTT solution, and the plates were then incubated for an additional 4 hours. When metabolically active cells reduced MTT levels, a color shift from colorless to blue was observed, indicating bacterial growth. The lowest concentration of the substance that successfully inhibited bacterial growth by avoiding this color shift was defined as the minimum inhibitory concentration or MIC. The experimental layoutBacterial strainThe L. monocytogenes field strain was selected from the isolated strains, which were maintained at a temperature of −80 °C for its samples. The final concentration was approximately 1.3×109 CFU/ml after activation by inoculation into Tryptone soya yeast extract broth (Oxoid) and incubation overnight at 37 °C. The experimental design is as follows:
Before the experiment, rabbits were bought from a local farm in the Giza governorate of Egypt. After allowing the rabbits to acclimate for 14 days and undergoing monitoring, we determined that the rabbits did not suffer from diarrhea.
Rabbits were divided into six groups of three replicates (20 rabbits /group):
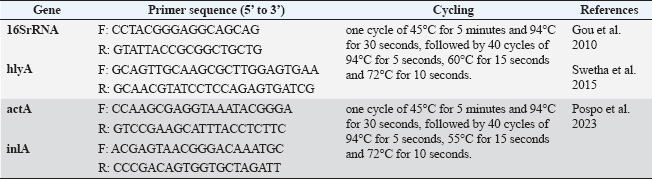
L. monocytogenes infection was induced in rabbits of Groups 2, 3, 4, 5, and 6 orally at a dose of 1.3 × 109 CFU/ml (single dose) administered at 10 weeks old. All L. monocytogene-infected groups were monitored until clinical signs appeared before the initiation of different treatment protocols. Antibiotic treatmentCiprofloxacin is a widespread and safe oral antibiotic for rabbits, so it was chosen as the treatment. Listeria monocytogenes viable counts in the liver and brain of slaughtered rabbits. We homogenized 25 g of each organ (liver and brain) in each group using a stomacher (Seward stomacher 80 Biomaster, England) and 225 ml of sterile peptone water broth (Oxoid). The materials were digested and serially diluted tenfold. One milliliter of each sample was plated immediately on 3 Listeria Chromogenic Agar Base plates using Ottaviani and Agosti (AlOA) agar medium. The inoculum was disseminated using a sterile bent glass streaking rod. To count L. monocytogenes, plates were incubated at 35ºC/48 hours after inoculation (International Organization for Standardization, 2017). Toxin gene relative expressionTissue extractionTo assess the impact of PP/NE on L. monocytogenes toxin gene expression, liver samples were randomly obtained from 10 rabbits in each treatment and control group on the 21st day of treatment (ciprofloxacin and PP/NE). Nucleic acid extractionFollowing the manufacturer’s instructions, liver samples were extracted using the PathoGene-spin™ DNA/RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea). As shown in Table 1, the samples were stored at −80°C after determining the RNA concentrations with the NanoDrop ND-1000 (NanoDrop, Wilmington, DE) and the synthetic oligonucleotide primers (Oligo) utilized in this study. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of L. monocytogenes toxin gene expression levelThe expression levels of hlyA, inlA, and actA were assessed by qRT–PCR in the excised spleens of each experiment group 21 days after inoculation. The tissue extracts were subjected to Trans Script® Green One-Step qRT-PCR SuperMix using aV2.2.2 software (AB Applied Biosystems) and the real-time PCR analysis primers listed in Table 1. Three sets of each sample were used. The toxin gene expression level estimates were normalized to the endogenous control (16S rRNA), and the fold changes in the target genes were computed using (Livak and Schmittgen, 2001) 2(−ΔΔ Ct) approach. Molecular dockingThe molecular docking analysis was performed to study the interaction of PP/NE compounds (1-Heptatriacotanol, 3-pentyl-2-oxiranyl undecanoate, trans-, Formic acid, and Bisabolol oxide B) [PP/NE was analyzed with LC-Mass-Mass chromatography] with the bacterial target prfA. The docking receptor with PDB ID: 6HCK was obtained from the Protein Data Bank (https://www.rcsb.org/structure/6HCK) on May 12, 2024. The molecular structures of 1-hepatocotanol, 3-pentyl-2-oxiranyl undecanoate, trans-, Formic acid, and ciprofloxacin (as a control) were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and the 3D structure of 1-hepatocotanol was predicted using Open Babel 2.3.2, http://openbabel.org (O’Boyle et al., 2011), and Avogadro 1.2.0 software was used to minimize the energy in their 3D designs (Hanwell et al., 2012). AutoDock Vina (Version 1.1.2) and AutoDock Tools (Version 1.5.7) were used to simulate the molecular docking. Specific docking was performed for all ligands. The receptor grid was created based on the positions of inhibitors cocrystallized with the protein, serving as the docking site for compounds in Glide. The grid box centered at coordinates −15.31, −11.241, and 10.807 was defined with dimensions of 40 points along the X, Y, and Z axes, with a grid spacing of 0.375 Å, to encompass the entire DNA molecule. The optimal DNA and ligand conformations were selected based on the lowest energy scores obtained from the docking simulations. The interactions and binding modes between the ligands and protein were analyzed using Discovery Studio. Statistical analysisData are presented as mean ± SD, with Student’s t-test applied as specified for each experiment. A probability (p) value of less than 0.05 was deemed statistically significant. Ethical approvalThe Institutional Animal Care and Use Committee (ARC-IACUC) at the Agricultural Research Center has approved the protocol (ARC-AHRI-5924) has approved study protocol from the ethical point of view and according to the 2014 Egyptian constitution article 45, the official decrees of the Ministry of Agriculture No. 27, 1967, the World Organization for Animal Health, and Guide for the Care and Use of Laboratory Animals 8th Edition 2011 (the Guide), which is reported in accordance to the ARRIVE guidelines 2.0: updated guidelines for reporting animal research (2020). ResultsStrain confirmationThe strain was Gram-positive and motile at 25 °C but nonmotile at 37 °C. It was positive for catalase and negative for oxidase. The strain did not produce acid from xylose or mannitol but rather generated acid from rhamnose. On sheep blood agar, a clear zone of hemolysis was observed, which was enhanced in proximity to a streak of Staphylococcus aureus, indicating a positive CAMP test. Table 1. qRT-PCR primers.
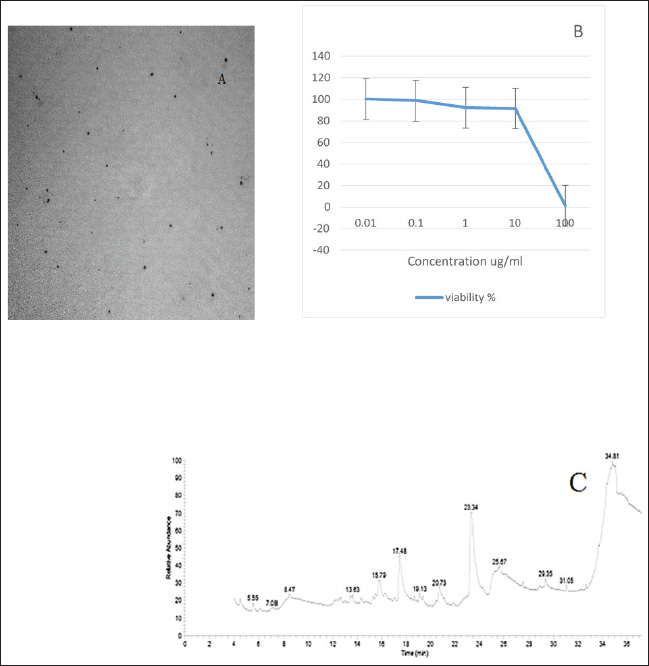
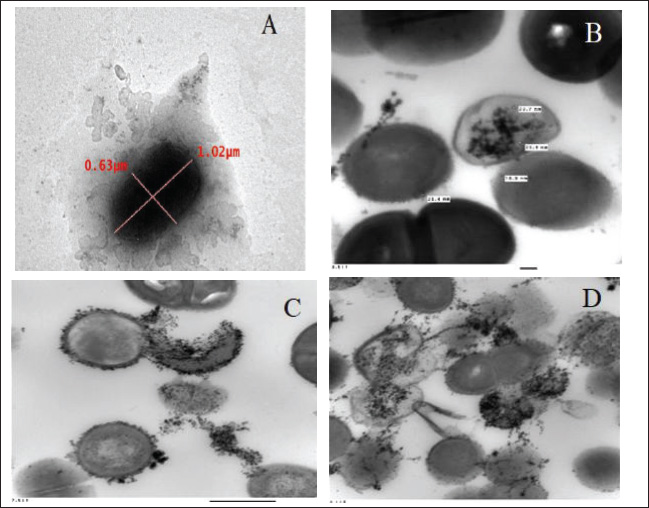
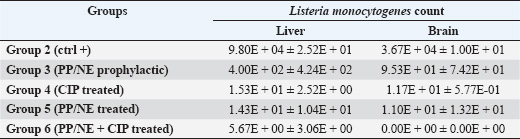
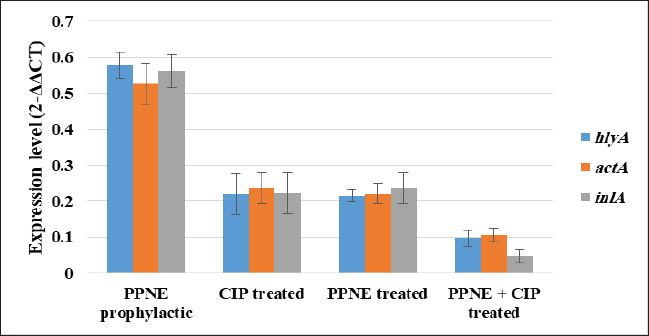
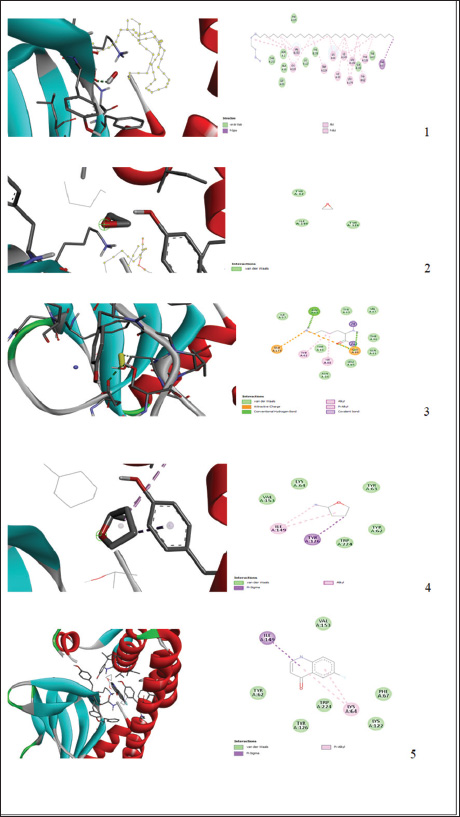
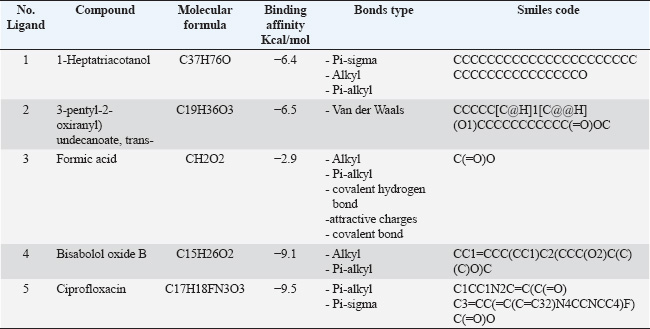
Preparation and characterization of PP/NEThe size distribution of the propolis pollen nanoemulsion (PP/NE) was limited, measuring 13.34 ± 2.102 nm. The results of the HRTEM investigation verified the spherical shape, size homogeneity, and lack of aggregation, as shown in Fig. 1A. Viability assessment (SRB assay)Viability Assessment (SRB Assay): The impact of PP/NE on cell viability varied with concentration. The value of IC50 was found to be 23.19 μg/ml, as shown in Fig. 1B. LC-MS/MS analysis of the nano-emulsion indicated the existence of 11-Heptatriacotanol; 2: 3-pentyl-2-oxiranyl) undecanoate, trans-; 3: Formic acid; and 4: bisabolol oxide B in high concentration. In addition, curcumadiol, Methyl oleate, Phenolic acids, 10,12-Pentacosadiynoic acid, Eupatorin, and quinindoline were used, as shown in Fig. 1C. The results of PP/NE effects on L. monocytogenes cells are indicated by TEM images after treatment for 2 hours, 4 hours, and 24 hours. TEM images showed that all untreated L. monocytogenes cells retained their typical round shape and exhibited smooth, undamaged surfaces. After 2 hours, PP/NE penetrated the L. monocytogenes bacteria, with PP/NE measuring 22 nm in size. After 4 hours, we observed the architectural image of cell wall breakdown and noted L. monocytogenes cells containing PP/NE. The bacterial cells ruptured, lost their shape, and were lysed after 24 hours (Fig. 2A–D). MIC of PP/NE on L. monocytogenesA striking shift in coloration was observed, providing a clear and unmistakable indication of the proliferation of L. monocytogenes. The minimum PP/NE concentration required for effective use was 187.5 µg/ml Experimental clinical signsGroup 2, which served as the positive control, exhibited nervous manifestations, including hind limb paralysis, erect ears, and intestinal disorders, such as diarrhea. Group 3, designated as the prophylactic group, exhibited intestinal disorders (diarrhea) but did not exhibit any nervous manifestations. In the other groups, group 4 did not exhibit any clinical signs after treatment with ciprofloxacin. Meanwhile, Groups 5 and 6, which received ciprofloxacin and a combination of ciprofloxacin and PP/NE, exhibited soft feces, potentially due to the effects of PP/NE. Listeria monocytogenes viable counts in the liver and brainAt the end of the experiment, conducted 21 days post-infection, the positive control group displayed high counts of L. monocytogenes, reaching 9.80E+04 ± 2.52E+01 log CFUs in the liver and 3.67E+04 ± 1.00E+01 log CFUs in the brain. In contrast, all treatment groups exhibited significant reductions in bacterial load (P ≤ 0.05, Student’s t-test). The PP/NE prophylactic group recorded reduced counts of 4.00E+02 ± 4.24E+02 in the liver and 9.53E+01 ± 7.42E+01 in the brain. The PP/NE-treated group exhibited even greater reductions, with counts of 1.43E+01 ± 1.04E+01 in the liver and 1.10E+01 ± 1.32E+01 in the brain. The ciprofloxacin-treated group showed similar declines, with counts of 1.53E+01 ± 2.52E+00 in the liver and 1.17E+01 ± 5.77E-01 in the brain. Notably, the group treated with both PP/NE and ciprofloxacin exhibited the most significant decrease in bacterial count, recording 5.67E+00 ± 3.06E+00 in the liver and complete bacterial clearance in the brain at 0.00E+00 ± 0.00E+00. These results indicate that the combination of PP/NE and ciprofloxacin was the most effective strategy for reducing the incidence of L. monocytogenes infection in both the liver and brain. Listeria monocytogenes could not be detected in the negative control group (Table 2). Results of the experiment’s toxin gene relative expressionRT-PCR measured expression levels of hlyA, inlA, and actA genes. In the PP/NE-prophy group, the relative mRNA expression levels of the hlyA, inlA, and actA genes decreased on 21 dpi to be 0.58 ± 0.04, 0.53 ± 0.05, and 0.56 ± 0.05, respectively. In the ciprofloxacin-treated group, the hlyA, inlA, and actA genes were 0.22 ± 0.06, 0.23 ± 0.04, and 0.22 ± 0.06, respectively, whereas those in the PP/NE-treated group were 0.21 ± 0.02, 0.22 ± 0.03, and 0.24 ± 0.04, respectively. The PPNE+ ciprofloxacin-treated group hlyA, inlA, and actA gene expression were 0.097 ± 0.022, 0.11 ± 0.02, and 0.046 ± 0.02, respectively (Fig. 3). Molecular dockingDocking simulationThe Avogadro software was used to prepare the protonated low-energy 3D ligand conformations. In addition, the protein-ligand interaction mechanism was demonstrated, and the binding energy was estimated using in silico molecular docking. Figure 4 and Table 3, respectively, show all of the effectively docked structures obtained from the molecular docking analysis of the investigated drugs (1-Heptatriacotanol, 3-pentyl-2-oxiranyl undecanoate, trans-, Formic acid, and ciprofloxacin) inside the prfA motif. Among the compounds that were studied, the predicted active site showed the highest binding affinity to the predicted active site of prfA bisabolol oxide B (−9.1 kcal/mol) and was comparable to the binding affinity of ciprofloxacin (control) (−9.5 kcal/mol). In addition, the binding free energy values observed for 1-Heptatriacotanol (−6.4 kcal/mol) and 3-pentyl-2-oxiranyl undecanoate (−6.5 kcal/mol) were almost identical. DiscussionThe evaluation of the internalization processes of nanoparticles in bacteria was greatly aided by TEM. These extremely thin bacterial cell cross-sections are used to characterize the internalization and endocytosis routes of nanoparticles. The size, shape, and basic composition of the nanoparticles, as well as their physical and chemical characteristics, were also examined (Phakatkar et al., 2022). Although conventional TEM has provided important insights into the mechanisms of nanoparticle internalization in bacteria, it is limited by the need to rely on snapshots taken at different time points during the process. In contrast, in-situ liquid TEM has great potential for real-time investigation of nanoscale endocytosis, which could be instrumental in developing new drug delivery systems to address multidrug resistance (Gao et al., 2021). There are currently two primary theories regarding silver nanoparticles: the release of ionic silver and direct contact following attachment on the bacterial cell wall (Gugala et al., 2022). Recent research has shown that mechanisms, including negative regulation porins, chromosomal resistance genes, or plasmids with resistance genes, may be responsible for the antibacterial efficacy of polymer-coated Ag-NPs when their concentration of silver ions (Ag) is reduced (Ashmore et al., 2018; Salas-Orozco et al., 2019). After incubating for 24 hours at 37 ºC, the PP/NE solution, comprising 10% propolis and 10% pollen concentration, exhibited remarkable antibacterial properties against L. monocytogenes in various dilutions. The minimum effective concentration of the solution was 187.5 µg/ml. These findings parallel those reported by Saad et al. (2024), who discovered that the propolis nanoemulsion was equally effective at the same concentration. Moreover, Mohdaly et al. (2015) noted that the optimal pollen extract concentration was 200 μg/ml. However, the superior efficacy of the PP/NE solution could be attributed to its unique nanoformula and the combination of propolis and pollen.
Fig. 1. (A): The transmission electron microscopy (TEM) study (A): The specimen had a spherical form, with an average size of nm. (B): The cell viability percentage of the nanoemulsion was evaluated using the SRB test. (C): LC-MS/MS analysis of nanoemulsion.
Fig. 2. Under the transmission electron microscopy (TEM) (A) L. monocytogenes bacteria morphology (B) Adsorption of PP/NE by L. monocytogenes bacteria after 2 hour (C) Rapture of L. monocytogenes bacteria cell due to PP/NE Effect after 4 hrs (D) Disappear of L. monocytogenes bacteria cell due to PP/NE effect after 24 hrs. Table 2. Listeria monocytogenes viable count (log CFU) from the liver and brain of slaughtered rabbits.
Fig. 3. Relative hlyA, inlA, and actA gene expression of L. monocytogenes in the PP/NE prophylactic group, PP/NE-treated group, ciprofloxacin-treated group, and PP/NE+ ciprofloxacin-treated groups. Values are expressed as the mean ± SD. The rabbit study demonstrated that the combination of PP/NE (phytochemical-based nanoemulsion) and ciprofloxacin significantly reduced L. monocytogenes loads in the liver (4.7-log reduction) and achieved complete sterilization in the brain (0 CFU), while also suppressing key virulence genes (hlyA, inlA, actA) by over 90%. These results are in good agreement with prior studies, such as Sahu et al. (2024), who reported a 2.8-log reduction in CFUs using thyme oil nanoemulsions in mice, and Van Bambeke et al. (2006), where ampicillin-gentamicin yielded a 3.5-log decrease in rats. Unlike these approaches, the PP/NE-ciprofloxacin combination uniquely prevents resistance and crosses the blood-brain barrier—an advantage over antisense RNA strategies (Toledo-Arana et al., 2009), which struggle with in vivo delivery. This research has revealed promising findings regarding the use of PP/NE against L. monocytogenes. PP/NE administration led to a remarkable decrease in the bacterial counts in both the liver and brain, significantly reducing the infection’s severity. In fact, the antibacterial efficacy of PP/NE closely matched that of ciprofloxacin, effectively targeting L. monocytogenes in these critical areas. While traditional antibiotics continue to show impressive antimicrobial properties, the emerging potential of PP/NE opens up exciting new avenues for disease management. This innovative approach could be a game-changer in our battle against infections. Antibiotics are well-known for their powerful antimicrobial effects, but the emerging potential of PP/NE offers some exciting benefits in disease management. Unlike conventional antibiotics, PP/NE is derived from natural products, significantly reducing the risk of developing antimicrobial resistance—an increasing concern in modern medicine. The innovative nanoemulsion delivery system enhances the bioavailability and targeted action of its bioactive compounds, ensuring a sustained therapeutic effect with minimal adverse effects (Preeti et al., 2023). This makes PP/NE an intriguing option as either an adjunct or alternative therapy, especially in situations where antibiotic resistance, toxicity, or long withdrawal periods are major hurdles. Embracing PP/NE could be a game-changer in the fight against disease (Wilson et al., 2022). Propolis is a natural substance that is effective against the bacterium L. monocytogenes, which is responsible for serious illnesses in humans. Research indicates that propolis alters the physical and chemical properties of the outer surface of bacteria, rendering it nonfunctional. This effect is primarily due to the accumulation and penetration of propolis components through the bacteria’s extracellular layer, leading to structural and morphological changes. These alterations increase surface permeability and disrupt the cell wall, ultimately leading to bacterial death. Notably, the antibacterial activity of propolis is largely physical, as it involves interactions between its constituents and bacterial cell wall structures. Overall, studies on propolis as a natural antimicrobial agent are promising and underscore the value of exploring natural solutions to combat infectious diseases (Vadillo-Rodríguez et al., 2021).
Fig. 4. 3D and 2D representations of the active sites 1: 1-Heptatriacotanol; 2: 3-pentyl-2-oxiranyl undecanoate, trans-; 3: Formic acid; 4: Bisabolol oxide B; 5: Ciprofloxacin active sites interaction between active sites and prfA active sites as observed with BIOVIA Discovery Studio. Table 3. Binding affinity, Bond type, and pi-pi stacking formed between the ligands and the protein residues at the binding site.
Bee pollen is an extraordinary substance rich in flavonoids and phenolic acids and known for its amphiphilic properties. These properties allow bee pollen to penetrate bacterial cell walls and disrupt the permeability of the cytoplasmic membrane. This damage prevents bacteria from accessing essential nutrients for survival. In addition, the compromised cytoplasmic membrane causes proteins to leak from the cell. This makes bee pollen highly effective at inhibiting bacterial growth and supporting overall health and wellness (Farhadi et al., 2019; Shamsudin et al., 2022). The combination of PP/NE and ciprofloxacin has shown a synergistic effect, suggesting that their joint use may be a hopeful therapeutic approach for treating L. monocytogenes infections. It is believed that propolis inhibits DNA polymerase, which could explain its enhanced effectiveness when used alongside agents that prevent the synthesis of proteins (Almuhayawi, 2020). The expression of toxins was significantly decreased in the PP/NE + ciprofloxacin group across different tissues during the experiment, and PP/NE exhibited an effect near the antibiotic (ciprofloxacin) on gene expression. Research has indicated that the natural compounds flavonoids found in propolis and pollen play a significant role in the inhibitory effect of PP/NE. These flavonoids have been found to influence the function of ribosomes, leading to the suppression of protein synthesis and affecting bacterial gene expression. Furthermore, studies have shown that propolis and pollen can hinder protein synthesis and RNA in both Gram-positive and Gram-negative bacteria. Numerous studies have demonstrated that flavonoids, which are abundantly present in propolis and pollen, significantly contribute to the antimicrobial activity of the PP/NE formulation. These compounds exert their action through multiple mechanisms, including interference with bacterial ribosomal function and the suppression of protein synthesis. For instance, quercetin and apigenin—flavonoids commonly found in propolis—bind to the bacterial ribosome, disrupting translation and inhibiting peptide elongation (Górniak et al., 2019). Additionally, flavonoids downregulate bacterial gene expression involved in virulence and cell wall synthesis (Almuhayawi, 2020). Propolis extracts have demonstrated a significant reduction in bacterial RNA and protein content, indicating the inhibition of transcription and translation pathways in both Gram-positive and Gram-negative bacteria (Nafea et al., 2022). These findings underscore the role of flavonoids in modulating core cellular processes in bacteria, supporting their contribution to the antimicrobial efficacy of PP/NE formulations. α-Bisabolol, a notable monocyclic-sesquiterpene, is naturally sourced from the essential oils of various edible and decorative plants. It has wide-ranging pharmacological benefits, among them antibacterial, neuroprotective, cardioprotective, anticancer, and antinociceptive qualities, which were recently shown in much research (Silva-Beltrán and Umsza-Guez, 2021). In this study, Bisabolol oxide B exhibited strong binding affinity for the active sites of the L. monocytogenes prfA protein, with a binding energy of −9.1 kcal/mol, indicating its potential to inhibit this key regulatory protein. Subsequent examination of the interactions between alpha-guanine and the active sites showed that the ligands interacted with the receptor in hydrophobic ways, including alkyl and pi-alkyl interactions. These interactions occurred at an average distance of 5 Å. The average distance at which these interactions occurred was 5 Å. In organic compounds, alkyl interactions—which are usually weak—occur between comparable, nonreactive carbon groups. Between aromatic and aliphatic groups, pi-alkyl interactions occur when the electron density of an alkyl group overlaps with the pi-electron cloud of an aromatic ring. ConclusionThe propolis-pollen nanoemulsion has demonstrated promising efficacy in reducing L. monocytogenes populations in rabbits. The nanoemulsion formulation enhances the bioavailability and penetration of propolis and pollen, making it more effective. Docking studies of the identified phytocompounds against the bacteria were conducted to complement the in vitro findings. The findings of this study highlight the promising potential of this innovative nanoemulsion as a safe and effective alternative for treating listeriosis in rabbits. The results suggest that this novel treatment could offer a viable solution for combating this severe infection while ensuring the well-being of rabbits. Moreover, propolis-pollen nanoemulsion shows potential as a natural antibacterial agent and preservative, contributing to a safer food supply while reducing the risk of antibiotic resistance. AcknowledgmentsThanks and appreciation to Prof. Dr. Anwar A. Eissa, Plant Protection Research Institute, for providing propolis and pollen. Thanks to the Science, Technology, and Innovation Funding Authority of Egypt for funding this work. FundingThis work was supported by the Science, Technology, and Innovation Funding Authority of Egypt. Innovation Grants (STDF-IG) call 8, proposal ID “43574”. Authors contributionAll authors were responsible for designing the experiment. Dalia Elmasry conducted the synthesis and characterization of nanomaterials, while Nayera, Mai Heba, and Shaimaa were in charge of the bacteriology part. Aalaa was responsible for the biotechnology and gene expression section, while NME, HF, MMM, SHA, and ASS performed the experiments and collected data. Each author contributed a unique section of the work. All authors have reviewed and approved the final manuscript. Conflicts of InterestThe authors declare that they do not have any conflicts of interest. Data availabilityAll authors declare that the materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for noncommercial purposes without breaching participant confidentiality. The docking receptor with PDB ID: 6HCK was obtained from the Protein Data Bank (https://www.rcsb.org/structure/6HCK) on May 12, 2024. The molecular structures of 1-hepatocotanol, 3-pentyl-2-oxiranyl undecanoate, trans-, Formic acid, and ciprofloxacin (as a control) were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and the 3D structure of 1-hepatocotanol was predicted using Open Babel 2.3.2, http://openbabel.org ReferencesAlmuhayawi, M.S. 2020. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 27(11), 3079–3086. Ashmore, D., Chaudhari, A., Barlow, B., Barlow, B., Harper, T., Vig, K., Miller, M., Singh, S., Nelson, E., and Pillai, S. 2018. Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Rev. Instit. Med. Trop. São Paulo 60, 18. Asres, A. and Amha, N. 2014. Effect of stress on animal health: a review. J. Biol. Agric. Healthc. 4, 116–121. Carpentier, B. and Cerf, O. 2011. Review--Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food. Microbiol. 145, 1–8. EFSA (European Food Safety Authority), 2023. The European Union one health 2022 zoonoses report. EFSA J. 21, e8442. El-Samadony, H.A., Mekky, H.M., Ghetas, A.M. and Saad, A.S. 2021. Molecular characterization of some isolates of rabbit viral hemorrhagic disease (VHD) in Egypt from 2014 to 2019. J. Adv. Vet. Anim. Res. 8, 396–403. Elsayed, M.M., Elkenany, R.M., Zakaria, A.I. and Badawy, B.M. 2022. Epidemiological study on Listeria monocytogenes in Egyptian dairy cattle farms’ insights into genetic diversity of multi-antibiotic-resistant strains by ERIC-PCR. Environ. Sci. Pollut. Res. 29, 54359–54377. Farhadi, F., Khameneh, B., Iranshahi, M. and Iranshahy, M. 2019. Antibacterial activity of flavonoids and their structure—Activity relationship: an update review. Phytotherapy Res. 33, 13–40. Ferreira, V., Wiedmann, M., Teixeira, P. and Stasiewicz, M.J. 2014. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J. Food Prot. 77, 150–170. Gaballa, A., Sunil, S., Doll, E., Murphy, S.I., Bechtel, T., Guariglia-Oropeza, V. and Wiedmann, M. 2021. Characterization of the roles of activated charcoal and Chelex in the induction of PrfA regulon expression in complex medium. PLoS One 16, e0250989. Gao, F., Shao, T., Yu, Y., Xiong, Y. and Yang, L. 2021. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 12, 745. Górniak, I., Bartoszewski, R. and Króliczewski, J. 2019. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272. Gou, J., Lee, H.Y., Ahn, J. 2010. Inactivation kinetics and virulence potential of Salmonella Typhimurium and Listeria monocytogenes treated by combined high pressure and nisin. J. Food Prot. 7, 2203–2210. Gugala, N., Salazar-Alemán, D.A., Chua, G. and Turner, R.J. 2022. Using a chemical genetic screen to enhance our understanding of the antimicrobial properties of copper. Metallomics, 14, mfab071. Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E. and Hutchison, G.R. 2012. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 4, 1–17. International Organization for Standardization. 2017. Microbiology of food chain: horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria Spp.- Part 1: Detection method. International Organization for Standardization, Geneva. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-CT method. Methods 25, 402–408. Mohdaly, A.A., Mahmoud, A.A., Roby, M.H., Smetanska, I. and Ramadan, M.F. 2015. Phenolic extract from propolis and bee pollen: composition, antioxidant and antibacterial activities. J. Food Biochem. 39, 538–547. Nafea, E.A., Yousef, A.D., Dereny, S.H.E., Abdel-Hameed, K.H.M., Mahfouz, H.M. and Farghaly, D.S. 2022. Impact of propolis on Escherichia coli and Bacillus subtilis based on total DNA, RNA and protein levels. Pak. J. Biol. Sci. 25, 859–866. Najdenski, H. and Vesselinova, A. 2002. Experimental mixed infection of rabbits with Yersinia enterocolitica and Listeria monocytogenes. J. Vet. Med. B. 49, 97–104. O’Boyle, N.M., Banck, M., James, C.A., Morley, C., Vermeersch, T. and Hutchison, G.R. 2011. Open Babel: an open chemical toolbox. J. Cheminf. 3, 1–14. OIE (World Organisation for Animal Health). 2022. Manual of diagnostic tests and vaccines for terrestrial animals. Geneva: World Organisation for Animal Health. Phakatkar, A.H., Ghildiyal, P., Wang, Y., Zachariah, M.R., Shokuhfar, T. and Shahbazian-Yassar, R. 2022. In-situ TEM studies on nanoparticle interactions with bacterial cells. Microsc. Microanal. 28(S1), 1104–1106. Pospo, T.A., Sultana, S., Rokon-Uz-Zaman, M., Mozumder, M.R., Parvin, M.S., Mohanta, U.K., Ahmed, M.M. and Islam, M.T. 2023. Hazard identification and characterization of Listeria monocytogenes in salad vegetables and milk products in Mymensingh district in Bangladesh. Appl. Food Res. 3, 100307. Pownall, W.R., Imhof, D., Trigo, N.F., Ganal-Vonarburg, S.C., Plattet, P., Monney, C., Forterre, F., Hemphill, A. and Oevermann, A. 2021. Safety of a novel Listeria monocytogenes-based vaccine vector expressing NcSAG1 (Neospora caninum surface antigen 1). Front. Cell. Infect. Microbiol. 11, 675219. Preeti, S.S., Malik, R., Bhatia, S., Al Harrasi, A., Rani, C., Saharan, R., Kumar, S., Geeta. and Sehrawat, R. 2023. Nanoemulsion: an emerging novel technology for improving the bioavailability of drugs. Scientifica 2023, 6640103. Rendueles, E., Mauriz, E., Sanz-Gómez, J., Adanero-Jorge, F. and García-Fernandez, C. 2023. Antimicrobial activity of Spanish propolis against Listeria monocytogenes and other Listeria strains. Microorganisms 11, 1429. Requena, R., Vargas, M. and Chiralt, A. 2019. Study of the potential synergistic antibacterial activity of essential oil components using the thiazolyl blue tetrazolium bromide (MTT) assay. LWT 101, 183–190. Rodrigues, I.C., Ribeiro-Almeida, M., Silveira, L., Prata, J.C., de Carvalho, A.P., Roque, C., Gomes, J.P., Borges, V., Pista, Â. and Martins da Costa, P. 2024. Unveiling a Listeria monocytogenes outbreak in a rabbit farm: clinical manifestation, antimicrobial resistance, genomic insights and environmental investigation. Microorganisms 12, 785. Rodriguez, C., Taminiau, B., García-Fuentes, E., Daube, G. and Korsak, N. 2021. Listeria monocytogenes dissemination in farming and primary production: sources, shedding and control measures. Food Control, 120, 107540. Rupp, S., Aguilar-Bultet, L., Jagannathan, V., Guldimann, C., Drögemüller, C., Pfarrer, C., Vidondo, B., Seuberlich, T., Frey, J. and Oevermann, A. 2015. A naturally occurring prfA truncation in a Listeria monocytogenes field strain contributes to reduced replication and cell-to-cell spread. Vet. Microbiol. 179, 91–101. Rzepecka-Stojko, A., Stojko, J., Kurek-Górecka, A., Górecki, M., Kabała-Dzik, A., Kubina, R., Moździerz, A. and Buszman, E. 2015. Polyphenols from bee pollen: structure, absorption, metabolism and biological activity. Molecules 20(12), 21732–21749. Saad, A.S., Ali, T.H.A., Alatfeehy, N. and Elmasry, D. 2024. Impact of propolis nanoemulsion on Listeria monocytogenes contaminating chilled stored breaded chicken panne. Adv. Anim. Vet. Sci. 12, 297–304. Sahu, A., Parai, D., Choudhary, H.R. and Singh, D.D. 2024. Essential oils as alternative antimicrobials: current status. Recent Adv. Anti-Infect. Drug Discov. 19, 56–72. Salas-Orozco, M., Niño-Martínez, N., Martínez-Castañón, G.A., Méndez, F.T., Jasso, M.E.C. and Ruiz, F. 2019. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: a literature review. J. Nanomater. 2019, 7630316. Shamsudin, N.F., Ahmed, Q.U., Mahmood, S., Shah, S.A.A., Khatib, A., Mukhtar, S., Alsharif, M.A., Parveen, H. and Zakaria, Z.A. 2022. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules 27, 1149. Silva-Beltrán, N.P., Umsza-Guez, M.A., Ramos Rodrigues, D.M., Gálvez-Ruiz, J.C., de Paula Castro, T.L. and Balderrama-Carmona, A.P. 2021. Comparison of the biological potential and chemical composition of Brazilian and Mexican propolis. Appl. Sci. 11, 11417. Sorour, H.K., Hosny, R.A. and Elmasry, D.M. 2021. Effect of peppermint oil and its microemulsion on necrotic enteritis in broiler chickens. Vet. World 14, 483. Swetha, C.S., Rao, T.M., Babu, A.J., Kumar, E. 2015. Process optimization for the detection of Listeria monocytogenes and listeriolysin O from spiked chicken meat by polymerase chain reaction. J. Meat Sci. 10, 7–15. Toledo-Arana, A., Dussurget, O., Nikitas, G., Sesto, N., Guet-Revillet, H., Balestrino, D., Loh, E., Gripenland, J., Tiensuu, T., Vaitkevicius, K. and Barthelemy, M. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature, 459, 950–956. Vadillo-Rodríguez, V., Cavagnola, M.A., Pérez-Giraldo, C. and Fernández-Calderón, M.C. 2021. A physico-chemical study of the interaction of ethanolic extracts of propolis with bacterial cells. Colloids Surf. B Biointerfac. 200, 111571. Van Bambeke, F., Barcia-Macay, M., Lemaire, S. and Tulkens, P.M. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Dev. 9(2), 218 Vichai, V. and Kirtikara, K. 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 1, 1112–1116. Wang, J., Deng, L., Chen, M., Che, Y., Li, L., Zhu, L., Chen, G. and Feng, T. 2024. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: a review of the literature of the last decade. Anim. Nutr. 244–264. Wiktorczyk-Kapischke, N., Skowron, K. and Wałecka-Zacharska, E. 2023. Genomic and pathogenicity islands of Listeria monocytogenes—overview of selected aspects. Front. Mol. Biosci. 10, 1161486. Wilson, R.J., Li, Y., Yang, G. and Zhao, C.X. 2022. Nanoemulsions for drug delivery. Particuology 64, 85–97. | ||
| How to Cite this Article |
| Pubmed Style Elmasry DMA, Alatfeehy NM, Morsy MM, Shaltot SH, Farouk H, Saad ASA. Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Vet. J.. 2025; 15(7): 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 Web Style Elmasry DMA, Alatfeehy NM, Morsy MM, Shaltot SH, Farouk H, Saad ASA. Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. https://www.openveterinaryjournal.com/?mno=266030 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.10 AMA (American Medical Association) Style Elmasry DMA, Alatfeehy NM, Morsy MM, Shaltot SH, Farouk H, Saad ASA. Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Vet. J.. 2025; 15(7): 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 Vancouver/ICMJE Style Elmasry DMA, Alatfeehy NM, Morsy MM, Shaltot SH, Farouk H, Saad ASA. Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 Harvard Style Elmasry, D. M. A., Alatfeehy, . N. M., Morsy, . M. M., Shaltot, . S. H., Farouk, . H. & Saad, . A. S. A. (2025) Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Vet. J., 15 (7), 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 Turabian Style Elmasry, Dalia M. A., Nayera M. Alatfeehy, Mai M. Morsy, Shaimaa H. Shaltot, Heba Farouk, and Aalaa S. A. Saad. 2025. Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Veterinary Journal, 15 (7), 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 Chicago Style Elmasry, Dalia M. A., Nayera M. Alatfeehy, Mai M. Morsy, Shaimaa H. Shaltot, Heba Farouk, and Aalaa S. A. Saad. "Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits." Open Veterinary Journal 15 (2025), 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 MLA (The Modern Language Association) Style Elmasry, Dalia M. A., Nayera M. Alatfeehy, Mai M. Morsy, Shaimaa H. Shaltot, Heba Farouk, and Aalaa S. A. Saad. "Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits." Open Veterinary Journal 15.7 (2025), 2993-3005. Print. doi:10.5455/OVJ.2025.v15.i7.10 APA (American Psychological Association) Style Elmasry, D. M. A., Alatfeehy, . N. M., Morsy, . M. M., Shaltot, . S. H., Farouk, . H. & Saad, . A. S. A. (2025) Highlight the role of propolis-pollen-nanoemulsion effect of Listeria monocytogenes infection in rabbits. Open Veterinary Journal, 15 (7), 2993-3005. doi:10.5455/OVJ.2025.v15.i7.10 |